Capsule Summary
This is the first study examining serotype-specific IgG responses following immunization with the polysaccharide vaccine Pneumovax® in infants aged 12 months in the absence of prior pneumococcal conjugate vaccine priming.
Keywords: Pneumococcal, immunogenicity, antibody, antigen specific immune response, children, infants
To the Editor
Streptococcus pneumoniae is a major cause of bacterial pneumonia, meningitis, bacteraemia and otitis media causing an estimated one million deaths per year worldwide in children under 5-years. The capsule of Streptococcus pneumoniae is the major virulence factor by which pneumococci cause invasive disease. Immunity against S. pneumoniae is mediated by phagocytosis of the organism in the presence of complement and serotype-specific antibody. Capsular polysaccharide antigens, classified as T-independent type II antigens (TI-2), are considered to directly stimulate B-lymphocytes to produce antigen-specific opsonic antibody without inducing immunological memory or affinity maturation. Polysaccharide antigens, with bound complement C3d, are recognised directly by the B lymphocyte through CD21, which associates with CD19 to enhance and prolong antigen signalling1. Based on a limited number of small studies, the ability to mount T-independent responses is considered to be absent in the neonate and poor in children less than 2-years of age2–4. This may be due to lower CD21 expression on neonatal and infant B cells and/or the immaturity of the splenic marginal zone5.
Pneumovax®, the 23-valent pneumococcal polysaccharide vaccine (23vPPV), contains 25μg of polysaccharide from each of 23 serotypes representing 85–90% of the serotypes causing invasive pneumococcal disease (IPD) in the USA. Current guidelines define an adequate response to 23vPPV immunization as a 4-fold increase on pre-immunization titre and/or a post-immunization titre ≥1.3μg/ml to at least 50% of serotypes tested for children aged 2–5 years and at least 75% of serotypes for older children and adults6. These guidelines are based on results from small study cohorts or studies examining a limited number of serotypes2–4, 6. Previous studies suggest that infants 6–12 months of age can respond to some antigens in 23vPPV; however, these studies measured responses to only a few serotypes in a small number of subjects, and used less specific older generation assay methods4–6. The ontogeny of the immune response to TI-2 antigens in early life has not been formally assessed in larger groups of children.
In this study, the immunogenicity of 23vPPV was evaluated in fifty-six healthy Fijian infants who received 23vPPV as their primary pneumococcal vaccination at 12 months of age. Serotype-specific IgG to the 23 serotypes in the vaccine was measured by a standardized ELISA using cell wall polysaccharide and serotype 22F double absorption7. The GMC and 95% confidence interval of serotype-specific IgG, prior to and 2-weeks post-immunization are reported in Table Ia. At 2-weeks post-23vPPV, a greater than 4-fold increase in IgG GMC was detected for 18 of 23 (78%) serotypes with a 2-fold increase in IgG GMC for serotypes 6B, 19A, 19F and 23F. There was a significant increase (p<0.05) over baseline at 2-weeks post-23vPPV for all serotypes except 14, 19F and 23F. This response was maintained at 5-months post-23vPPV for 13 of 23 (57%) serotypes (data not shown).
Table 1a.
Serotype-specific IgG GMC pre- and post-23vPPV immunization in infants at 12 months of age.
| Pre 23vPPV | 2-wks post 23vPPV | ||
|---|---|---|---|
| Serotype | GMC μg/mL (95% CI) | GMC μg/mL (95% CI) | p-value * |
| 1 | 0.17 (0.13, 0.22) | 2.04 (1.49, 2.80) | <0.0001 |
| 2 | 0.28 (0.23, 0.35) | 12.48 (9.66, 16.12) | <0.0001 |
| 3 | 0.37 (0.26, 0.53) | 13.74 (10.55, 17.91) | <0.0001 |
| 4 | 0.08 (0.06, 0.10) | 2.37 (1.76, 3.20) | <0.0001 |
| 5 | 0.26 (0.20, 0.34) | 2.77 (2.15, 3.57) | <0.0001 |
| 6B | 0.14 (0.12, 0.17) | 0.31 (0.23, 0.42) | <0.05 |
| 7F | 0.10 (0.08, 0.14) | 2.58 (1.98, 3.37) | <0.0001 |
| 8 | 0.26 (0.20, 0.32) | 13.59 (10.72, 17.23) | <0.0001 |
| 9N | 0.12 (0.09, 0.15) | 3.06 (2.18, 4.30) | <0.0001 |
| 9V | 0.09 (0.07, 0.12) | 1.21 (0.90, 1.62) | <0.0001 |
| 10A | 0.20 (0.16, 0.24) | 0.87 (0.65, 1.17) | <0.0001 |
| 11A | 0.10 (0.08, 0.13) | 2.21 (1.58, 3.08) | <0.0001 |
| 12F | 0.06 (0.05, 0.08) | 0.39 (0.28, 0.54) | <0.0001 |
| 14 | 0.20 (0.16, 0.25) | 0.41 (0.29, 0.59) | ns |
| 15B | 0.17 (0.14, 0.22) | 0.79 (0.59, 1.05) | <0.0001 |
| 17F | 0.11 (0.09, 0.13) | 1.39 (0.98, 1.97) | <0.0001 |
| 18C | 0.07 (0.05, 0.08) | 1.23 (0.88, 1.72) | <0.0001 |
| 19A | 0.29 (0.24, 0.36) | 0.79 (0.55, 1.12) | <0.05 |
| 19F | 0.47 (0.36, 0.62) | 1.15 (0.81, 1.62) | ns |
| 20 | 0.10 (0.08, 0.12) | 0.90 (0.61, 1.34) | <0.0001 |
| 22F | 0.36 (0.29, 0.46) | 5.23 (3.47, 7.88) | <0.0001 |
| 23F | 0.19 (0.15, 0.25) | 0.42 (0.30, 0.57) | ns |
| 33F | 0.14 (0.11, 0.17) | 2.01 (1.44, 2.80) | <0.0001 |
Number of infants = 56
comparison of serotype-specific IgG pre- and 2-weeks post-23vPPV immunization (paired t-test)
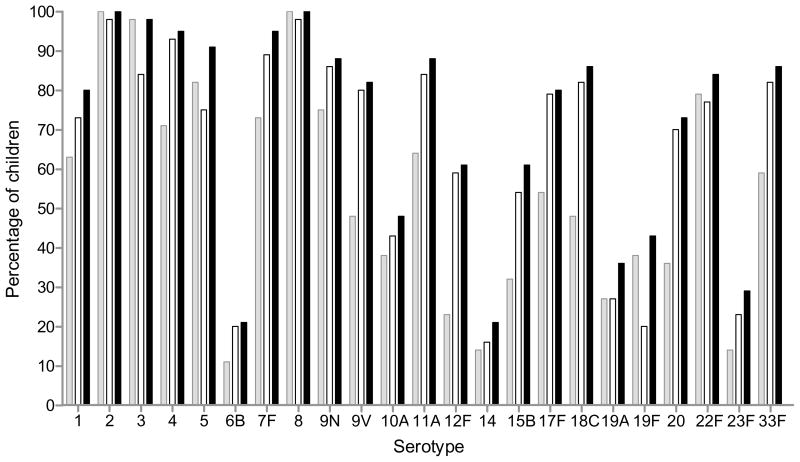
The percentage of infants achieving an ‘adequate response’ (either a 4-fold increase over baseline titre or a post titre ≥1.3μg/ml) to each serotype is shown in Figure 1. All infants responded with a post-titre ≥1.3μg/ml to serotypes 2 and 8. Ninety-eight percent of infants mounted an adequate response to 11 of 23 serotypes (Table Ib). Thirty-two infants (54%) responded to at least 17 of 23 serotypes. Two infants (4%) responded to all 23 serotypes. All infants responded to at least 9 of 23 serotypes. Serotypes 6B, 14 and 23F were the least immunogenic in this age group, with twelve infants (21%) producing an adequate response to serotypes 6B and 14 while 16 infants (29%) produced an adequate response to serotype 23F. Previous studies have also reported the low immunogenicity of serotypes 6B and 23F3.
Figure 1.
Proportion of infants at one year of age (n=56) with an adequate serotype-specific IgG response 2-weeks following 23vPPV immunization. An adequate response is defined as a post-immunization titre ≥1.3μg/ml and/or a 4-fold increase over baseline titre. Grey bars represent ≥1.3μg/ml; white bars represent a 4-fold increase on baseline titre and black bars represent either ≥1.3μg/ml and/or a 4-fold increase on baseline titre.
Table 1b.
The proportion of infants with a post-immunization titre ≥ 1.3 ug/ml, a 4-fold increase from baseline titre and an adequate response 2-weeks post-23vPPV immunization against the number of serotypes to which they respond.
| Number of serotypes | Post immunization titre > 1.3 ug/ml | A 4-fold increase from baseline titre | Adequate responsea |
|---|---|---|---|
| 22 | 2% | 5% | 5% |
| 19 | 5% | 16% | 25% |
| 17 | 14% | 39% | 54% |
| 14 | 41% | 73% | 82% |
| 11 | 64% | 89% | 98% |
Number of infants = 56
adequate response defined as either a post-immunization titre ≥1.3μg/ml and/or a 4-fold increase from baseline titres.
The number and selection of serotypes to be tested are critical to the evaluation of an adequate immune response to 23vPPV. The hierarchy of responses to pneumococcal serotypes 2-weeks post-23vPPV in this population confirms that the selection of serotypes for measurement of specific IgG by laboratories is critical. If serotype-specific immune responses in this cohort were evaluated for 12 of the more immunogenic serotypes (e.g. 2, 3, 4, 5, 7F, 8, 9N, 9V, 11A, 18C, 22F and 33F), an adequate response would be detected in all infants. Conversely, if immune responses to 12 of the least immunogenic serotypes were assessed (e.g. 1, 6B, 10A, 12F, 14, 15B, 18C, 19A, 19F, 20F and 23F), 38% or 21 infants would be considered to have an inadequate response. If it is impossible to test all serotypes in the vaccine, the decision on which serotypes to assay should be based on the patient population and the serotypes causing disease in that population.
In 1998, Shann8 suggested that a controlled trial of 23vPPV for children under 2-years of age was warranted considering the morbidity and mortality caused by S. pneumonia in this age group, the increasing antibiotic resistance of many of the bacterial strains and the cost and the limited availability of pneumococcal conjugate vaccines in developing countries. We have shown 23vPPV to be immunogenic in this age group with all infants enrolled in the study capable of producing adequate serotype-specific IgG to at least 9 of the 23 serotypes at 12 months of age.
Nevertheless there are several issues concerning the use of 23vPPV in children less than 2-years that require further clarification. It will be important to monitor the kinetics of the immune response over time, as recent studies and our own data raise the possibility that early administration of full dose 23vPPV may result in immune hyporesponsiveness to subsequent challenge with polysaccharide antigens9. Further investigations including disease incidence and nasopharyngeal carriage data are required to clarify this issue.
Acknowledgments
Sources of Funding:
Funding was provided by NIAID (2 U01 AI052337-05) and the Australian National Health and Medical Research Council (251648). Pneumovax ® was kindly donated by CSL Biotherapies, Australia.
Abbreviations
- 23vPPV
23-valent pneumococcal polysaccharide vaccine
- CD
Cluster of Differentiation antigen
- ELISA
Enzyme-linked immunosorbent assay
- IPD
Invasive Pneumococcal Disease
- TI-2
T-independent type II antigen
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breukels MA, Zandvoort A, Rijkers GT, Lodewijk ME, Klok PA, Harms G, et al. Complement dependency of splenic localization of pneumococcal polysaccharide and conjugate vaccines. Scand J Immunol. 2005;61:322–8. doi: 10.1111/j.1365-3083.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- 2.Blum MD, Dagan R, Mendelman PM, Pinsk V, Giordani M, Li S, et al. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine. 2000;18:2359–67. doi: 10.1016/s0264-410x(00)00021-9. [DOI] [PubMed] [Google Scholar]
- 3.Zielen S, Buhring I, Strnad N, Reichenbach J, Hofmann D. Immunogenicity and tolerance of a 7-valent pneumococcal conjugate vaccine in nonresponders to the 23-valent pneumococcal vaccine. Infect Immun. 2000;68:1435–40. doi: 10.1128/iai.68.3.1435-1440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomat WS, Lehmann D, Sanders RC, Lewis DJ, Wilson J, Rogers S, et al. Immunoglobulin G antibody responses to polyvalent pneumococcal vaccine in children in the highlands of Papua New Guinea. Infect Immun. 1994;62:1848–53. doi: 10.1128/iai.62.5.1848-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peset Llopis MJ, Harms G, Hardonk MJ, Timens W. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J Allergy Clin Immunol. 1996;97:1015–24. doi: 10.1016/s0091-6749(96)80078-9. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen RU, Leiva LE, Javier FC, 3rd, Sacerdote DM, Bradford N, Butler B, et al. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol. 1998;102:215–21. doi: 10.1016/s0091-6749(98)70089-2. [DOI] [PubMed] [Google Scholar]
- 7.Balloch A, Licciardi PV, Leach A, Nurkka A, Tang ML. Results from an inter-laboratory comparison of pneumococcal serotype-specific IgG measurement and critical parameters that affect assay performance. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Shann F. Pneumococcal vaccine: time for another controlled trial. Lancet. 1998;351:1600–1. doi: 10.1016/S0140-6736(05)77683-2. [DOI] [PubMed] [Google Scholar]
- 9.Russell FM, Carapetis JR, Balloch A, Licciardi PV, Jenney AW, Tikoduadua L, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]