The thalidomide tragedy of 1957–1961 was one of the greatest medical disasters of modern times. Worldwide the drug was prescribed to pregnant women to alleviate morning sickness, but it resulted in widespread spontaneous abortion and severe birth defects in over 10,000 babies, 40% of whom died before one year of age1. Many thalidomide victims are still alive, and their characteristic limb deformities are a potent reminder to society of the cost of an incomplete understanding of drug mechanisms and metabolism and inadequate in vivo testing before clinical use. Thalidomide’s safety was originally tested on mice, but, when administered to pregnant mice, the drug does not reach teratogenic levels in embryos as it does in other vertebrates.
Thalidomide was taken off the market in 1961 and remained so for over 30 years. Since then, many studies addressing the cause of thalidomide-induced birth defects have uncovered a plethora of biological activities for this drug1. What they found is that the limb deformities caused by thalidomide in human embryos result from the drug’s ability to inhibit angiogenesis—the growth of new blood vessels1,2. Consequently, thalidomide was adopted by oncologists as an antiangiogenic agent for the treatment of certain cancers, including multiple myeloma, leukemia and, more recently, prostate cancer3. Because of its anti-inflammatory effects, thalidomide has also been used under strictly controlled circumstances for the treatment of specialized inflammatory lesions that occur in leprosy.
In this issue of Nature Medicine, Lebrin et al.4 reveal another potential clinical application of this drug, namely prevention of severe bleeding in individuals with the genetic disorder hereditary hemorrhagic telangiectasia (HHT). These findings are great news for people with HHT, whose quality of life suffers greatly due to excessive bleeding from the nose and gastrointestinal tract. The findings might also have broader implications for those ten percent of stroke victims whose stroke is due to the rupture of weak or malformed vessels in the brain.
HHT affects between 1 in 10,000 and 1 in 1,330 individuals. Symptoms, which typically begin in adolescence, include severe nose bleeds and multiple cutaneous telangiectases. Telangiectases are dilated and thin-walled vascular dysplasias of the capillary bed that are prone to heavy bleeding from the nasal passages and the gastrointestinal tract. Some affected individuals also develop more dangerous internal vascular lesions, called arteriovenous malformations, in the liver, lung or brain. These large, dilated and tortuous vessels replace the normal vascular architecture of the organ, reducing vessel function and predisposing to hemorrhage and stroke.
Excessive angiogenesis is thought to cause telangiectases and may contribute to formation of arteriovenous malformations in HHT. Indeed, anecdotal studies of the antiangiogenic drug bevacizumab, an antibody against the vascular endothelial growth factor (VEGF), have indicated considerable benefit to patients with HHT5. One patient, on the waiting list for a liver transplant owing to her severe hepatic HHT, no longer required the transplant after bevacizumab treatment6.
Because thalidomide is an antiangiogenic drug, Levin et al.4 examined its potential role in treating HHT. HHT is mainly caused by mutations in genes encoding receptors for ligands in the transforming growth factor-β (TGF-β) superfamily. The mutations that cause HHT lower the expression of one of two genes, ACVRL1, which encodes the activin-like receptor kinase-1, an endothelial cell–specific TGF-β type I receptor, or ENG, which encodes endoglin, an auxiliary receptor that promotes signaling in response to a variety of TGF-β superfamily ligands, including TGF-β1.
Normally, in response to TGF-β, the classical TGF-β type I receptor signals through the proteins Smad2 and Smad3 to inhibit the growth of endothelial cells and to stimulate myogenic differentiation of adjacent pericytes—connective tissue cells that serve to support blood vessels. In contrast, in endothelial cells TGF-β signaling can also occur via the alternative type I receptor, Alk-1, with subsequent phosphorylation of the proteins Smad1 and Smad5, resulting in an activated cellular state associated with angiogenesis. Endoglin, which comes in several flavors due to its differential splicing and membrane shedding, can modulate both TGF-β type I receptor and Alk-1 signaling, resulting in complex patterns of TGF-β signal pathway activation.
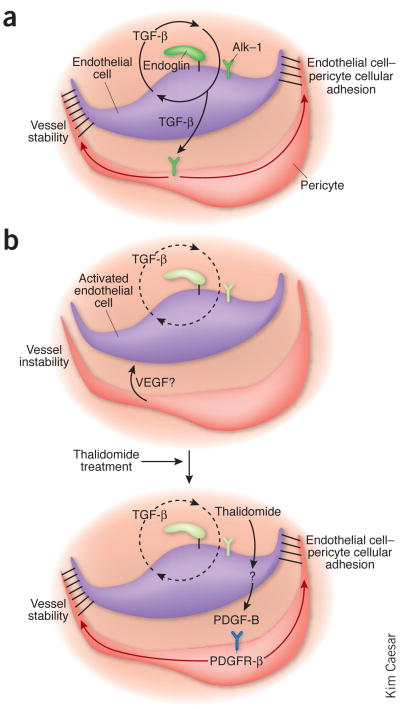
In the model presented by Lebrin et al.4, the endoglin–Alk-1 signaling pathway promotes TGF-β secretion from endothelial cells, which enhances close endothelial cell–pericyte apposition, possibly via platelet-derived growth factor-B (PDGF-B)7. The endothelial cell–pericyte interaction potentiates TGF-β activation8 and contributes to a positive feedback regulation through the classical TGF-β–Smad2-Smad3 pathway (Fig. 1). In Eng+/− mice, an animal model of HHT, the classical TGF-β–Smad2-Smad3 pathway is attenuated both in endothelial cells and in adjacent pericytes, resulting in increased endothelial cell proliferation and decreased pericyte differentiation.
Figure 1.
Thalidomide bypasses the requirement of the TGF-β1–endoglin–Alk-1 signaling pathway for vascular stability. (a) In normal developing vessels, TGF-β1 signals via endoglin, TGF-β type I receptor and Alk-1, in a complicated positive feedback loop, to enhance endothelial cell–pericyte contact, vessel quiescence and stabilization. TGF-β1 also directly stimulates pericyte differentiation. (b) In HHT, signaling by the endoglin–Alk-1 receptor complex is reduced, disrupting this positive regulatory loop and leading to a breakdown of endothelial cell–pericyte contacts and increased angiogenesis. Thalidomide administration in HHT stimulates PDGF-B expression, independently of the TGF-β1–endoglin–Alk-1 pathway, thereby bypassing a requirement for this regulatory pathway in vessel stability. The quiescent state of the endothelial cell is re-established by molecular interactions between the closely apposed pericyte and endothelial cell and the downregulation of angiogenic factors, such as VEGF. The molecular interactions stimulated by the intimate cellular contact between endothelial cell and pericyte redresses the balance from active angiogenesis toward a stable functional blood vessel.
In their study, Lebrin et al.4 used in vitro angiogenesis models and elegant in vivo studies of vascular development in genetically engineered mice to show that thalidomide inhibits excessive angiogenesis and stabilizes blood vessels. Eng+/− mice, which have lost one copy of the gene encoding endoglin, have increased endothelial angiogenesis and vascular branching in their developing retinas. Giving Eng+/− pups 75 mg per kg body weight thalidomide once daily normalized the vasculature, such that it resembled the vasculature of a wild-type retina. But at 150 mg per kg body weight, angiogenesis was strongly inhibited in both Eng+/− and wild-type mice, to such an extent that the pups were considerably stunted in growth. Most importantly, thalidomide increased the number of pericytes and their recruitment to blood vessels, enhancing the apposition between the inner endothelial and supportive pericyte layers and resulting in vessel stabilization in both wild-type and Eng+/− vessels.
Next, Lebrin et al.4 addressed the molecular mechanisms by which thalidomide inhibits angiogenesis in the Eng+/− mice. Previous studies, in zebrafish and humans, suggested that thalidomide inhibits angiogenesis by suppressing VEGF and its receptors9. In the current study, thalidomide-treated mouse retinas unexpectedly showed only marginally reduced Vegf mRNA levels compared to untreated controls. The authors then examined PDGF-B transcript levels4, as this growth factor is known to have a key role in pericyte chemotaxis and the promotion of tight endothelial cell–pericyte contacts. Importantly, there was a marked and rapid increase in Pdgfb mRNA levels in response to thalidomide, specifically within the endothelial tip cells of the developing retina, which are actively undergoing angiogenesis.
The observation that the antiangiogenic effects of thalidomide were prevented by concurrent administration of imatinib, a kinase inhibitor that blocks PDGF receptor-β but not VEGF receptor-1 or VEGF receptor-2 signaling, suggests a functional role for PDGF-B in this thalidomide-stimulated reduction in angiogenesis. However, it cannot be ruled out that this correction may be mediated via the c-Kit or Abl kinases, which are also inhibited by imatinib and might also have an impact on angiogenesis. Nevertheless, transgenic mice harboring a mutant, inactive Pdgfb gene did not show increased retinal pericyte coverage in response to thalidomide treatment. These findings support the notion that PDGF-β released from endothelial cells in response to thalidomide acts on surrounding pericytes and their precursors to stimulate vessel maturation, endothelial cell–pericyte connectivity and the resultant vascular quiescence (Fig. 1).
The study by Lebrin et al.4 sheds light on another mode of thalidomide action on the vascular system by demonstrating that the drug attenuates angiogenesis by bypassing the need for the endoglin–Alk-1 pathway in vessel stabilization. Targeting angiogenesis via this mechanism might prove effective in the treatment of HHT.
Despite these exciting findings, thalidomide should still be used with extreme caution in humans, not only because of the drug’s potent teratogenic effects within the dose range administered in the current study but also because fairly small differences in doses seem to result in dramatic differences in vascular outcomes both in vitro and in mice4. Moreover, many antiangiogenesis agents, including bevacizumab and thalidomide, have been associated with an increased risk of thrombosis in some patients. It is also likely that each patient will show distinct angiogenic responses to thalidomide treatment because of innate genetic variation among individuals. Certainly, in different mouse strains, genetic modifiers have been shown to influence growth factor–induced corneal angiogenesis10, vascular development in Tgfb1−/− embryos11 and the severity of vascular lesions in mature Eng+/− mice12. Most pertinent to this discussion, Robert d’Amato and his colleagues have shown that there are large strain differences in response to thalidomide-mediated inhibition of angiogenesis13.
Although thalidomide might not ultimately be the drug of choice for the treatment of HHT or other conditions, the value of probing deeper into its molecular mechanisms of action cannot be understated. Knowing how drugs like thalidomide work will help inform decisions about other therapies. For example, the angiogenesis inhibitor sunitinib should be avoided for vascular normalization in HHT, as it targets both VEGF and PDGF-B signaling. With the development of numerous new ‘smart’ drugs that inhibit specific signaling pathways, clinicians possess a panoply of tools with which to treat patients.
Drug selection should be made in an educated fashion. Understanding the molecular pathways that regulate both cell-autonomous and cell-to-cell cross-talk mechanisms of vascular stabilization should lead to new therapeutic combinations that may have fewer adverse side effects than bevacizumab or thalidomide.
Footnotes
Thalidomide, a drug reviled in the 1960s for its teratogenic effects, has been revived in recent years for cancer and leprosy therapy. A study now finds another use for this drug in vascular disease, providing further insights into the drug’s mechanisms of action (pages 420–428).
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Vargesson N. Bioessays. 2009;31:1327–1336. doi: 10.1002/bies.200900103. [DOI] [PubMed] [Google Scholar]
- 2.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Proc Natl Acad Sci USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeken JF, et al. Pharmacogenomics J. 2009 December 29; doi: 10.1038/tpj.2009.57. advance online publication. [DOI] [Google Scholar]
- 4.Lebrin F, et al. Nat Med. 2010;16:420–428. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 5.Flieger D, Hainke S, Fischbach W. Ann Hematol. 2006;85:631–632. doi: 10.1007/s00277-006-0147-8. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell A, et al. Liver Transpl. 2008;14:210–213. doi: 10.1002/lt.21417. [DOI] [PubMed] [Google Scholar]
- 7.Daniel TO, Gibbs VC, Milfay DF, Williams LT. J Biol Chem. 1987;262:11893–11896. [PubMed] [Google Scholar]
- 8.Hirschi KK, Burt JM, Hirschi KD, Dai C. Circ Res. 2003;93:429–437. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 9.Li X, et al. Anticancer Res. 2003;23:2481–2487. [PubMed] [Google Scholar]
- 10.Rogers MS, Rohan RM, Birsner AE, D’Amato RJ. FASEB J. 2003;17:2112–2114. doi: 10.1096/fj.03-0246fje. [DOI] [PubMed] [Google Scholar]
- 11.Tang Y, et al. Hum Mol Genet. 2003;12:1579–1589. doi: 10.1093/hmg/ddg164. [DOI] [PubMed] [Google Scholar]
- 12.Bourdeau A, et al. Am J Pathol. 2001;158:2011–2020. doi: 10.1016/s0002-9440(10)64673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rohan RM, Fernandez A, Udagawa T, Yuan J, D’Amato RJ. FASEB J. 2000;14:871–876. doi: 10.1096/fasebj.14.7.871. [DOI] [PubMed] [Google Scholar]