Abstract
P120 catenin (p120ctn) belongs to the family of Armadillo repeat-containing proteins, which are believed to have dual functions of cell-cell adhesion and transcriptional regulation. In vascular endothelium, p120ctn is mostly recognized for its cell-cell adhesion function through its ability to regulate VE-cadherin. The current study investigated whether p120ctn in endothelial cells also has the capability to signal transcription events. Examination of several endothelial cell types indicated that Kaiso, a p120ctn-binding transcription factor, was abundantly expressed, with a predominant localization to the perinuclear region. Immunoprecipitation of endothelial cell lysates with a p120ctn antibody resulted in p120ctn-Kaiso complex formation, confirming the interactions of the two proteins. Transfection of the KBS (Kaiso-binding sequence) luciferase reporter plasmid into endothelial cells resulted in a 40% lower reporter activity compared to the mutant Kaiso-insensitive construct or empty vector pGL3, indicating that the suppressed reporter activity was attributed to endogenous Kaiso. The knock-down of p120ctn increased the KBS reporter activity 2-fold over control, but had no effects on the mutant KBS reporter activity. Further, p120ctn knock-down also reduced Kaiso expression, suggesting that p120ctn functioned to stabilize Kaiso. Overall, the findings provide evidence that in endothelial cells, p120ctn has a transcription repression function through regulation of Kaiso, possibly as a cofactor with the transcription factor.
Keywords: Armadillo repeat-containing proteins, p120 catenin, VE-cadherin, Kaiso, endothelial cells, transcription repression
INTRODUCTION
P120 catenin (p120ctn) is an adherens junction-associated protein belonging to the subfamily of Armadillo repeat-containing proteins. Structure-function studies of p120ctn show that it has two putative nuclear localization signals (Kelly et al., 2004) and a nuclear export signal (Van Hengel et al., 1999), suggesting that p120ctn is capable of trafficking into and out of the nucleus. It has also been reported that p120ctn can bind directly with at least two transcription factors, Kaiso (Daniel and Reynolds, 1999; Daniel et al., 2002) and Glis2 (Hosking et al., 2007), implicating a possible function as a cofactor for these transcription factors.
In vascular endothelium, the most understood function of p120ctn is its ability to promote cell-cell adhesion through direct binding with the cytoplasmic juxtamembrane domain (JMD) of VE-cadherin, which prevents cadherin from being internalized and degraded (Davis et al., 2003; Ireton et al., 2002; Xiao et al., 2003). This binding is also believed to induce lateral clustering of cadherins which increases cell-cell adhesive strength (Yap et al., 1998), and provides the important function of maintenance of proper fluid balance between the circulation and extravascular tissues. Emerging evidence from fields in cancer (Davis and Reynolds, 2006; Reynolds and Roczniak-Ferguson, 2004; Van Hengel and van Roy, 2007) and development (Mccrea and Park, 2007; Perez-Moreno et al., 2006; Kim et al., 2002) suggests that endothelial p120ctn may additionally harbor role(s) in transcriptional regulation. The involvement of p120ctn in signaling transcriptional activities in vascular endothelium is unknown, but a recent report by Beckers and coworkers (Beckers et al., 2008) observed that thrombin stimulated translocation of p120ctn to the nucleus in human umbilical vein endothelial cells. Although the significance of such translocation was not further investigated in the study, findings by Kondapalli and workers (Kondapalli et al., 2004) indicated that laminar shear stress upregulated both p120ctn and Kaiso at wound borders of coronary artery endothelial cells. Such co-regulation implicates a potential regulatory role between p120ctn with Kaiso in endothelial cells. Therefore, the goal of the study is to investigate the hypothesis that p120ctn regulates transcriptional activity in endothelial cells in addition to its well-established cell-cell adhesion function.
MATERIALS and METHODS
Materials
General reagents were obtained as follows: MCDB 131, RPMI 1640, DMEM, L-glutamine 100× solution, penicillin-streptomycin 100× solution, sodium pyruvate, phosphate-buffered saline (PBS), MEM nonessential amino acids, MEM Vitamins, and Lipofectamine 2000 (Invitrogen Corp.; Carlsbad, CA); fetal bovine serum (FBS) (Hyclone; Logan, UT); human epidermal growth factor (EGF), hydrocortisone, endothelial cell growth supplement (ECGS), heparin, protease inhibitor cocktail, phenylmethylsulfonyl fluoride (PMSF), leupeptin, pepstatin A and aprotinin (Sigma Chemical Co.; St. Louis, MO); ECL Kit (Amersham Pharmacia Biotech; Piscataway, NJ); Luciferase Activity kit (Promega Corporation; Madison, WI); Coomassie Plus Protein Assay (Pierce; Rockford, IL).
The following are antibodies used: Monoclonal anti-p120ctn antibody (Ab) (BD Transduction Laboratories; San Jose, CA); monoclonal anti-Kaiso Ab (MBL International Corporation, Woburn, MA); anti-β-actin Ab (Sigma Chemical Co.; St. Louis, MO); anti-mouse horseradish peroxidase-conjugated (HRP) Ab (Amersham Pharmacia Biotech; Piscataway, NJ).
The wild-type (wt) KBS (Kaiso-binding sequence) luciferase reporter plasmid, pwtKBS-Luc, was generated by inserting four tandem copies of an oligonucleotide possessing the consensus KBS element (5’ AGATCTGCACTAATCCTGCTAACCGCTC 3’) between the XhoI and KpnI sites in the multiple cloning site of the pGL3-control reporter vector (Promega); the mutant KBS reporter (pmuKBS-Luc) was generated by inserting four copies of the mutated KBS (underscored nucleotide) (5’ AGATCTGCACTAATCATGCTAACCGCTC 3’) into the pGL3-control vector (Spring et al., 2005). The pmuKBS-Luc construct does not bind Kaiso, and as with the empty reporter vector, pGL3-control vector, serves as experimental controls. These experimental control vectors, which are insensitive to Kaiso, produce higher luciferase activity relative to the Kaiso-sensitive wtKBS vector (Spring et al., 2005). The pwt- and pmu-KBS-Luc reporter plasmids were a kind gift from Dr. Juliet M. Daniel (McMaster University, Canada). Replication-deficient adenovirus containing GFP-tagged JMD (juxtamembrane domain) fragment of VE-cadherin (AdJMD) was generated from bovine VE-cadherin (Genbank accession No. AY363224) as described (Iyer et al., 2004). Adenoviruses lacking the insert served as control (AdNull). All other reagents are indicated in the text accordingly.
Cell culture
Bovine pulmonary artery endothelial cells, BPAEC (VEC Technologies, Inc, Rensselaer, NY), were maintained in culture and used experimentally at up to population doubling 12. BPAEC were grown in MCDB 131 medium, supplemented with 10% FBS, 10 µg/L EGF, 1 mg/L hydrocortisone, 90 mg/L heparin and 1% penicillin-streptomycin. Human lung microvascular endothelial cells (HLMEC; Lonza) were cultured in supplemented EGM2-MV base growth medium (Lonza) and used at population doublings 8–12. Human dermal microvascular cells (HDMEC) were isolated from human foreskin and grown in EGM2-MV growth medium and used at population doublings 8–12. Human brain microvascular endothelial cells (HBMEC) were cultured in RPMI 1640 supplemented with 10% FBS, 10% NuSerum (Becton Dickinson, Bedford, MA), ECGS (30 µg/ml), heparin (5 U/ml), 1 mM sodium pyruvate, 1 mM MEM nonessential amino acids, 1 mM MEM vitamins, 1% L-glutamine, and 1% penicillin-streptomycin and used at population doublings 30–40 as previously described (Qiao et al., 2006). These latter cells were immortalized by SV40 large T antigen and retain the endothelial cell phenotypic and functional characteristics (Stins et al., 1997a; Stins et al., 1997b). The cells were positive for von Willebrand factor, carbonic anhydrase IV, and Ulex Europeus agglutinin I, took up fluorescent labeled acetylated LDL, and expressed γ-glutamyl transpeptidase, demonstrating their brain endothelial cell characteristics.
Promoter reporter activity
Transfection protocol
Promoter reporter plasmid DNA was transfected into endothelial cells based on a modified protocol as previously described (Lum et al., 1999). Endothelial cells were grown in 12-well tissue culture dishes for transfection at ~70% confluence under antibiotic-free conditions. The cells were incubated for 3–4 hr with plasmid DNA pre-mixed with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) at a 1:3 ratio (µg DNA:µl Lipofectamine). A mock transfection control was included using same volume of Lipofectamine 2000 without DNA. Following incubation, the cells were washed and replaced with complete medium containing 2× FBS, incubated overnight, washed twice with Ca2+-free and Mg2+-free PBS and collected in reporter lysis reagent (Promega, Madison, WI) for luciferase activity assay.
Luciferase reporter assay
The collected cell lysate was clarified by centrifugation, and the supernatant was collected for analysis. Sample aliquots in duplicates were assayed for reporter activity as relative light units in a luminometer (Zylux FB12, Maryville, TN) according to manufacturer’s protocol (Luciferase Assay Kit; Promega, Madison, WI). Remaining aliquots were determined for protein determination, which was used for normalization of luciferase activity.
Targeted silencing of endogenous p120ctn with siRNA
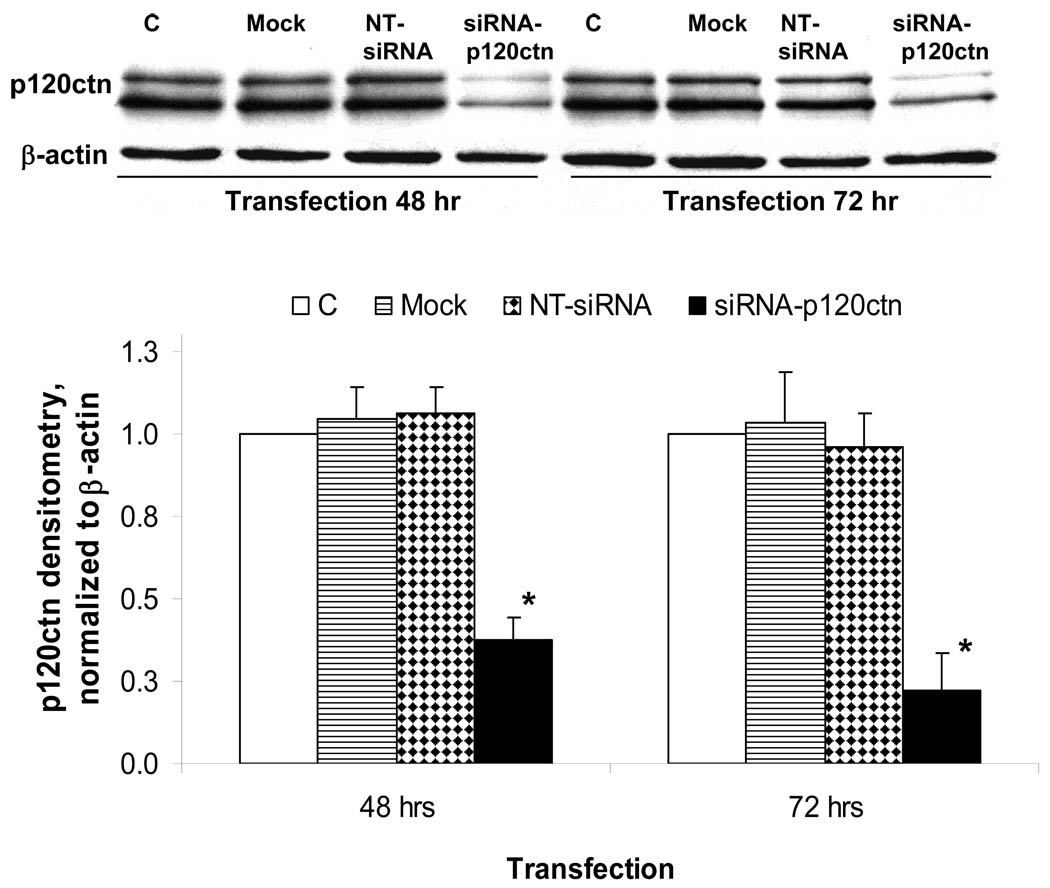
The knock-down of p120ctn expression in endothelial cells was made using a mixture of four siRNA oligonucleotide duplexes (siRNA-p120ctn) made commercially (Target-Plus SMARTpool, Dharmacon, Inc.; Lafayette, CO) based on the human p120ctn gene sequence, accession number NM_001331: (i) GAAUGUGAUGGUUUAGUUUU, (ii) UAGCUGACCUCCUGACUAAUU, (iii) GGACCUUACUGAAGUUAUUUU, and (iv) GAGGUAAGCUCGCCGGAAAUU). For transfection, endothelial cells were grown in 12-well culture dishes to 70–80% confluence and incubated for 24–72 hr with pre-mixed siRNA-p120ctn duplexes in Dharmafect1 siRNA transfection reagent at a ratio of 1.5 µl of Dharmafect1 to 0.1 – 0.4 nmol siRNA per well. During incubation with siRNA, overt cell toxicity was monitored by phase microscope observations. The confirmation of p120ctn knock-down was determined by Western blot analysis in separate studies, and was reprobed with anti-β-actin Ab for evaluation of equal loading for all groups (see Fig. 5). The siCONTROL Non-Targeting siRNA (Dharmacon, Inc) (NT-siRNA), which has no known mRNA targets, served as control for specificity of knock-down. Endothelial cells treated with Dharmafect1 transfection medium alone (Mock) served as an additional control for siRNA transfection.
Fig. 5. SiRNA-mediated knock-down of p120ctn.
HBMEC were transfected with siRNA-120ctn target sequences to human p120ctn (siRNA-p120ctn), non-target scramble oligonucleotides (NT-siRNA), or Mock as negative controls (see Materials and Methods), and the extent of silencing determined by Western blot analysis at 48 hr or 72 hr post transfection. C indicates untransfected control. Equal loading of protein per lane was evaluated by reprobing for β-actin. Top: representative Western blot showing p120ctn and β-actin. Bottom: densitometric analysis of Western blots; densitometric units normalized to β-actin and values reported as mean ± SE; * p<0.05, compared with NT-siRNA; n = 4.
Immunoprecipitation
Confluent endothelial cells grown in 60 mm dishes were washed with ice-cold PBS and lysed in RIPA buffer (50 mM Tris pH7.4, 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS) plus protease inhibitor cocktail. The cell lysates were passed through a 27-gauge needle eight times and centrifuged at 4°C at 10,000×g for 10 min. The supernatant was incubated with mouse anti-p120ctn Ab for 1 hr at 4°C on rocker, then 20 µl Protein G agarose was added and incubated overnight at 4°C. The immunoprecipitated protein complex was collected by centrifugation at 2,500 rpm at 4°C for 5 min, washed four times with PBS plus protease inhibitor cocktail, boiled in 1× electrophoresis sample buffer and separated by SDS-PAGE. As negative control, a separate group of cells was used for immunoprecipitation without the precipitating Ab.
Western blot
Following the experimental protocol, endothelial cells were collected and prepared for routine Western blot analysis and protein concentration determination. In the case of human umbilical vein endothelial cells (HUVEC), the cell lysate was directly obtained for Western blot analysis. All samples were loaded at constant protein concentrations for separation by SDS-polyacrylamide gel electrophoresis in 8 or 12.5% acrylamide as needed, and electrotransferred to nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris buffered saline with 0.05% Tween-20 (TBST), and incubated with the appropriate primary Ab diluted in TBST with 1% nonfat dry milk for overnight at 4°C in a rocker. The blot was washed five times with TBST and incubated with the appropriate anti-IgG secondary Ab conjugated with horseradish peroxidase. The bands were detected using the ECL kit and quantified by densitometry (NIH Image J program).
Indirect immunofluorescence
Endothelial cells were plated at a density of 8×104 cells/coverslip onto coverslips (13 mm) or chamber slides, both were precoated with 5 µg/ml fibronectin. The next day, the cells were washed three times with PBS, followed by fixation with 3.7% formaldehyde for 20 min at room temperature (RT) and permeabilization with 0.5% Triton-X 100 for 5 min at RT. The cells were washed three times with PBS, blocked with 5% goat serum for 60 min, and incubated with primary Ab for 1–2 hr at RT. Following washes with PBS, the cells incubated with fluorochrome-conjugated secondary Ab (i.e., goat anti-mouse IgG1 (r1) conjugated with Cy2) for 1 hr at RT in the dark. The cells were washed in PBS and mounted with Immunomount (Shandon, Pittsburg, PA) for fluorescence microscopy evaluation.
Statistical analysis
Single sample data were analyzed by the two-tail t test. Anova was used for comparisons of experimental groups with a single control group (SPSS 15.0).
RESULTS
Endothelial p120ctn interactions with Kaiso
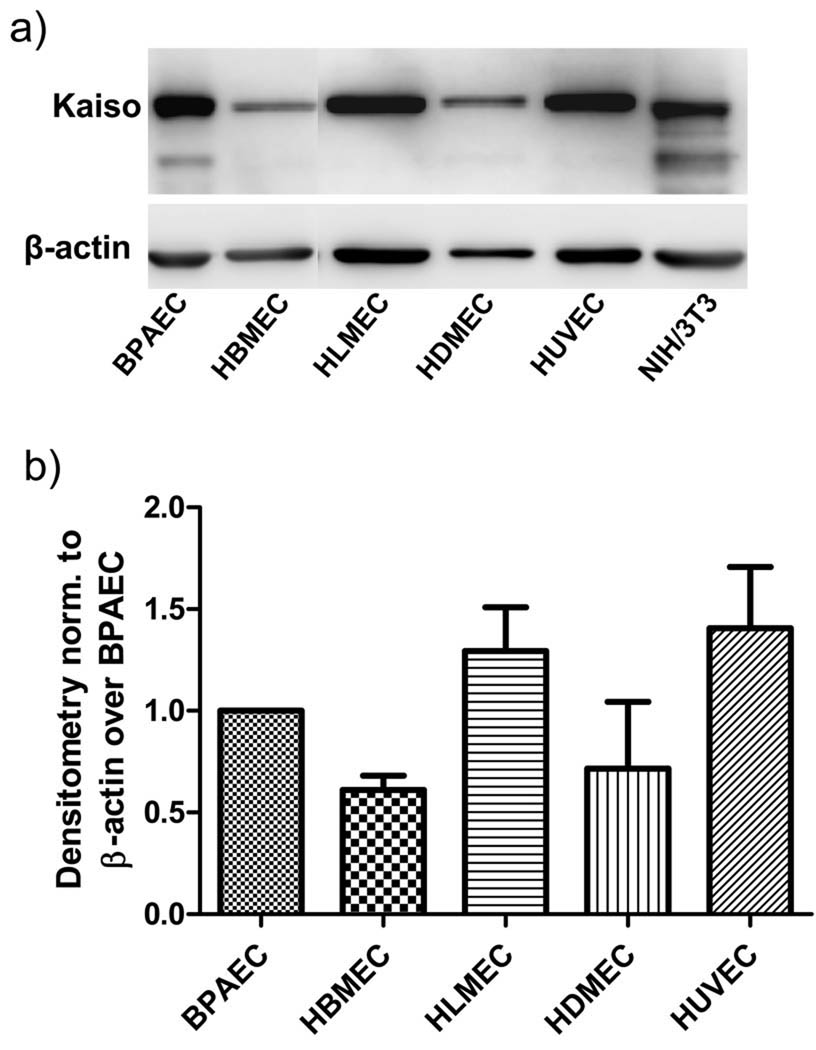
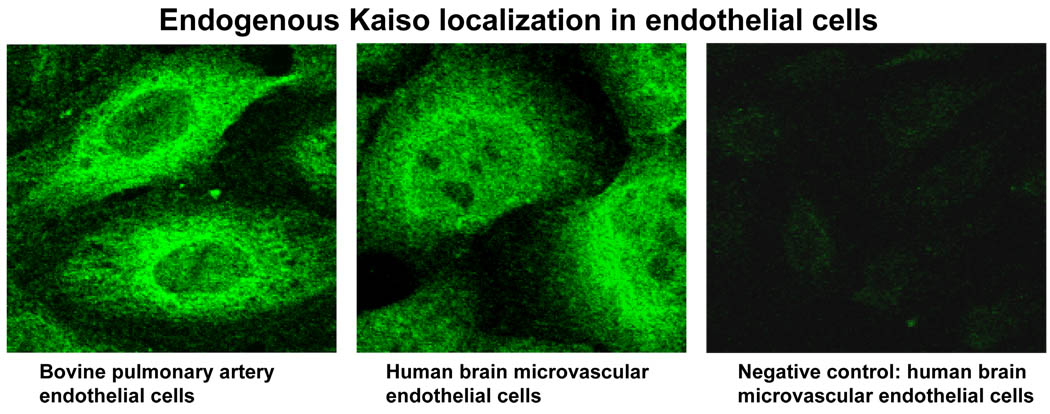
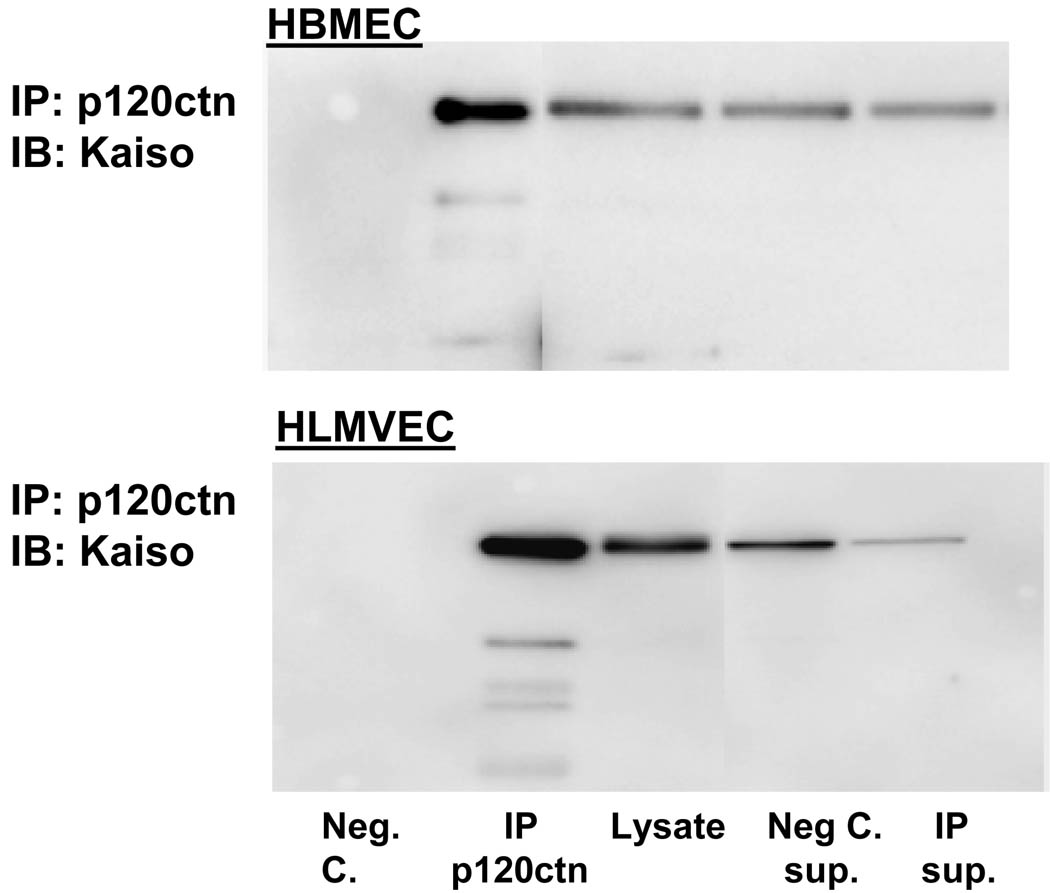
In the current study, Western blot analyses indicated that a wide range of endothelial cell types (BPAEC, HBMEC, HLMEC, HDMEC, HUVEC) show a robust expression of Kaiso (100 kDa), the level varying among the cell types (Fig. 1). The intracellular distribution of Kaiso was further investigated by indirect immunofluorescence, which showed a predominant localization in the cytosolic and perinuclear regions and lower expression within the nucleus in HBMEC as well as BPAEC (Fig. 2). Kaiso is a binding partner of p120ctn, and this interaction has been shown to prevent Kaiso’s ability to suppress transcription (Daniel et al., 1999; Daniel, 2007). We determined whether p120ctn interacts with Kaiso by immunoprecipitation studies using anti-p120ctn Ab of two different endothelial cell types, HBMEC and HLMEC. Western blot analysis of the immunoprecipitated proteins indicated that p120ctn and Kaiso form immunocomplexes (Fig. 3), implicating potential p120ctn-kaiso interactions in transcriptional regulation in endothelial cells.
Fig. 1. Western blot analysis of Kaiso in endothelial cells.
a) Representative Western blot of Kaiso expression in different endothelial cell types (BPAEC, HBMEC, HLMEC, HDMEC, HUVEC); NIH/3T3 indicates mouse fibroblasts; b) Densitometric analysis reported as normalized to β-actin over BPAEC; mean±SE; n = 3
Fig. 2. Intracellular localization of Kaiso in endothelial cells.
Indirect immunofluorescent microscopy was prepared for detection of Kaiso in HBMEC and BPAEC using Cy2-conjugated anti-mouse IgG (see Materials and Methods); representative of 3 independent experiments. Negative control detected with secondary Ab alone; 600× original magnification.
Fig. 3. P120ctn interaction with Kaiso.
Endothelial cell lysates from HBMEC and HLMVEC were immunoprecipitated with anti-p120ctn Ab, and separated proteins were analyzed by Western blot for Kaiso. Negative control (Neg. C.) group was in the absence of anti-p120ctn Ab; Sup. indicates supernatant after immunoprecipitation; lysate group included for comparison with the immunoprecipitated group; blot shown is representative of 3 independent experiments.
Endothelial cells show Kaiso-sensitive transcriptional activity
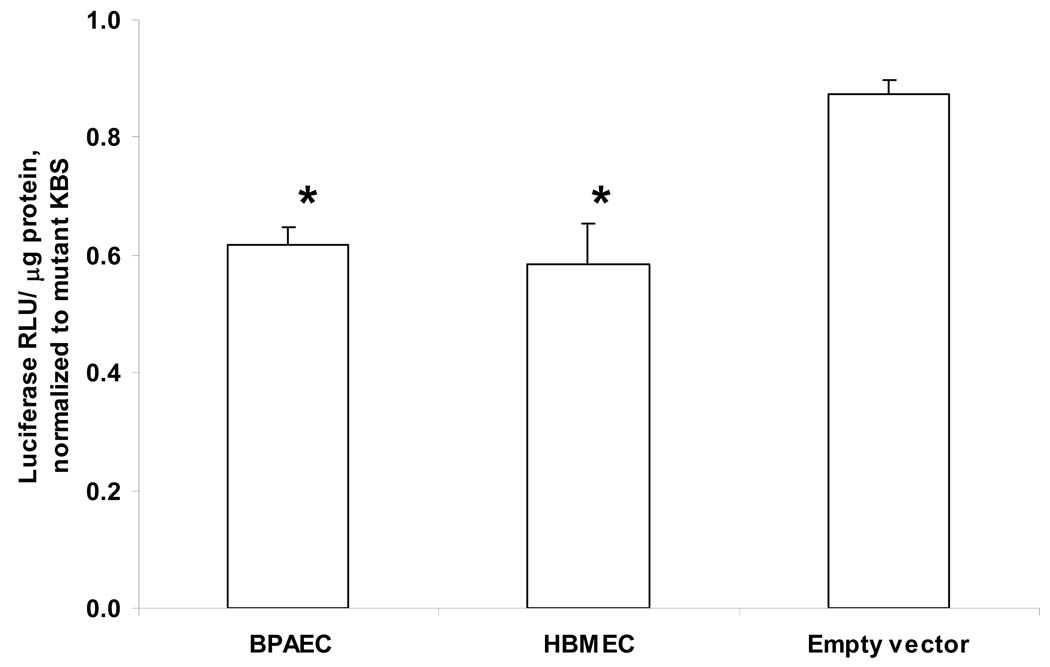
The transcription factor Kaiso has been reported to suppress transcriptional activity in non-endothelial cells, and binding by p120ctn can prevent such suppression (Spring et al., 2005; van Roy and Mccrea, 2005). Therefore, we investigated whether p120ctn in endothelial cells regulate transcriptional repression through interactions with Kaiso. We evaluated the functional significance of Kaiso in endothelial cells by determining Kaiso-driven transcriptional activity using KBS luciferase reporter plasmids [pwtKBS-Luc (wild-type) and pmuKBS-Luc (mutant) (see Materials and Methods). Results show that BPAEC and HBMEC transfected with the wtKBS have ~40% lower luciferase reporter activity relative to the Kaiso-insensitive mutant KBS (Fig. 4). The luciferase activity of the empty vector (absence of KBS) control reporter (pGL3) relative to the mutant KBS was near 90–100% (Fig. 4), further supporting the Kaiso-specificity of the suppression response. This decreased basal KBS reporter activity suggests that in endothelial cells Kaiso is a repressor of transcriptional activity.
Fig. 4. Repression of basal KBS promoter reporter activity.
Endothelial cells (HBMEC, BPAEC) were transfected with reporter plasmids pwtKBS-Luc or pmuKBS-Luc overnight, and collected for measures of luciferase activity (Materials and Methods). Empty reporter vector (pGL3) was transfected in BPAEC. Luciferase relative light units were normalized to protein (RLU/μg protein) and reported as relative to muKBS (mean ± SE; * p<0.01); n = 6.
Effects of p120ctn knock-down on KBS reporter activity
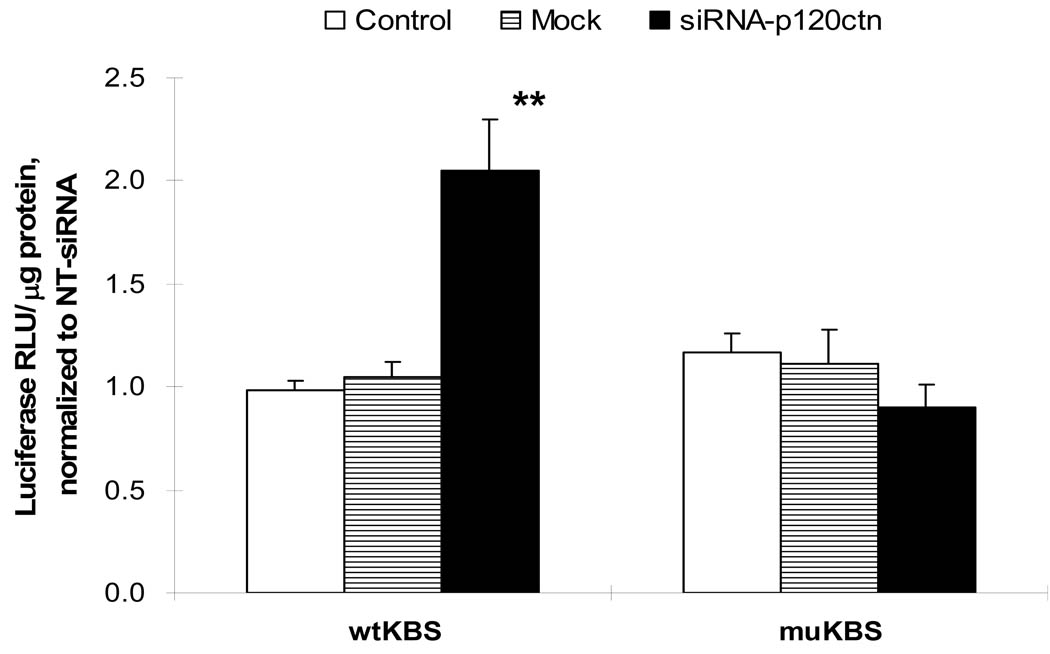
The involvement of p120ctn in regulation of the KBS reporter activity was investigated by silencing p120ctn expression in endothelial cells with siRNA sequences targeted to human p120ctn (siRNA-p120ctn; see Materials and Methods). HBMEC treated with siRNA-p120ctn for 48 or 72 hr presented with decreased p120ctn protein expression, 60 and 80% respectively, compared to the NT-siRNA (non-targeting siRNA), Mock (Dharmafect1 alone), and Control (absence of any treatment) (Fig. 5). Using this knock-down protocol, we determined the consequences of the decreased p120ctn expression on KBS reporter activity. Endothelial cells were treated with the siRNA-p120ctn, NT-siRNA, or Mock for 48 hr as described, followed by transfection with either pwtKBS-Luc or control pmuKBS-Luc reporter plasmid for 24 hr, and the cells collected for luciferase assay. In the wtKBS transfectants, the knock-down of p120ctn induced a 2-fold increase in KBS reporter activity relative to NT-siRNA group (Fig. 6); but not in the Mock and Control groups (Fig. 6). However, in the muKBS transfectants, the knock-down of p120ctn did not alter the KBS reporter activity, which was similar to Mock and Control groups (Fig. 6). The findings indicated that loss of p120ctn expression stimulated KBS reporter activity in endothelial cells.
Fig. 6. P120ctn knock-down increased KBS promoter reporter activity.
HBMEC were transfected with siRNA target sequences to human p120ctn (siRNA-p120ctn) (see Materials and Methods) for 48 hr, followed by transfection with reporter plasmids, pwtKBS-Luc or pmuKBS-Luc, for 24 hr, then collected for luciferase reporter activity determination. Control groups were transfected with non-target scramble oligonucleotides (NT-siRNA) or Mock as negative controls. Luciferase relative light units were normalized to protein (RLU/µg protein) and reported as relative to NT-siRNA values (mean ± SE); * p<0.01, compared to either Control (no siRNA) or Mock control; n = 8.
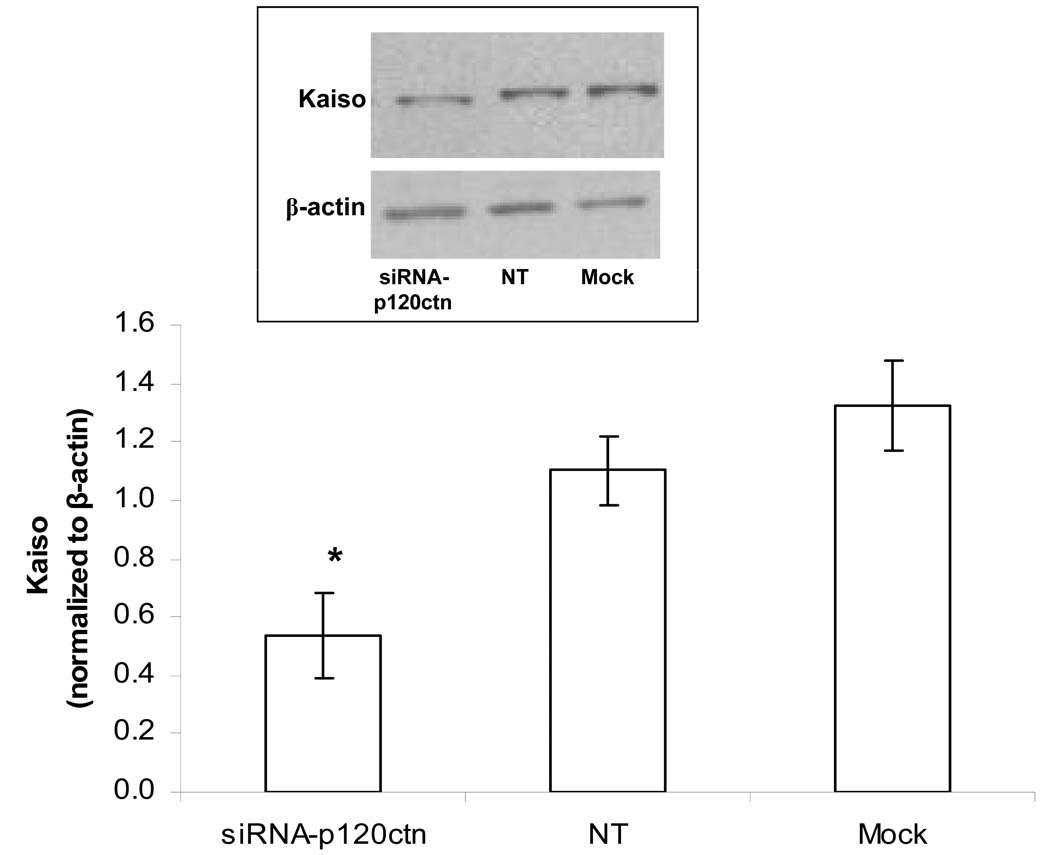
To determine whether this upregulated KBS reporter activity was associated with altered levels of Kaiso expression, the cell lysate was analyzed by Western blot for Kaiso protein. The results indicated that loss of p120ctn was also associated with loss of Kaiso expression (Fig. 7), indicating that p120ctn interaction with Kaiso may exert a stabilization effect on Kaiso expression. Importantly, this finding suggests that the transcriptional repression by Kaiso requires interaction of Kaiso with p120ctn.
Fig. 7. P120ctn knock-down decreased Kaiso expression.
HBMEC were transfected with siRNA target sequences to human p120ctn (siRNA-p120ctn), non-target scramble oligonucleotides (NT) or Mock as described, and prepared for Western blot analysis for Kaiso. Top: representative Western blot showing kaiso and β-actin. Bottom: Densitometry was performed and relative units reported as normalized to β-actin (mean±SE relative units); * p<0.05, when compared to either NT-siRNA or Mock; n = 3.
Effects of VE-cadherin peptide competition on transcription repression
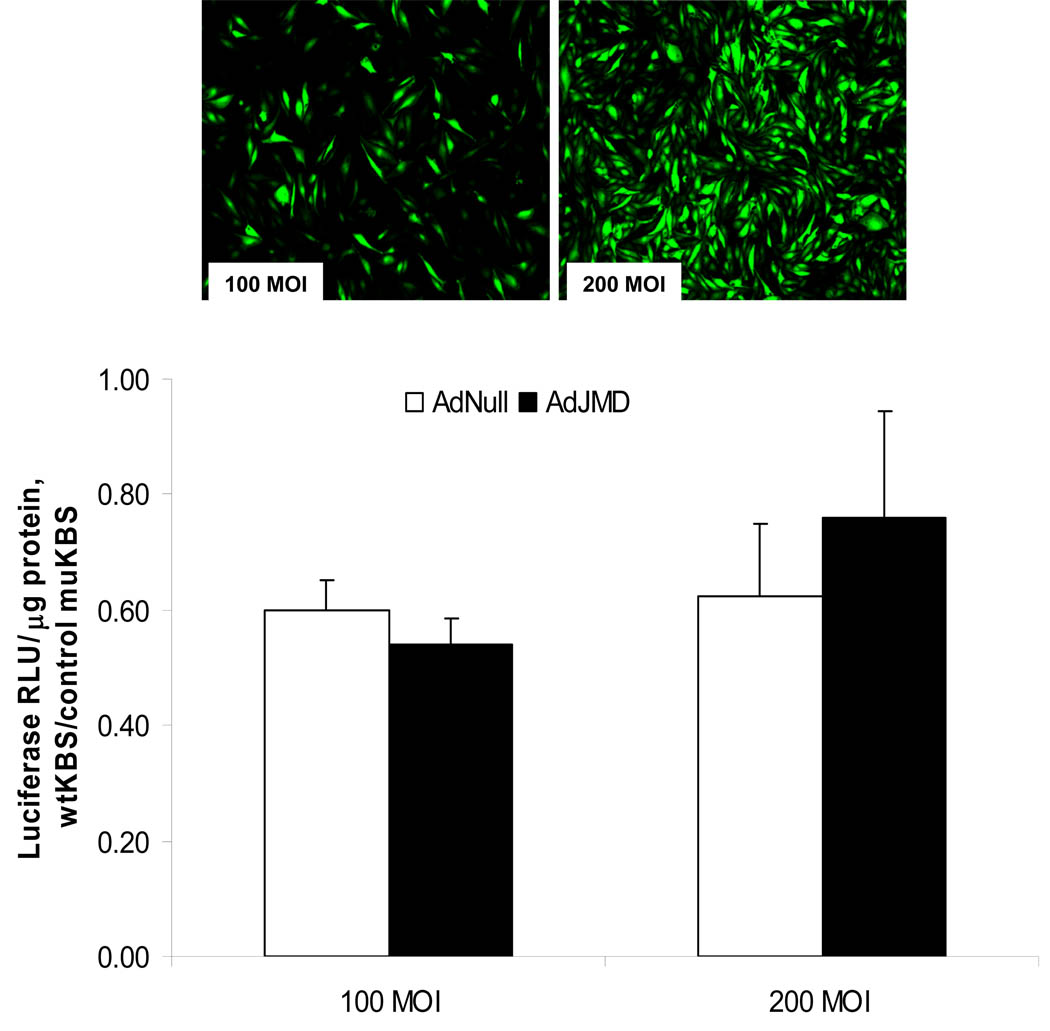
We further tested the role of p120ctn-Kaiso interactions in regulation of transcriptional repression by competition for p120ctn binding. Both Kaiso and the cadherin JMD sequence bind to p120ctn in the region spanning Armadillo repeats 1–7 (Daniel et al., 1999; Ireton et al., 2002). This suggests that the cadherin JMD should compete with Kaiso for binding on p120ctn. Using the recombinant adenovirus containing JMD coding sequence (AdJMD), endothelial cells were infected with AdJMD to overexpress the peptide (Materials and Methods) to compete with Kaiso for binding on p120ctn. Infection of confluent endothelial cells with AdJMD at 100 and 200 MOI (multiplicity of infection) for 48 hr resulted in >95% of the cells expressing GFP at the higher MOI (Fig. 8). For study, the cells were infected at the higher MOIs of 100 or 200 with AdJMD or the control AdNull, after which the cells were transfected with the pwtKBS-Luc or pmuKBS-Luc reporter plasmid for 24 hr, and collected for luciferase assay. As expected, in the AdNull-infected control group, the KBS luciferase reporter activity was repressed ~40% relative to the mutant KBS reporter activity (Fig. 8). However, infection with AdJMD at MOIs of 100 and 200 did not alter the repressed KBS reporter activity (Fig. 8). The findings suggest that the Kaiso-dependent repression may not require p120ctn interactions, or alternatively, the JMD peptide was ineffective in competing with Kaiso for binding on p120ctn. A more in-depth discussion is provided in the Discussion section.
Fig. 8. Effects of VE-cadherin peptide overexpression on KBS reporter activity.
a) BPAEC were infected with AdJMD (bovine) tagged with GFP at 100 or 200 MOI (multiplicities of infection) for 48 hrs. The percent of GFP positive cells relative to total cell number of the monolayer was estimated to be, respectively, 50% and >95%; n=3. b) Bar graph showing wtKBS reporter luciferase activity from BPAEC infected with AdJMD or Adnull for 48 hrs at the 100 or 200 MOI; luciferase activity (relative light units) reported as normalized to protein (RLU/µg protein) relative to mutant KBS (muKBS) values (mean ± SE); n = 4.
DISCUSSION
In vascular endothelium, most studies of the Armadillo repeat-containing protein, p120ctn, have been limited to its role in junctional integrity at the plasma membrane. The current study presents novel evidence that p120ctn in endothelial cells has an additional function which suppressed Kaiso-dependent transcription. This conclusion is based on three key observations: i) the basal wild-type KBS-driven reporter luciferase activity was lower than mutant KBS-driven reporter activity, ii) knock-down of p120ctn elevated KBS reporter activity and iii) knock-down of p120ctn was associated with loss of Kaiso expression. The findings support the notion that loss of p120ctn removes the p120ctn-mediated repression, leading to activation of transcriptional events. Although the pathophysiological significance of p120ctn-kaiso interactions in vascular endothelium is not presently clear, patients with aggressive tumors have associated reduced p120ctn expression (Davis et al., 2006; Reynolds et al., 2004; Van Hengel et al., 2007). Further, mice with conditional knock-out of p120ctn in keratinocytes present with a hyperproliferative and proinflammatory tissue phenotype (Perez-Moreno et al., 2006).
Analysis of endothelial cell types from a wide range of tissues indicated that Kaiso protein expression was relatively abundant, with a particular localization in the perinuclear region. Kaiso has also been reported to be expressed in several tissue types (including spleen, lung, liver, muscle, and kidney) (Daniel et al., 1999), indicating that it is ubiquitously expressed. Kaiso belongs to the family of POZ-ZF (BTB/POX-zinc finger) transcription factors (van Roy et al., 2005; Spring et al., 2005), and emerging evidence implicates important roles in development and cancer (Ruzov et al., 2004; Prokhortchouk et al., 2006). To date, there is no information regarding the role of Kaiso in endothelial cells, and the target genes regulated by the p120ctn-Kaiso pathway remain yet to be elucidated.
We show that in endothelial cells p120ctn and Kaiso formed immunocomplexes. The region of Kaiso which binds p120ctn has been mapped to sequences lying close to DNA binding motifs of the transcription factor (Daniel et al., 1999; Daniel et al., 2002), the juxtaposition of these functional domains suggesting possible facilitation by p120ctn of Kaiso-regulated transcriptional activities. In non-endothelial cell types such as COS-7 cells and fibroblasts, it has been found that binding of p120ctn on Kaiso serves to relieve the Kaiso-mediated transcription repression (Spring et al., 2005; Daniel et al., 1999; van Roy et al., 2005). Our findings suggest that this was not the case in endothelial cells, but rather p120ctn and Kaiso likely acted in concert for transcriptional repression since p120ctn knock-down increased Kaiso-sensitive transcriptional events. Further, the p120ctn knock-down induced loss of Kaiso expression, implicating a possible cofactor role to maintain Kaiso stability to mediate transcription repression.
Evidence indicates that Kaiso and the cadherin JMD peptide may bind to p120ctn in a mutually exclusive manner, suggesting that relative levels of Kaiso and cadherin can influence 120ctn function. Therefore, taking advantage of this proposed relation, we tested the requirement for p120ctn-Kaiso interaction in transcription repression by overexpression of JMD peptide to compete off Kaiso binding. However, JMD overexpression did not alter KBS-mediated repression of reporter activity in endothelial cells. It is unlikely that the negative findings were attributed to insufficient JMD expression, since high transfection efficiency (>95%) was achieved using the recombinant adenoviruses in endothelial cells as we (Lum et al., 1999) and others (Iyer et al., 2004) have previously shown. We suspect that the inability of the JMD peptide to relieve the repressed transcription activity was likely attributed to ineffective competition with Kaiso and not indicative of p120ctn-independent transcription regulation. Kaiso is reported to bind Armadillo repeats 1–7 of p120ctn (Daniel et al., 1999; Daniel et al., 2002); whereas classic cadherins bind to Armadillo repeats 1–5 and 7 (Ireton et al., 2002). This difference could be sufficient to preclude effective competition for binding between Kaiso and JMD. Further, there is evidence that the interaction between cadherin and p120ctn is relatively weak, although this point remains controversial (Anastasiadis and Reynolds, 2000).
These latter findings do not exclude the possibility that p120ctn also regulated Kaiso through indirect mechanisms in addition to direct binding with Kaiso. Loss of p120ctn within the adherens junctions, such as observed during p120ctn knock-down, could destabilize the junctions by inducing VE-cadherin degradation (Xiao et al., 2003; Davis et al., 2003; Ireton et al., 2002), which in turn would release β- and γ-catenins to function as transcriptional cofactors and cross-talk with Kaiso (Miravet et al., 2002; Park et al., 2005). Alternatively, p120ctn has been reported to contain putative binding sites for Rho and Rho-related proteins (Castano et al., 2007; Wildenberg et al., 2006), whose binding with p120ctn is believed to signal downstream cellular activities including transcriptional events.
In summary, our findings provide the first evidence that in endothelial cells, p120ctn functions to repress transcriptional activity. Here, we show that p120ctn has a cofactor role, possibly through direct interaction with Kaiso to mediate the transcription repression. Our results also suggest that pathological loss or dysfunction of p120ctn in vascular endothelium could lead to removal of such transcriptional repression, leading to a pathological endothelial phenotype.
ACKNOWLEDGMENTS
This work was supported by National Heart Lung and Blood Institute Grant HL093715 (HL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113(Pt 8):1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- Beckers CM, Garcia-Vallejo JJ, van Hinsbergh VW, Nieuw Amerongen GP. Nuclear targeting of beta-catenin and p120ctn during thrombin-induced endothelial barrier dysfunction. Cardiovasc Res. 2008;79:679–688. doi: 10.1093/cvr/cvn127. [DOI] [PubMed] [Google Scholar]
- Castano J, Solanas G, Casagolda D, Raurell I, Villagrasa P, Bustelo XR, Garcia dH, Dunach M. Specific phosphorylation of p120-catenin regulatory domain differently modulates its binding to RhoA. Mol Cell Biol. 2007;27:1745–1757. doi: 10.1128/MCB.01974-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso. Biochim Biophys Acta. 2007;1773:59–68. doi: 10.1016/j.bbamcr.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The catenin p120(ctn) interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol Cell Biol. 1999;19:3614–3623. doi: 10.1128/mcb.19.5.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Spring CM, Crawford HC, Reynolds AB, Baig A. The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 2002;30:2911–2919. doi: 10.1093/nar/gkf398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006;10:21–31. doi: 10.1016/j.devcel.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Hosking CR, Ulloa F, Hogan C, Ferber E, Figueroa A, Gevaert K, Birchmeier W, Briscoe J, Fujita Y. The Transcriptional Repressor Glis2 Is a Novel Binding Partner for p120 Catenin. Mol Biol Cell. 2007 doi: 10.1091/mbc.E06-10-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton RC, Davis MA, Van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1143–L1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- Kelly KF, Spring CM, Otchere AA, Daniel JM. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J Cell Sci. 2004;117:2675–2686. doi: 10.1242/jcs.01101. [DOI] [PubMed] [Google Scholar]
- Kim SW, Fang X, Ji H, Paulson AF, Daniel JM, Ciesiolka M, van Roy F, Mccrea PD. Isolation and characterization of XKaiso, a transcriptional repressor that associates with the catenin Xp120(ctn) in Xenopus laevis. J Biol Chem. 2002;277:8202–8208. doi: 10.1074/jbc.M109508200. [DOI] [PubMed] [Google Scholar]
- Kondapalli J, Flozak AS, Albuquerque ML. Laminar shear stress differentially modulates gene expression of p120 catenin, Kaiso transcription factor, and vascular endothelial cadherin in human coronary artery endothelial cells. J Biol Chem. 2004;279:11417–11424. doi: 10.1074/jbc.M306057200. [DOI] [PubMed] [Google Scholar]
- Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol Cell Physiol. 1999;277:C580–C588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- Mccrea PD, Park JI. Developmental functions of the P120-catenin sub-family. Biochim Biophys Acta. 2007;1773:17–33. doi: 10.1016/j.bbamcr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Miravet S, Piedra J, Miro F, Itarte E, Garcia dH, Dunach M. The transcriptional factor Tcf-4 contains different binding sites for beta-catenin and plakoglobin. J Biol Chem. 2002;277:1884–1891. doi: 10.1074/jbc.M110248200. [DOI] [PubMed] [Google Scholar]
- Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, Mccrea PD. Kaiso/p120-Catenin and TCF/beta-Catenin Complexes Coordinately Regulate Canonical Wnt Gene Targets. Dev Cell. 2005;8:843–854. doi: 10.1016/j.devcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Davis MA, Wong E, Pasolli HA, Reynolds AB, Fuchs E. p120-catenin mediates inflammatory responses in the skin. Cell. 2006;124:631–644. doi: 10.1016/j.cell.2005.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokhortchouk A, Sansom O, Selfridge J, Caballero IM, Salozhin S, Aithozhina D, Cerchietti L, Meng FG, Augenlicht LH, Mariadason JM, Hendrich B, Melnick A, Prokhortchouk E, Clarke A, Bird A. Kaiso-deficient mice show resistance to intestinal cancer. Mol Cell Biol. 2006;26:199–208. doi: 10.1128/MCB.26.1.199-208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Huang F, Naikawadi RP, Kim KS, Said T, Lum H. Lysophosphatidylcholine Impairs Endothelial Barrier Function through the G Protein-Coupled Receptor, GPR4. Am J Physiol Lung Cell Mol Physiol. 2006;291:L91–L101. doi: 10.1152/ajplung.00508.2005. [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Roczniak-Ferguson A. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene. 2004;23:7947–7956. doi: 10.1038/sj.onc.1208161. [DOI] [PubMed] [Google Scholar]
- Ruzov A, Dunican DS, Prokhortchouk A, Pennings S, Stancheva I, Prokhortchouk E, Meehan RR. Kaiso is a genome-wide repressor of transcription that is essential for amphibian development. Development. 2004;131:6185–6194. doi: 10.1242/dev.01549. [DOI] [PubMed] [Google Scholar]
- Spring CM, Kelly KF, O'Kelly I, Graham M, Crawford HC, Daniel JM. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the beta-catenin/TCF target gene matrilysin. Exp Cell Res. 2005;305:253–265. doi: 10.1016/j.yexcr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997a;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- Stins MF, Prasadarao NV, Zhou J, Arditi M, Kim KS. Bovine brain microvascular endothelial cells transfected with SV40-large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease [letter] In Vitro Cellular & Developmental Biology Animal. 1997b;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- Van Hengel J, van Roy F. Diverse functions of p120ctn in tumors. Biochim Biophys Acta. 2007;1773:78–88. doi: 10.1016/j.bbamcr.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Van Hengel J, Vanhoenacker P, Staes K, van Roy F. Nuclear localization of the p120(ctn) Armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc Natl Acad Sci U S A. 1999;96:7980–7985. doi: 10.1073/pnas.96.14.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roy FM, Mccrea PD. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer. 2005;5:956–964. doi: 10.1038/nrc1752. [DOI] [PubMed] [Google Scholar]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Buckley KM, Kottke MD, Vincent PA, Faundez V, Kowalczyk AP. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003;163:535–545. doi: 10.1083/jcb.200306001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]