Abstract
In order to generate substantial amounts of neoglycoconjugate needed for commercialization of diagnostic kits and high-throughput detection of leprosy, we developed a facile and high-yield synthesis of the corresponding disaccharide. Herein, the non-reducing disaccharide segment of phenolic glycolipid I from Mycobacterium leprae, O-(3,6-di-O-methyl-β-D-glucopyranosyl)-(1→4)-O-2,3-di-O-methyl-α-L-rhamnopyranose was synthesized by an improved procedure. The disaccharide was efficiently conjugated to bovine/human serum albumin, via acyl-azide intermediate, to form natural disaccharide-BSA/HSA neoglycoproteins that showed a high activity in serodiagnosis of leprosy. The disaccharide incorporated into the proteins was accurately measured by MALDI-ToF mass spectrometry. The serological activities of the neoglycoproteins against pooled human lepromatous leprosy were measured by ELISA and they were detectable at picogram amount.
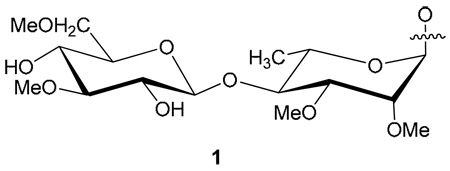
Leprosy, also known as Hansen’s disease, was the first human disease shown to be caused by bacterium. The prime infectious targets of Mycobacterium leprae (M. leprae), the causative pathogen of leprosy, are peripheral nerves and more specifically the Schwann cells where M. leprae colonizes by attaching to several receptors, such as α-dystroglycan, a laminin-2 receptor in the Schewann cell plasma membrane. 1 The bacterial ligand that binds to these receptors on host cell is a glycolipid termed as phenolic glycolipids I (PGL-I) that was only found in M. leprae. 2 The structure of PGL-I, a trisaccharide [O-(3,6-di-O-methyl-β-D-glucopyranosyl)-(1→4)-O-(2,3-di-O-methyl-α-L-rhamnopyranosyl)-(1→2)-3-O-methyl-α-L-rhamnopyranose] glycosidically linked to a phenolic phthiocerol core has been firmly established. 3 PGL-I is present in copious amounts in M. leprae and it invades human nerves by binding to the receptors on the Schwann cells, 2 and a specific antibody against PGL-I is produced in sera of leprosy patients. Therefore, the trisaccharide as well as the corresponding disaccharide [O-(3,6-di-O-methyl-β-D-glucopyranosyl)-(1→4)-O-2,3-di-O-methyl-α-L-rhamnopyranose], located at the non-reducing end of PGL-I, were found to be serologically active. 4 The possibility of using PGL-I based neoglycoconjugates (di-, or tri-saccharides) in the serodiagnosis of leprosy infection by an enzyme-linked immunosorbent assay (ELISA) prompted several research groups to synthesize the haptens and link them to the protein carrier bovine serum albumin (BSA). 2, 5–8 In this work, we have described an efficient and improved synthesis of the constituents of the natural disaccharide (ND) 1 from PGL-I. Furthermore, the number of glycosyl residues incorporated on the proteins was measured accurately using mass spectrometry.
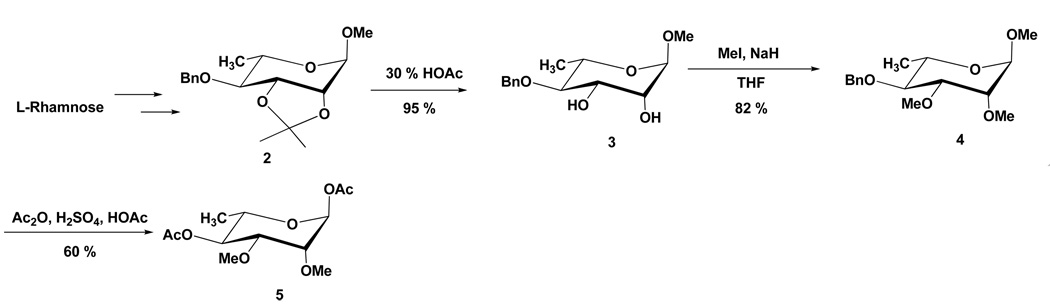
The key intermediate in our synthesis of the disaccharide 1 is 1,4-di-O-acetyl- 2,3-di-O-methyl-L-rhamnopyranose (5). L-Rhamnose was converted into methyl 4-O-benzyl-2,3-O-isopropylidene-α-L-rhamnopyrsnoside (2) by published methods. 9,10 Removal of the isopropylidene group followed by methylation yielded the corresponding 2,3-dimethoxy derivative 4. The main feature of this improved synthesis is the one-step conversion of 4 into the desired 1,4-diacetate 5 by acetolysis with 2% sulfuric acid in acetic anhydride and acetic acid (Scheme 1). Purification of the product by chromatography on silica gel removed a trace amount of a byproduct which was found to be the corresponding 1-acetoxy-4-O-benzyl derivative. Continued elution gave the main product 5 in 60% yield. 11
Scheme 1.
The other intermediate needed for the synthesis of the disaccharide is 1,2,4-tri-O-acetyl-3,6-di-O-methyl-D-glucopyranose (6). In the previous synthesis, this intermediate had been obtained by a nine-step process from 3-O-methyl-D-glucose. 12 In the present work, compound 6 was synthesized by a five-steps procedure starting from the commercially available 6,3-D-glucurono lactone as described. 13 Compound 6 was then converted to the chloroacetate derivative of 3,6-di-O-methyl-D-glucose (7, not shown).
Previously, 2,8 the 1,4-disaccharide was synthesized by coupling of a protected rhamnoside intermediate (a debenzylation product of compound 2) and a derivative of 3-O-methyl-D-glucosyl bromide. The methylation process was completed only after the deprotection of the disaccharide and selective methylation of the 2, 3-OH groups of rhamnose and the 6-OH of glucose. However, in this improved approach, the methyl groups were introduced before the coupling of the two hexose moieties. The building blocks 5 and 7 were obtained in high yield from compound 2 and 6,3-D-glucurono lactone, respectively.
The synthesis of disaccharide intermediate (11) to be conjugated to the BSA was initiated by the conversion of the building block 5 into the corresponding 8-methoxycarbonyloctyl glycoside 8 in 78% yield (Scheme 2). Subsequent deacetylation of compound 8 produced compound 9, which was coupled to the gluco-intermediate 7 to give the disaccharide derivative 10 in 67% yield for two steps of conversion. Finally, deacetylation of 10 quantitatively gave the methoxycarbonyloctyl derivative of the natural disaccharide (11).
Scheme 2.
Conjugation of the methoxycarboxyl-octyl intermediate (11, Scheme 3) to the carrier proteins (either BSA or HSA) proceeded by converting it into the corresponding hydrazide derivative 12 (which could be stored for several months at −20 ° C) in 81 % yield. The hydrazide was then converted into the acyl azide, which was immediately conjugated to BSA or HSA, according to the established procedure. 12,14 The carbohydrate content in these two neoglycoproteins, ND-O-BSA and ND-O-HSA was measured by the carbohydrate assay (phenol-H2SO4 assay) as described 5 and MALDI-ToF mass spectrometry. As shown in Figure 1, ND-O-HSA (B) exhibited higher heterogeneity compared with HSA (A) in the MALDI-ToF mass spectra. The single charged (m/z, 66372 and 87132) and double charged (m/z, 33186 and 43566) monomer of HSA and ND-O-HSA were the major components. The number of disaccharide molecules incorporated in the neoglycoproteins was calculated from the difference in the molecular weights of the neoglycoproteins and BSA or HSA. ND-O-HSA showed higher incorporation of the disaccharide (n=40) than ND-O-BSA (n=32). Thus, MALDI-TOF mass spectrometry provides an accurate and simple method to determine the degree of the conjugation of the disaccharides and the proteins.
Scheme 3.
Figure 1.
MALDI-TOF mass spectrum of HSA (A) and ND-O-HSA (B).
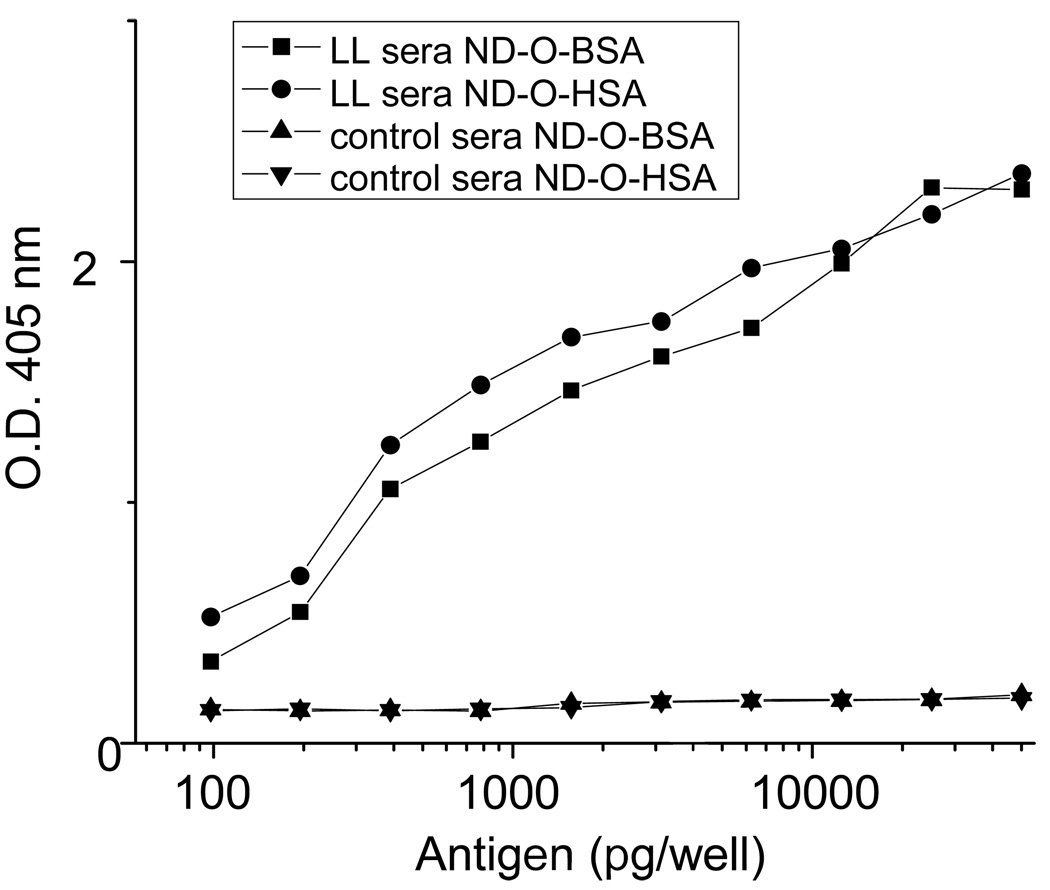
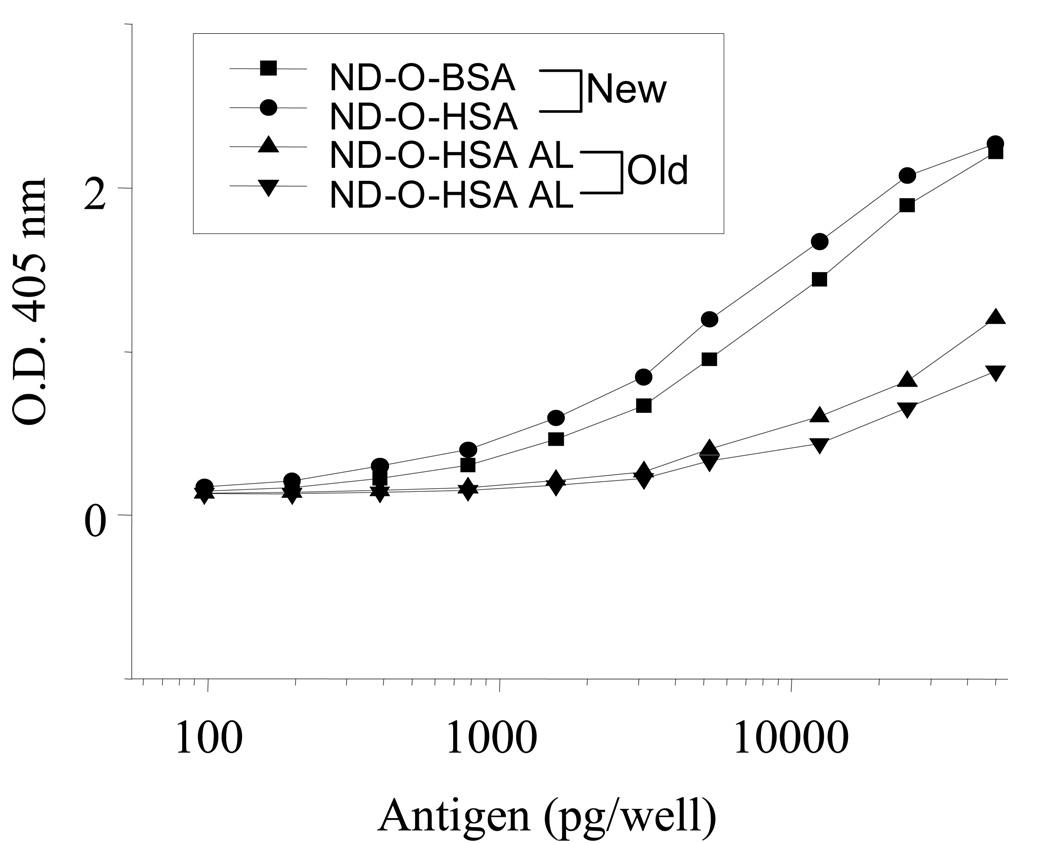
The serological activities of these two neoglycoproteins against pooled human lepromatous leprosy (LL) and normal sera were tested by ELISA. As shown in Figure 2, ND-O-BSA and ND-O-HSA showed the detection limit at 100 picogram (pg)/well, where the assays using LL sera had obvious difference of O.D.405nm compared to the assays using normal control sera. The higher sensitivity of ND-O-HSA compared to ND-O-BSA at the region of 100 to 10000 pg/well may be caused by its higher incorporation of ND onto HSA. In addition, the activity of the newly synthesized antigens were compared to the old batches provided by Leprosy contract (NIH N01 AI-25469). As shown in Figure 3, compared with the old batches, both of new ND-O-BSA and ND-O-HSA showed incremented activity along with the increase of antigen concentration from 100 pg to 50 nanogram (ng)/well. Especially when antigens were at the concentration of 50 ng/well, the new antigens showed double activity compared to the old ones.
Figure 2.
Reactivity of pooled lepromatous leprosy (LL) patient sera with a known high antibody titer to native PGL-I and normal control sera with ND-O-BSA (32 sugar residues per molecule) and ND-O-HSA (40 sugar residues per molecule) by ELISA. The wells were coated with two-fold serial dilutions of the antigen ranging from 50 ng to 100 picograms. The antigens were each tested with the pooled serum samples at a 1:200 dilution. The O.D. readings were determined using a plate reader at 405 nm.
Figure 3.
Reactivity of monoclonal antibody CS-48 specific for ND-O-BSA and ND-O-HSA by ELISA. The glycoconjugates tested were prepared by using an improved synthesis described in this work (New batches) or by an earlier coupling procedure (Old batches). The wells were coated with two-fold serial dilutions of the antigen ranging from 50 ng to 100 picograms. The O.D. readings were determined using a plate reader at 405 nm.
Due to call for large amount of leprosy diagnostic reagent, we started searching synthetic and sensitive analogues of PGL antigens. The newly made neoglycoproteins, ND-O-BSA and ND-O-HSA, carrying high natural disaccharide content (30~40 ND incorporated) showed higher sensitivity compared to the ones used before to detect M. leprae. Especially, the ND-O-HSA containing 40 natural disaccharides possessed the detection limit at pg grade and had the potentiality to be developed as a high-throughput diagnostic kit that can examine 2×105 serum samples by every 10 mg of the reagent. In addition, the synthetic antigens will serve as an ideal probe to fabricate carbohydrate microarray that will be used to diagnose infectious diseases.
Acknowledgements
The work was supported by NIH/NIAID, contract N01-AI25469, "Leprosy Research Support".
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Rambukkana A, Yamada H, Zanazzi G, Mathus T, Salzer JL, Yurchenco PD, Campbell KP, Fischetti VA. Science. 1998;282:2076–2079. doi: 10.1126/science.282.5396.2076. [DOI] [PubMed] [Google Scholar]
- 2.Ng V, Zanazzi G, Timpl R, Talts JF, Salzer JL, Brennan PJ, Rambukkana A. Cell. 2000;103:511–524. doi: 10.1016/s0092-8674(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 3.Hunter SW, Fujiwara T, Brennan PJ. J Biol Chem. 1982;257:15072–15078. [PubMed] [Google Scholar]
- 4.Chatterjee D, Cho SN, Stewart C, Douglas JT, Fujiwara T, Brennan PJ. Carbohydr Res. 1988;183:241–260. doi: 10.1016/0008-6215(88)84078-3. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee D, Cho SN, Brennan PJ, Aspinall GO. Carbohydr Res. 1986;156:39–56. doi: 10.1016/s0008-6215(00)90098-3. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara T, Aspinall GO, Hunter SW, Brennan PJ. Carbohydr Res. 1987;163:41–52. doi: 10.1016/0008-6215(87)80163-5. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara T, Hunter SW, Brennan PJ. Carbohydr Res. 1986;148:287–298. doi: 10.1016/s0008-6215(00)90396-3. [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara T, Hunter SW, Cho SN, Aspinall GO, Brennan PJ. Infect Immun. 1984;43:245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haines AH. Carbohydrate Research. 1969;10:466–467. [Google Scholar]
- 10.Liptak ANP, Neszmelyi A, Wagner H. Tetrahedron. 1980;36:1261–1268. [Google Scholar]
- 11.Synthesis of the diacetate 5: To a cold (ice-bath) solution of methyl 4-O-benzyl-2,3-di-O-methyl-α-L-rhamnopyranpside (380 mg) in acetic acid (2 mL) and acetic anhydride (2 mL) was added a 10% solution of sulfuric acid in acetic acid (1 mL) and the mixture was then stirred at room temperature overnight. Ice, solid sodium bicarbonate and ethyl acetate were added and the two layers were separated. The organic layer was washed with water, dried and evaporated. The residue was dried under vacuum and chromatographed on silica gel (70–230 mesh). Elution with ethyl acetate-petroleum ether 1:1 removed trace amount of 1-O-acetyl-4-O-benzyl-2,3-di-O-methyl-α-L-rhamnopyranoside. 1H NMR (CDCl3, 300 MHz): δ 7.36-7.34 (m, 5 H, aromatic), 6.18 (s, 1 H, H-1), 4.91, 4.62 (2 d, J gem= 10.8 Hz, OCH2Ph), 3.56, 3.55, (2 s, 3 H each, OCH3), 2.09 (s, 3 H, C(O)CH3), 1.31 (d, 3 H, J 6,5= 6.0 Hz, CH3-C5). Continued elution with the same solvent system gave product 5 (218 mg; 60%). 1H NMR (CDCl3, 300 MHz): δ 6.17 (d, J= 2.1 Hz, 1H, H-1), 5.08 (t, J= 9.9 Hz, 1H, H-4), 3.80 (dq, J= 6.3, 9.9 Hz, 1H, H-5), 3.48. 3.38 (2s, 6H, OMe), 2.08, 2.04 (2s, OAc), 1.13 (d, J=6.3, C5-Me).
- 12.Chatterjee D, Douglas JT, Cho S-N, Rea TH, Gelber RH, Aspinall GO, Brennan PJ. Glycoconjugate J. 1985;2:187. [Google Scholar]
- 13.Liav A, Goren MB. Carbohydr Res. 1986;149:C13–C16. doi: 10.1016/s0008-6215(00)90070-3. [DOI] [PubMed] [Google Scholar]
- 14.Synthesis of neoglycoproteins: a stirred solution of the hydrazide (11 µmol, 6 mg) in dry DMF (125 µL) was cooled to −30 °C, and 3.6 M HCl-1,4-dioxane (25 µL) was added. Then tert-butyl nitrite in DMF (1:10, 40 µL) was added, and the solution was stirred for 30 min at −30 °C. TLC in solvent (EtOAc/MeOH/H2O, 6:2:1) showed the disappearance of the hydrazide and formation of a new component. The excess of nitrous acid was neutralized with 0.5 M sulfamic acid (40 µL). The cold solution (−50 °C) of acyl-azide was added dropwise to a solution of BSA/HSA (8 mg, 0.1 µmol) in 0.08 M Na2B4O7 (0.8 mL) and 0.3 M KHCO3, pH 9.2 at 0 °C, stirred overnight at 0 °C, and dialyzed against four changes of de-ionized water in an ultrafiltration cell equipped with a PM-10 membrane. Gel filtration of dialysis retentate was conducted in a column (80 × 1.6 cm) of sephadex G-75 in Milli-Q water. The neoglycoproteins were lyophilized and stored at −10 °C.