Abstract
Objective
There is mounting evidence that the posterior parietal cortex (PPC) plays an important role in episodic memory. We previously found that patients with PPC damage exhibit retrieval-related episodic memory deficits. Our objective was to assess whether parietal lobe damage affects episodic memory on a different task: the Deese-Roediger-McDermott (DRM) false-memory paradigm.
Method
Two patients with bilateral PPC damage and matched controls were tested. In Experiment 1, the task was to remember words; in Experiment 2 the task was to remember pictures of common objects. Prior studies have shown that normal participants have high levels of false memory to words, low levels to pictures.
Results
The patients exhibited significantly lower levels of false memory to words. The patients' false memories were accompanied by reduced levels of recollection, as tested by a Remember/Know procedure. It is unlikely that a failure of gist processing accounts for these results, as patients accurately remembered thematic elements of short vignettes, but failed to remember details. These results support the view that portions of the PPC play a critical role in objective and subjective aspects of recollection.
Keywords: false memory, subjective recollection, attention, parietal lobe, memory retrieval, Balint's syndrome, simultanagnosia
There is growing evidence that the posterior parietal cortex (PPC) plays an important role in episodic memory retrieval. Functional MRI studies have reported lateral and medial PPC activations across a wide range of episodic memory retrieval tasks (reviewed in Cabeza, 2008; Cabeza, Ciaramelli, Olson, & Moscovitch, 2008; Ciaramelli, Grady, & Moscovitch, 2008; Wagner, Shannon, Kahn, & Buckner, 2005; (Vilberg & Rugg, 2008).
The functional involvement of the PPC in episodic memory has been verified by a small number of focal lesion studies (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007; Davidson, et al., 2008; Simons, Peers, Mazuz, Berryhill, & Olson, 2009; reviewed in Olson & Berryhill, 2009). For instance, we previously tested two patients with bilateral parietal lobe damage, one patient with damage that was mostly in the inferior parietal lobe (IPL), the other had damage extending into the superior parietal lobe, on a study examining autobiographical memory (Berryhill, Phuong, Picasso, Cabeza, & Olson, 2007). Patients and matched controls were asked to select a memory from five designated epochs in their lifetimes and then describe the memory in as much detail as possible. Following this free recall stage, a series of specific probe questions were asked that were aimed at elaborating on the number of details mentioned. The free recall and specific probe data were subjected to a detailed text analysis to categorize, tally, and rate the details mentioned by study participants (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002). The results demonstrated a surprising dissociation in the patients' performance between the free recall and the specific probe stages. Their freely recalled memories were significantly impoverished in terms of the number of details they referenced. In contrast, during the specific probe stage, patients responded with as much detail as the control subjects. Because the task did not involve encoding, the patients' abnormally low performance is best characterized as a memory retrieval deficit.
In another study (Davidson, et al., 2008), a patient with unilateral left PPC damage was tested in a series of tasks including the classic Deese-Roediger-McDermott (DRM) false-memory paradigm (Deese, 1959; Roediger & McDermott, 1995). In the standard DRM, participants are presented with lists of conceptually related words, and later, they are asked to perform an old/new recognition memory task. At retrieval, most participants claim to have seen or heard words that in fact, had never appeared on the list (“false memories”). This occurs when the words are closely related to the core theme of the words on the learned list. It is thought that such false memories arise from automatic activation of conceptually related words, or “gist” information (Schacter & Slotnick, 2004). Such false memories are typically accompanied by a strong subjective sense of recollection. Meta-memory processes operating at retrieval can also shape expectations about what true memories feel like, further modulating false memory rates.
The Davidson, et al., (2008) report described a unique pattern of deficits in their unilateral PPC patient: she remembered relatively fewer items and made relatively fewer false alarms. The authors interpret this as evidence that the patient was not activating the ‘gist’ information associated with each semantically related list. Davidson's patient also reported a decreased sense of subjective recollection, a finding that has been reported by two other groups using different tasks and stimuli (Ally, Simons, McKeever, Peers, & Budson, 2008; Simons, Peers, Mazuz, Berryhill, & Olson, 2009).
It is important to point out that there are several negative findings in this small literature. For instance, three studies have reported that unilateral and bilateral parietal lobe damage has no deleterious effect on source memory accuracy (Davidson et al., 2008; Simons et al., 2009) even when the lesions overlap with regions activated by a source-memory task in an fMRI study (Simons et al., 2008). There is also evidence that unilateral parietal lobe damage or has no measureable effect on item-recognition memory, such as recognition of a long series of words, pictures, or sounds (Haramati, Soroker, Dudai, & Levy, 2007).
In summary, the existing data indicates that parietal lobe damage affects episodic memory in some cases but not others. Because there have only been a small number of studies on this topic, and those that exist offer uneven evidence for parietal involvement in episodic memory, it is difficult to make informed hypotheses about the parietal lobes' functional role in episodic memory. Also, with a few exceptions, most reported findings have not been replicated. The goal of this study is to bolster the literature by replicating and extending the DRM findings reported by Davidson and colleagues.
Davidson's finding is valuable because the DRM task has been used on several different patient populations, thus allowing one to compare the effects of different focal lesions on task performance. In the present study, two patients with bilateral parietal lobe damage are tested on two different DRM tasks. After conducting our primary analyses, we conduct a brief qualitative meta-analysis in which the performance of our patients is compared to the performance of patients with medial temporal lobe (MTL) damage.
Experiment 1: False Memory with Auditory Word Stimuli
Method
Lesion Patients
Two patients, EE555 and TQ591, with bilateral parietal lobe damage were tested in this study. Both patients are highly personable, alert and attentive participants. They have been discussed extensively in prior studies (Berryhill & Olson, 2008; Berryhill et al., 2007); we summarize their neurological profiles here. Patient EE555 EE555 is a 40-year-old former teacher with 16 years of education. In 2004, she suffered three infarcts in the watershed between the posterior and middle cerebral arteries. Her physical and perceptual symptoms are currently stable. EE555's MRI revealed symmetrical lesions in lateral aspects of the inferior parietal lobe, extending from superior aspects of the occipital lobe through the angular gyrus (Brodmann areas (BA) 39) in and around inferior and middle portions of the intraparietal sulcus (IPS). Damage does not encroach into the midline (e.g. precuneus). EE555's lesions are depicted in Figure 1.
Figure 1.
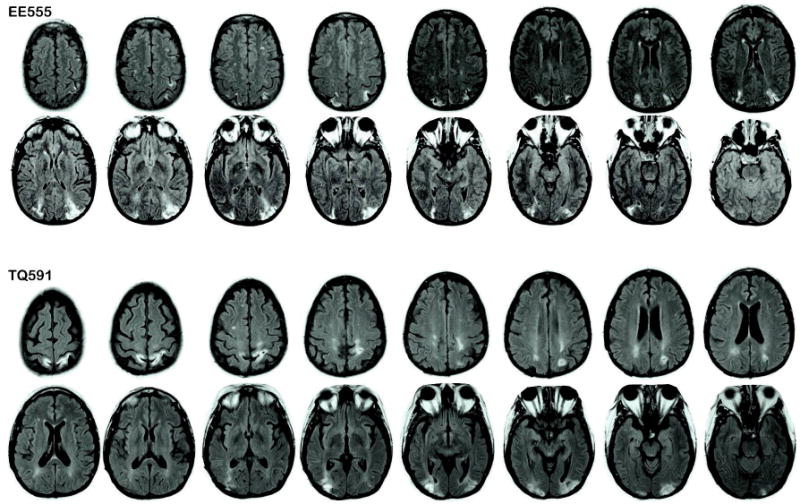
Patient lesion traces. Images are T2 fluid-attenuated inversion recovery (FLAIR) images in which the lesions appear as white higher intensity patches in parietal regions. Right is on the left.
Patient EE555's primary deficit is simultanagnosia. When shown a line drawing of a visual scene she describes parts of the picture, ‘there is a woman’, and ‘I see water’, without attaining a global understanding of the scene. In line cancellation tasks, she crosses off items only at the center, ignoring peripheral items. She only reports the local elements when shown Navon letters. Language comprehension and speech fluency were unimpaired as assessed by her conversational skills, and by ceiling performance on the auditory tests of the Western Aphasia Battery. She finds reading of long words or text passages impossible due to her simultanagnosia. Her eyesight is normal.
Patient TQ591
TQ591 is a 49-year-old former preschool assistant teacher with 15 years of education. She suffered bilateral parieto-occipital damage due to CNS cerebral vasculitis in March 2006. TQ591's MRI revealed signs of previous subacute posterior cerebral artery infarctions. The primary lesions are in bilateral parietal regions; see Figure 1. The left parietal lesion extends into IPS (BA 39) and precuneus (BA 7). There are two right lesion sites: the inferior lesion is in superior aspects of the occipital lobe (BA 18 and 19), and the superior lesion is in the superior parietal lobe (BA 7). In both hemispheres, the lesions extend slightly into occipital (BA 19) regions and parietal white matter.
TQ591's primary deficit is simultanagnosia. When shown pictures of scenes, TQ591 is slow to describe them and complains that parts of scenes ‘disappear’ when she looks away or blinks. In line cancellation tasks, she only identifies a few lines within a narrow visual field. She has a local bias with Navon letters. Language comprehension and speech fluency were unimpaired as assessed by her conversational skills, and ceiling performance on the auditory tests of the Western Aphasia Battery. Reading is somewhat impaired due to her simultanagnosia. Her vision is corrected-to-normal.
Neuropsychological Evaluation of Memory and Language
Patients were administered several standardized memory, language, and vision tests (see Table 1). First, to establish that patients are able to follow verbal instructions we conducted auditory subtests of the Western Aphasia Battery (WAB-R, Harcourt Assessment); they both performed very well. To assess episodic and working memory, auditory subtests of the Wechsler Memory Scale (WMS-III, The Psychological Corporation) were administered. EE555 performed at least 1 standard deviation (SD) below the mean on all tests, and was 2 SD below normal on the auditory delayed component. TQ591's performance was in the normal range (see Table 1).
Table 1.
Neuropsychological evaluation of patients EE555 and TQ591. The AMI personal semantic score is out of a total of 63 (normal = 54 - 63), the autobiographical semantic measure is out of a total of 27 (normal = 19 - 27). WMS scores are index scores from auditory subtests in which the population mean is 100 and the sd = 15. We consider performance on the WMS abnormal if it deviates by 2 SD, on the AMI if it deviates from the population mean by 1 SD. The Western Aphasia Battery (WAB) measures were scored out of 60, 80 and 100 possible points. WM=working memory. Abnormal scores are denoted with an ‘*’.
| Test | Subtest | EE555 | TQ591 |
|---|---|---|---|
| AMI | Personal semantic | 49.5* | 60 |
| Autobiographical incidents | 21 | 17* | |
| WMS | Immediate | 86 | 80 |
| Delayed | 77 | 97 | |
| WM | 83 | 79 | |
| Recognition delayed | 55* | 110 | |
| WAB | Auditory verbal comprehension | 60 | 60 |
| Sequential Commands | 80 | 76 | |
| Repetition | 100 | 98 |
The Logical Memory I and II subtests of the WMS-III provide estimates of gist and item memory. Participants read short vignettes and then freely recalled the events immediately and later, after a lengthy delay. Performance measures evaluate gist memory based on how many thematic components are retold, and item memory based on how many detail components are retrieved. At the immediate retelling stage, patients retold a greater proportion of the gist information than details or item information (Item versus gist memory: EE555 = 41.3% vs. 78.3%; TQ591 = 45.3% vs. 82.6%). This pattern was also observed after the delay period (Item versus gist memory: EE555 = 36.0% vs. 86.7%; TQ591 = 36.0% vs. 73.3%)1. These data indicate that gist memory was generally preserved whereas item memory was impoverished.
As an initial assessment of parietal involvement in autobiographical memory, a standard autobiographical memory test, the Autobiographical Memory Inventory (AMI) (Kopelman, Wilson, & Baddeley, 1989), was administered. This test measures memory for personal semantic and autobiographical incidents in a short-answer format. The results showed that both patients were subtly abnormal on this test. EE555's semantic recollections were scored as ‘probably abnormal’ (see Table 1), but her description of autobiographical events was in the ‘acceptable’ range. TQ591 showed the reverse pattern. A more complex assessment of autobiographical memory was also administered (Levine et al., 2002) and both patients performed abnormally low on free recall of life events, but performed normally on cued recall (Berryhill et al., 2007). Other tests revealed that both patients exhibit visual working memory impairments when tested by old/new recognition (Berryhill & Olson, 2008).
Control Participants
Twelve normal controls (8 males, 4 females) that were matched in age (M = 51.5, range = 39-67) and education (M = 14.5, range = 12 - 18) to the two patients were tested. There were no differences between patients and controls in terms of age and education (p > .05). All control participants were given a short questionnaire to verify that they were not experiencing any neurological or psychiatric disorders at the time of testing. All participants were compensated for their participation in the experiment.
Equipment
Stimuli were recorded using Apple's GarageBand software (Cupertino, CA), and presented on a 1.83 GHz Intel Core Duo MacBook Pro laptop computer with a 15-inch monitor. Each word list was presented using iTunes software (Cupertino, CA).
Stimuli
The stimuli consisted of 24 digitized sound-file word lists, each containing 15 words taken from Roediger and colleagues (Roediger & McDermott, 1995). Each list contained words that were linked through one common theme (i.e. sleep, spider, bread), with the false alarm being the “category” of the list. A male and female speaker each spoke one half of the recordings.
Design
The design, depicted in Figure 2, closely followed the design of Roediger and colleagues (H. L. I. Roediger & McDermott, 1995). During the 45-minute testing session, participants heard 16 word lists. The word lists were determined by a random number generator with an even distribution of lists spoken by male and female speakers. Immediately following the presentation of each list, the participant was asked to perform either free-recall or simple arithmetic problems, (i.e. addition or subtraction with whole number values ranging from 0-99). The determination of whether the free-recall or simple arithmetic that followed the presentation of each list was also done using a random number generator. After all the word lists had been presented, a recognition test was conducted. The researcher read aloud 96 words; 3 words from each of the 16 word lists the participant heard and the ‘lure’ word for each list, 3 words from the 8 word lists the participant did not hear and the ‘lure’ word for these lists as well. The participant then made an old/new judgment as to whether the word had been heard at study. If the participant made an‘old’ response, the participant then made a remember/know decision about the presented word. Participants were instructed to provide a ‘remember’ response when they were able to vividly recall the word with high confidence and to make a ‘know’ response when they had a sense of familiarity for the word but perhaps lower confidence.
Figure 2.
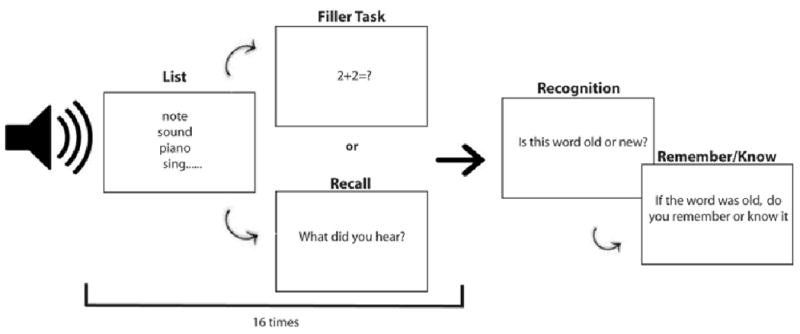
A schematic illustration of the DRM task used in Experiment 1.
Analysis
The same analyses were used for Experiments 1 and 2. Averages for controls were compared to patients in both free recall and recognition tasks using z-tests to determine significance (z > +/-1.96 SD). Corrected true recognition was calculated by subtracting false alarms (“old” responses to unstudied items) from hits (“old” responses to studied items) (Melo, Winocur, & Moscovitch, 1999; Schacter, Verfaellie, Anes, & Racine, 1998). Corrected false recognition (CFR) scores were calculated with the formula: CFR = hits (“old” responses to critical lures) – false alarms (“old” responses to lure-controls, i.e., critical lures of unstudied lists); see Table 2.
Table 2.
Data from Experiment 1, verbal DRM study, recognition portion of the task. This table mimics Table 4 of Davidson et al. (2008) to allow comparison between parietal patients. Based on standard high-threshold procedures (Melo et al., 1999; D. L. Schacter et al., 1998) corrected true recognition (CTR) was calculated using the formula: CTR = hits (“old” responses to targets) – false alarms (“old” responses to target-controls, i.e. items from unstudied lists). Corrected false recognition (CFR) scores were calculated with the formula: CFR = hits (“old” responses to critical lures) – false alarms (“old” responses to lure-controls, i.e., critical lures of unstudied lists). R and K responses were computed as a proportion of the old responses thus they do not add up to 100 vertically but do horizontally. Z scores below -1.96 are considered impaired and are demarked with an ‘*’.
| Old responses | R response | K responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TQ591 | EE555 | Controls | TQ591 | EE555 | Controls | TQ591 | EE555 | Controls | |
| A. Targets (heard words) | 0.58 | 0.29* | 0.63 | 0.61 | 0.64 | 0.70 | 0.39 | 0.36 | 0.30 |
| B. Target controls (never heard words) | 0.00 | 0.00 | 0.14 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.78 |
| Corrected true recognition | 0.58 | 0.29* | 0.49 | 0.61 | 0.64 | 0.80 | 0.39 | 0.36 | 0.20 |
| C. Critical lures (lures corresponding to heard lists) | 0.38* | 0.38* | 0.69 | 0.16* | 0.50* | 0.72 | 0.84* | 0.50 | 0.28 |
| D. Lure controls (lures from never heard lists) | 0.00 | 0.00 | 0.16 | 0.00 | 0.00 | 0.25 | 0.00 | 0.00 | 0.75 |
| Corrected false recognition | 0.38 | 0.38 | 0.53 | 0.16* | 0.50* | 0.79 | 0.84* | 0.50 | 0.21 |
Results and Discussion
Free Recall
In our first analysis we evaluated the free recall portion of the task, by comparing the accuracy of control participants and patients. Although the controls correctly recalled more of the words than the patients (M = .42 versus M = .33), when z-scores were analyzed, both patients performed within 1 SD of controls (EE555: M = .33, z = -.78; TQ591: M = .32, z = -.94). The rate at which participants recalled non-studied lure words, or in other words the degree of false recall, was 44% for both controls and patients. These findings show that the patients' DRM recall performance was normal.
Recognition
Recognition performance is shown in Figure 3. Patient EE555's hit rate of 29% and false alarm rate of 38% were both abnormally low (z = -2.15; z = -2.41). Patient TQ591's hit rate of 58% was in the normal range (z = .27) but her false alarm rate of 38% was abnormally low (z = -2.41).
Figure 3.
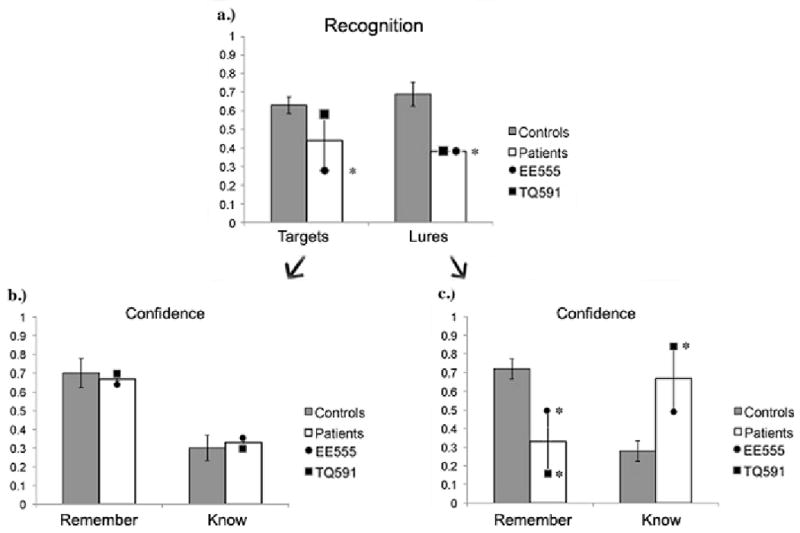
Results from (a) the recognition portion of the DRM task used in Experiment 1; (b) R/K responses to targets; and (c) R/K responses to lures. Data are raw accuracy for targets and lures. Each patient's mean accuracy is illustrated by a symbol overlaid on the column. Error bars represent the standard error of the mean.
We examined participants' remember (R)/know (K) responses following each ‘old’ response (see Figure 3). Both patients had R/K profiles resembling that of the controls for target words. However, patients' recollection of lure words was abnormal. Patient EE555 supplied significantly fewer R responses (.50 versus .79, z = -2.27) than controls. Her K responses were in the normal range. Patient TQ591 supplied significantly fewer R responses (.16 versus .79, z = -3.44), and significantly more K responses (.84 versus .21, z = 3.54), relative to controls.
The results of Experiment 1 provide us with several insights into parietal lobe memory processes. The patients had normal levels of true and false memory on the recall portion of the task. However, a different picture emerges when the recognition data were assessed. One patient exhibited decreased recognition memory for target words. Both patients exhibited decreased levels of false memory as illustrated by their low false alarm rates, and they lacked confidence in their false memories as shown by their reluctance to supply remember responses. Interestingly, patient EE555's performance on the DRM was very similar to the performance of a patient with a left PPC lesions on the DRM (Davidson et al., 2008). Davidson and colleagues attribute their patients' deficit on the DRM to impaired memory for gist driven by a general problem with recollection.
Experiment 2: False Memory with Visual Objects
The goal of Experiment 2 was to replicate the findings of Experiment 1 and to explore one reason for the diminished levels of false memories and recollection observed in Experiment 1. Reduced false memory rates can be reflect problems at several different stages of memory (Johnson & Raye, 2000). Problems with gist extraction or gist memory, such as a failure to understand the semantic theme of a word list or a problem in retrieving the overall gist (Ciaramelli, et al., 2006; Melo, et al., 1999; Schacter, et al., 1998) can decrease false memory rates. Unusually good item-specific memory or source memory can also decrease false memory rates because subjects can accurately distinguish studied words from similar lures. Last, meta-memory processes can influence criteria used to make memory decisions at retrieval. Several such processes have been documented, such as the meta-memory belief that self-generated information is more memorable than heard information (Johnson & Raye, 1981). A similar process can explain the finding that false recognition is significantly reduced by using pictorial stimuli compared to word stimuli (Dodson & Schacter, 2002; Israel & Schacter, 1997; Schacter, Israel, & Racine, 1999; Smith, Lozito, & Bayen, 2005). This effect may be due to differences between the semantic associations of words as compared to pictures, pictures have a smaller semantic network, or it can be explained by a meta-memory belief termed the “distinctiveness heuristic” (e.g. Israel & Schacter, 1997; Schacter, Israel, & Racine, 1999) where individuals expect to remember distinctive information following picture encoding. The distinctiveness heuristic is thus a strategy that normal participants adopt in which the failure to remember expected information, in this case, distinctive image-based information, signals that the event did not occur. As such, the use of pictures in a DRM paradigm tends to make normal participants more conservative, since they tend to rely heavily on item-specific recollection to reject lures.
In Experiment 2 we assessed whether parietal lobe damage affects the ability to use distinctiveness to modulate false recognition. If found, it would suggest that parietal lobe damage affects recollection and/or the strategic use of distinctive information.
Method
Lesion Patients
The same patients tested in Experiment 1 were tested.
Control Participants
Twelve normal controls (7 males, 5 females) that were matched to the patient in age (M = 49, range = 37-66) and education (M = 14, range = 12-16) were tested. There were no differences between patient and controls in terms of age and education (both p's > .05). All control participants were given a short questionnaire to verify that they were not experiencing any neurological or psychiatric disorders at the time of testing. All participants were compensated for their participation in the experiment.
Equipment
Stimuli were presented on a 2.39 GHz Intel Core Duo Dell Optiplex 745, and utilized a 22-inch Viewsonic 2245 monitor for display of images. All stimuli were presented using E-Prime 2.0 (Psychology Software Tools, PA, USA).
Stimuli
The stimuli consisted of 24 picture lists, each containing 15 pictures that were semantically related. Each list contained pictures that were linked through one common theme (i.e. baby, clown, school), with the false alarm being the “category” of the list (see Appendix 1). Picture stimuli consisted of color photographs of real objects. Pictures were manipulated in Adobe Photoshop to make them approximately the same size, about 200 × 200 pixels.
Design
The design closely followed the design of Experiment 1 with the one exception of presenting picture lists instead of auditory word lists. The 45-minute testing session consisted of three parts: picture encoding, recall testing, and recognition testing. Part 1 began with the presentation of 16 of the 24 picture lists. Different participants were shown different lists during the testing session following an order determined by a random number generator. Each picture was presented for 1500 ms followed by a 700 ms inter-stimulus interval (ISI).
At the end of each 15-item list, one of two things happened with equal likelihood in pseudorandom order. On one half of trials, participants were required to perform a free-recall memory task where they listed all of the items they had just seen. On the other half of trials, they were given simple arithmetic problems consisting of addition or subtraction with whole number values ranging from 0-99. The presentation of another 15-item picture list was then presented for encoding. This continued until all 16 picture lists had been presented.
After all the pictures lists had been presented, a surprise recognition test was administered. Ninety-six pictures were presented; 3 pictures from each of the 16 pictures lists that the participant had previously seen and the ‘lure’ picture for each list, 3 pictures from the 8 picture lists that the participant had not seen, and the ‘lure’ pictures from the unseen lists as well. Each picture was presented for 1500 ms and followed by a 700 ms ISI. The task was to make an old/new judgment as to whether the picture had previously been seen or not. If the participant selected ‘old’, a remember/know decision about the presented picture was elicited. Participants were instructed to provide a ‘remember’ response when they were able to vividly recall the picture and to make a ‘know’ response when they had a sense of familiarity for the picture.
Results and Discussion
Free Recall
In our first analysis, we evaluated performance on the free recall portion of the task by comparing the accuracy of control participants and each patient. Patient EE555 recalled significantly fewer pictures (control M = .51, EE555: M = .32, z = -2.02), but patient TQ591 was not significantly impaired (M = .48, z = -.38). We also assessed the rate at which participants recalled non-studied lure items, in other words the degree of false recall. Again, patient EE555 performed abnormally by recalling significantly more lure items than control subjects (controls M = .13, EE555: M = .38, z = 1.79). Patient TQ591 performed no differently than controls (TQ591: M = .13, z = 0). The patients made few wrong responses or intrusions (3 intrusions each), but across all control participants there were only a total of 11 intrusions. The results of the recall analysis indicate that patient EE555 had diminished free recall but elevated false recall of pictures, whereas patient TQ591 performed within the normal range on both measures.
Recognition
Recognition performance is shown in Figure 4. Similar to the findings reported in Experiment 1, patient EE555's hit rate of 77% and false alarm rate of 44% were both abnormal (M controls = 92%, z = -2.36; M controls = 19%, z = -1.96). In this case however, her false alarm rate was significantly higher than that of controls. Patient TQ591's hit rate of 79% was also abnormally low (z = -2.02). She had a low false alarm rate of 0%, which was numerically lower than that of controls. However, the low false alarm rate of the control participants hampered our ability to detect a statistical difference in patient TQ591 (z = -1.46).
Figure 4.
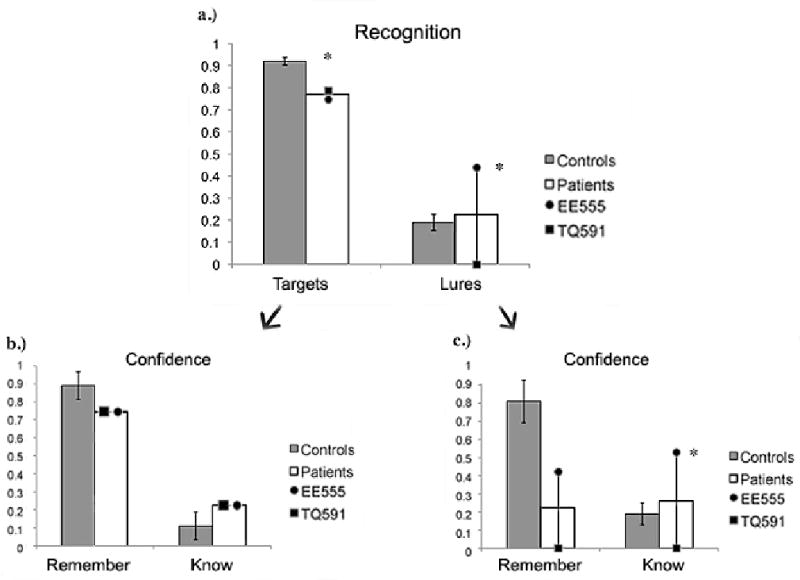
Results from (a) the recognition portion of the Visual DRM task used in Experiment 2; (b) R/K responses to targets; and (c) R/K responses to lures. Data are raw accuracy for targets and lures. Error bars represent the standard error of the mean.
As noted earlier, prior studies have shown that normal adults tend to exhibit relatively low levels of false recognition for pictorial stimuli as compared to verbal stimuli. This finding was apparent in our data: the corrected false recognition rate of normal controls dropped significantly between Experiments 1 and 2 (Experiment 1 M = .53; Experiment 2 M = .08, t 22= 6.56; p < .0004). This improvement in corrected false recognition was observed in patient TQ591, but it was not observed in patient EE555. However, both patients were better able to discriminate between old and new items in Experiment 2 than in Experiment 1 as indicated by the larger difference between their corrected true and false recognition scores (Experiment 1 M = .06, Experiment 2 M = .38).
The overall pattern for R/K responses to the lure items was similar to what was observed in Experiment 1 (see Figure 4). Patients exhibited normal levels of recollection for old items; their R/K distributions for lures was more interesting. Patient EE555's R/K profile was dominated by Know responses (control M = .39, EE555: M = .71, z = 2.70), with a numerically lower number of Remember responses relative to controls (control M = .61, EE555: M = .29, z = -.77). Patient TQ591's R/K profile was impossible to assess as she only made two false alarms to lure control items and none to lure items.
To summarize, the results of Experiment 2 show that patient EE555 had lower levels of true memory on both the recall and recognition portions of the task. Unlike control participants, she failed to benefit from distinctiveness and thus showed an elevated false recognition rate. Her false recognition responses were characterized by reduced recollection. In contrast, patient TQ591 performed relatively normally on the recall portion, and somewhat abnormally on the recognition portion of the task. Her false recall and recognition scores were normal.
These results suggest that patient EE555 failed to adopt a normal metamemory strategy that would allow one to benefit from item-specific recollection processes that tend to accompany pictorial stimuli. Alternatively, EE555's memory for pictorial stimuli may have been so impoverished in detail that even if a conservative response strategy were adopted, the quality of the remembered information was insufficient to support its implementation. The second alternative is supported by the finding that she had abnormally low levels of true memories as assessed by both recall and recognition. As in Experiment 1, EE555 lacked confidence in her false memories. In contrast, patient TQ591 appeared to benefit from the pictorial stimuli, and although she was less accurate at recognizing previously seen pictures, she was not prone to making false alarms.
General Discussion
It is well known that our recollection of the past is not a veridical simulacrum of what we have experienced, but rather, is a flawed re-creation of bygone events. The flaws in our memory are such that people frequently claim to have seen or heard something never experienced, a phenomenon captured by false memory tasks. False memories are thought to arise when target words cause the automatic activation of semantically and conceptually related gist information (Schacter, Verfaellie, & Koutstaal, 2002), leading to a false sense of recollection. In this study we used a standard false memory task, the DRM, to ask whether the parietal lobe plays any meaningful role in true memories and false memories.
Experiment 1 used a classic DRM task with auditorily presented verbal stimuli. Two patients with bilateral parietal lobe damage were tested. Both patients exhibited normal levels of word recall after relatively short delays. The recognition task occurred after a longer delay period. One of the patients, EE555, had decreased levels of true memory on the recognition task. Nevertheless, both she and the second patient exhibited normal levels of confidence in their old responses. A different picture emerged when the lure trials were examined. Both patients exhibited low numbers of false memories and their false memories were accompanied by few reports of recollection as measured by their R/K profile. For example, patient TQ591 gave remember responses to lures only 16% of the time while the normal control participants gave remember responses to lures 72% of the time.
In Experiment 2, we tested a variant of the classic verbal DRM in which the stimuli were pictures rather than words. Prior studies of normal adults have found that false recognition is significantly reduced by using pictorial stimuli compared to word stimuli (Israel & Schacter, 1997; Schacter et al., 1999). Both patients were more accurate when recognizing target pictures than when remembering words. However, the patients' false recognition rates differed. Patient EE555's false recognition rates remained constant across both experiments, suggesting that she did not benefit from the distinctiveness heuristic. Furthermore, her false memories were accompanied by an abnormal R/K profile, causing her to have a statistically lower level of confidence on the lure trials. In contrast, TQ591's false recognition rate decreased, suggesting she did benefit from the distinctiveness heuristic similarly to controls.
It is important to emphasize that the observed deficits do not appear to be linked to the patients' perceptual deficits – such as difficulty perceiving spatial information, especially information that is spatially arrayed, such as a visual scene. The stimuli used in Experiment 1 were auditory words without meaningful spatial attributes. The stimuli used in Experiment 2 were single common objects presented at central fixation, which were easily perceived by all participants. We have shown in the past that the patients tested here do not have global mental imagery impairments (Berryhill, et al., 2007) so it is unlikely the case that deficient mental imagery problems account for these findings. As such, we turn to mnemonic explanations for our findings.
Parietal Lobe Memory Mechanisms
There is fMRI evidence linking parietal lobe activity to both episodic memory encoding and retrieval. In the former case, subsequent memory paradigms have shown encoding related activity in the inferior parietal lobe that predicts poor memory retrieval, whereas more superior lateral parietal activity predicted good memory retrieval (Uncapher & Wagner, 2009). Other fMRI findings strongly indicate that inferior and superior aspects of the parietal lobe play distinct roles in memory retrieval (Cabeza et al., 2008).
False memories can arise from many sources including the activation at encoding of overlapping representations which lead to the formation of a strong gist representation due to semantic relatedness (e.g. Brainerd, Yang, Reyna, Howe, & Mills, 2008) or associative strength (e.g. Roediger, Watson, McDermott, & Gallo, 2001). However, it is difficult to explain the abnormal false memory rates in Experiment 1 as a problem specific to gist encoding. Memory for the thematic content of stories, a form of gist, was specifically tested in the WMS-III. The results showed that the patients' memory for the gist of short stories was normal but their item-specific memory, abnormal (see Experiment 1 Methods). Of course, it is possible that our patients can extract gist from stories, which are context-rich, while failing to activate semantic associates from single words, but here too, we have observed normal levels of semantic priming on an implicit priming task, indicating that these patients have normal access to semantic associates. Although the possibility remains that parietal lobe memory deficits arise at encoding, the neuropsychological data that we have collected to date do not support this contention.
Instead, our findings tend to favor a retrieval account. It is well known that abnormal false memory rates can reflect problems at retrieval (Johnson & Raye, 2000). For instance, meta-memory processes operating at retrieval bias expectations about what true memories feel like, thus modulating false memory rates. Also, the amount of retrieved perceptual detail modulates subjective memory states (Johnson & Raye, 2000).
The subjective feeling of fully re-experiencing or recollecting the event typically accompany remember responses (Tulving, 1985). Many studies, including our own, have shown that normal individuals experience false memories accompanied by strong feelings of recollection, as indicated by a high number of remember responses. The bilateral PPC patients experienced false memories accompanied by weak feelings of recollection. This behavior should not be perceived of as a general outcome of brain damage however. Both patients had normal levels of recollection to the target words in Experiment 1. Also, in three source memory tasks in which confidence ratings were collected, the same patients had normal levels of confidence for old/new recognition judgments, but abnormally low levels of confidence when making source memory judgments (Simons et al., 2009).
The issue of memory confidence has typically been conceived of as inherently intertwined with the process of remembering: high confidence responses (to targets) are thought to accompany recollection whereas low confidence responses are thought to mainly accompany familiarity. Another possibility is that these processes are different facets of memory, with only subjective aspects of memory being linked to IPL function (Ciaramelli, Lin, & Moscovitch, 2009). This observation is based on the fact that several studies have reported intact source recollection accompanied by depressed subjective memory states after parietal lobe damage (Davidson, et al., 2008; Simons, et al., 2008; Simons, et al., 2009). There are several possible explanations for the patients' decreased subjective memory states. One possibility is that the parietal lobe may play be involved in recollecting perceptual details. Consequently, when the parietal lobe is damaged, the memory lacks detail and leads to reduced memory confidence (Johnson & Raye, 2000). Alternatively, the parietal lobe may also play a role in bottom-up internal attention. This type of attention may be required for memory retrieval. Parietal lesions may impair the automatic retrieval of memories and since memory retrieval is less spontaneous the patient's recollection, or sense of reexperiencing the memory is lowered (Cabeza, 2008; Cabeza et al., 2008).
Relationship to fMRI Findings
The present findings have some concordance with neuroimaging findings. A robust finding within neuroimaging is the ‘old/new effect’ (reviewed in (Wagner, Shannon, Kahn, & Buckner, 2005). This effect refers to the observation that in memory paradigms, increased parietal activity is observed whenever an item is endorsed as ‘old’ – even when that response is incorrect. This finding supports the view that parietal structures are involved in providing a signal corresponding to perceived oldness. The prediction from these findings is that parietal damage should decrease patient's ability to assess perceived oldness. Also, fMRI studies have frequently reported that superior PPC activations correlate with familiarity and low-confidence responses whereas inferior PPC activations correlate with recollection and high-confidence responses (reviewed in (Cabeza et al., 2008; Vilberg & Rugg, 2008). In the DRM task, responses to targets are thought to reflect some combination of recollection and familiarity while responses to lures mostly reflect familiarity. fMRI findings thus predict that inferior PPC damage should decrease recollection (hits) and high-confidence responses (remember responses). In line with this, patient EE555 had significantly lower memory for target words and pictures mostly due to a reluctance to supply old responses. Also, her R/K profile on lure trials was dominated by low confidence Know responses.
Comparison to Other Patient Populations
The cognitive neuroscience of false memory has concentrated on two brain areas: the lateral frontal lobe and the medial temporal lobe (MTL). Patients with focal lateral frontal lobe lesions perform more variably on an array of false memory paradigms, but generally have elevated levels of false recognition (Budson, Daffner, Desikan, & Schacter, 2000; Budson, et al., 2002; Curran, Schacter, Norman, & Galluccio, 1997; Parkin, Ward, Bindschaedler, Squires, & Powell, 1999; Schacter, Curran, Galluccio, Milberg, & Bates, 1996; (Stuss & Levine, 2002) whereas patients with hippocampal or diencephalic lesions tend to show abnormally low levels of false memories (for a review see Schacter et al., 2002). Similarly, patients with Alzheimer's disease who possess some combination of hippocampal and frontal lobe pathology tend to exhibit diminished levels of both true and false recognition (Balota, et al., 1999; Budson, et al., 2002), which has variably been attributed to impoverished gist memory or lack of item-specific recollection. This can be contrasted to normal aging in which there is a reduced tendency to accurately retrieve target words, but an increased tendency to erroneously retrieve lure words (Balota et al., 1999).
Only one other study has examined true and false memory in patients with parietal lobe damage. Davidson and colleagues (2008) tested one patient with unilateral left parietal lobe damage on a verbal DRM task and found that false recognition was significantly decreased, similar to the findings reported in Experiment 1. Davidson's findings (2008) indicate that the left PPC more than the right, may be critical for normal performance on the standard (verbal) DRM.
One shortcoming of our study is that a lesion-control population was not tested. Perhaps the most interesting comparison group would be with patients sustaining bilateral MTL damage because they also exhibit diminished true and false memory. To compare the magnitude of the effect observed in those patients to the effects reported in PPC patients, we plotted difference scores – performance of matched controls minus patients – in Figure 5. Only studies that used the identical DRM task – the standard verbal version - were included in order to constrain stimulus and task differences. Figure 5 shows that the magnitude of the false memory impairment exhibited by both of our patients is of a similar magnitude to Davidson et al.'s patient (2008) and to the MTL patients tested by one group, Melo et al. (1999). In the future it will be important to directly compare the performance of patients with PPC damage to patients with medial temporal and frontal lobe pathology in order to determine the relative contribution of each region to true and false recognition.
Figure 5.
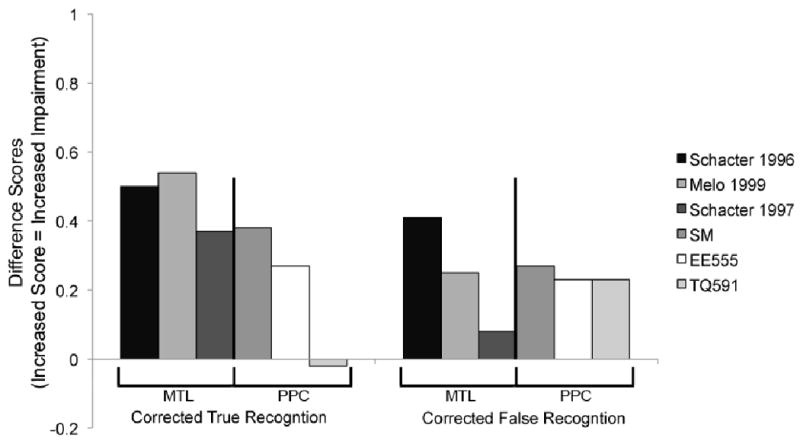
Review of focal lesion studies of the standard verbal DRM. Each bar shows the difference scores (control performance minus patient performance) for corrected true and false recognition for MTL lesion patients (Melo, et al., 1999; Schacter, et al., 1997; Schacter, et al., 1996) or PPC lesion patients (patient SM from Davidson, et al., 2008); this paper). Higher bars indicate worse performance.
As with any experimental methodology there are inherent limitations; neuropsychological research is no exception. In this study, we tested a small number of bilateral PPC patients due to their scarcity. The issue of small sample size is a frequent limitation in lesion studies, particularly when a rare patient group is studied. Other limitations associated with neuropsychological research include the likelihood of post-insult brain reorganization, the possibility of damage to other brain structures that remains invisible or unnoticed, and patients' use of different strategies to perform cognitive tasks. However, the current findings can serve as a useful guide when interpreting data from other sources such as the powerful, but correlational, method of functional neuroimaging.
Table 3.
Data from Experiment 2, DRM visual study, recognition portion of the task. This table mimics Table 4 of Davidson et al. (2008) to allow comparison between parietal patients. Based on standard high-threshold procedures (Melo et al., 1999; Schacter et al., 1998) corrected true recognition (CTR) was calculated using the formula: CTR = hits (“old” responses to targets) – false alarms (“old” responses to target-controls, i.e. items from unstudied lists). Corrected false recognition (CFR) scores were calculated with the formula: CFR = hits (“old” responses to critical lures) – false alarms (“old” responses to lure-controls, i.e., critical lures of unstudied lists). R and K responses were computed as a proportion of the old responses thus they will not add up to 100 vertically but will horizontally. Z scores below -1.96 are considered impaired and are demarked with an ‘*’.
| Old responses | R response | K responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TQ591 | EE555 | Controls | TQ591 | EE555 | Controls | TQ591 | EE555 | Controls | |
| A. Targets (heard words) | 0.79* | 0.77* | 0.92 | 0.76 | 0.76 | 0.89 | 0.24 | 0.24 | 0.11 |
| B. Target controls (never heard words) | 0.08 | 0.04 | 0.03 | 0.91 | 0.91 | 0.53 | 0.09 | 0.09 | 0.47 |
| Corrected true recognition | 0.71* | 0.73* | 0.89 | 0.69 | 0.69 | 0.93 | 0.31 | 0.31 | 0.07 |
| C. Critical lures (lures corresponding to heard lists) | 0.00 | 0.44* | 0.19 | 0.00 | 0.44 | 0.78 | 0.00 | 0.56* | 0.22 |
| D. Lure controls (lures from never heard lists) | 0.25 | 0.00 | 0.10 | 1.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 |
| Corrected false recognition | -0.25 | 0.44* | 0.09 | 1.00 | 0.29 | 0.61 | 0.00 | 0.71* | 0.39 |
Acknowledgments
We would like to thank Marianna Stark for recruiting participants, Anjan Chatterjee and Branch Coslett for providing the patient database, and David Wolk, Jason Chein, and Roberto Cabeza for helpful discussions. We would also like to thank Yonatan Mazuz for assistance in testing control participants and Cara Shay for stimulus development. This research was supported by RO1 MH071615 to I. Olson and NRSA NS059093 to M.Berryhill.
Appendix 1
| Stapler | Car | Refrigerator | Clown | Hammer | Baseball | Dog | School | |
| Pen | Train | Fork | Trapeze | Nail | Diamond | Bone | Desk | |
| Pencil | Segway | Peeler | Cotton Candy | Screwdriver | Base | Rabbit | Teacher | |
| Pencil Sharpener | Tractor | Knife | (Big Top)Tent | Toolbox | Pitcher | Cat | Chalkboard | |
| Tape | Helicopter | Spoon | Elephant | Saw | Catcher | Frisbee | Microscope | |
| Scissors | Traffic Light | Mixer | Balloon Animal | Tape Measure | Hotdog | Wolf | Calculator | |
| Paperclip | Hot Air Balloon | Plate | Clown Car | Level | Cracker Jacks | Bowl | Locker | |
| Ruler | Submarine | Stove | Juggler | Axe | Bat | Fleas | Apple | |
| Highlighter | Bicycle | Oven Mitt | Tightrope | Drill | Glove | Collar | Eraser | |
| Filing Cabinet | Motorcycle | Microwave | Ringmaster | Mallet | Umpire | Leash | Notebook | |
| Desk | Truck | Pan | Bozo Shoes | Wrench | Scoreboard | Dog House | Bus | |
| Clipboard | Tricycle | Pot | Peanuts | Pliers | Stadium | Paw (Print) | Backpack | |
| Rolledex | Bus | Blender | Unicycle | Socket Wrench | Cards | Bed | Crayon | |
| Briefcase | Buggy | Coffee Pot | Wig | Crowbar | Helmet | Kibbles & Bits | Hopscotch | |
| Scanner | Scooter | Colander | Acrobat | Ladder | Cleats | Lint Roller | Lunchbox | |
| Office Chair | Skateboard | Faucet (Sink) | Bear | Vice | Cap | Pooper Scooper | Bell | |
| Baby | Computer | Lion | Bird | Rose | Butterfly | Priest | Shell | |
| Stork | CD | Rhinoceros | Worm | Daisy | Grasshopper | Church | Starfish | |
| Bottle | Copier | Tiger | Binoculars | Violet | Bee | Bible | Volleyball | |
| Balloons | Fax Machine | Africa | Beak | Leaf | (Insect) Wings | Angel | Bikini | |
| Rattle | Floppy Disk | Savannah | Wing | Plant | Beatle | Jesus | Beach Chair | |
| Safety pin | Joystick | Camera | Talon | Stem | Jar | Star (of David) | Sunglasses | |
| (Rocking)Chair | Keyboard | Hyena | Nest | Soil | Net | Cross | Pail | |
| High Chair | Power Strip | Hippopotamus | Eggs | Tree | Fly | Dove | Sandastle | |
| Diapers | Mouse | Antelope | Feather | Petal | Cocoon | Nun | Lighthouse | |
| Doll | Monitor | Joshua Tree | Bath | Tulip | Dragonfly | Wafer | Flippers | |
| Stroller | Speakers | Alligator | Cage | Vase | Caterpillar | Pope | Scuba Mask | |
| Bib | Cables | Zebra | Food | Bouquet | Spider | Pipe Organ | Seagull | |
| Pacifier | Apple Symbol | King | Sylvester the Cat | Pot | Ladybug | Wine Cup (Chalice) | Sandal | |
| Crib | Windows Symbol | Canoe | Cucu Clock | Watering Can | Mosquito | Altar | Palm tree | |
| Mobile | Processor/chip | Flashlight | South (Direction) | Roots | Peacock | Candles | Kite | |
| Teddy Bear | Webcam | Monkey | Flying V | Seed | Tinkerbell (fairy) | Holy Water | Sailboat | |
| Hotel | Tire | Film | Whiskey | Shirt | Earth | Cigarette | Airplane | |
| Bed | Rearview mirror | Popcorn | Beer Mug | Socks | Asteroid | Smoke | Pilot | |
| Toiletries | Steering wheel | Candy | Martini | Sweater | Moon | Doctor | Flight Attendant | |
| Phonebook | Gearshift | Ticket | Police men | Pants | Astronaut | Pipe | Cockpit | |
| Maid | Emergency Brake | Seat | Flask | Bra | Telescope | Red Bull can | Luggage | |
| Bellboy | Windshield Wiper | Screen | Grey Goose | Belt | Galaxy | No Smoking Sign | Baggage Claim | |
| Suitcase | Brake Light | Usher | Absolut | Scarf | Comet | Matches | Wing | |
| Do not disturb tag | Door | Arcade Game | Beer (Miller Lite) | Shorts | Globe | Pills | Tray Table | |
| Mints (on pillow) | Odometer | Clacker | Shot (glass) | Boots | Spaceship | Ashtray | Plane Seat | |
| Towels | Radio | Camera | Pub | Dress | Satellite | Marlboro Logo | Parachute | |
| Bathrobe | Gas Pedal | Stagelights | Slot Machine | Cowboy Hat | Sun | Cigar | Life Jacket | |
| Slippers | Glove Compartment | Stage | Bourbon | Gloves | Constellation | Joe the Camel | Lavatory/bath room | |
| Room service tray | Headlight | Microphone | Tequilla | Jacket | Saturn | Lighter | Propeller | |
| Minibar | Car key | Ticket Booth | Cognac | Overalls | Star | Lungs | Seatbelt | |
| Pool | Sun Roof | Theater | Wine (Glass) | Shoe | Rocket | Needle | Oxygen Mask | |
| Doorman | Sideview Mirror | Director's Chair | Barrel | Tie | Mars | Convenience Store | Runway |
Footnotes
Age-standardized percentile scores were available however percentile ranks were not available.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE. Parietal contributions to recollection: electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia. 2008;46(7):1800–1812. doi: 10.1016/j.neuropsychologia.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Cortese MJ, Duchek JM, Adams D, Roediger HL, III, McDermott KB, et al. Veridical and false memories in healthy older adults and in dementia of the alzheimer's type. Cognitive Neuropsychology. 1999;16:361–384. [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for working memory. Neuropsychologia. 2008;46(7):1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Yang Y, Reyna VF, Howe ML, Mills BA. Semantic processing in “associative” false memory. Psychon Bull Rev. 2008;15(6):1035–1053. doi: 10.3758/PBR.15.6.1035. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46(7):1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nature Reviews Neuroscience. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Ghetti S, Frattarelli M, Ladavas E. When true memory availability promotes false memory: Evidence from confabulating patients. Neuropsychologia. 2006;44:1866–1877. doi: 10.1016/j.neuropsychologia.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Lin O, Moscovitch M. Episodic memory for spatial context biases spatial attention. Experimental Brain Research. 2009;192(3):511–520. doi: 10.1007/s00221-008-1548-9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy M, Shulman G. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim ASN, Murphy KJ, et al. Does parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46(7):1743–1755. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deese J. On the prediction of occurence of particular verbal intrusions in immediate recall. Journal of Experimental Psychology. 1959;58(1):17–22. doi: 10.1037/h0046671. [DOI] [PubMed] [Google Scholar]
- Gallo DA, Weiss JA, Schacter DL. Reducing false recognition with criterial recollection tests: Distinctiveness heuristic versus criterion shift. Journal of Memory and Language. 2004;51:473–493. [Google Scholar]
- Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Israel L, Schacter DL. Pictorial encoding reduces false recognition of semantic associates. Psychonomic Bulletin & Review. 1997;4(4):577–581. [Google Scholar]
- Johnson MK, Raye CL. Reality monitoring. Psychological Review. 1981;88(1):67–85. [Google Scholar]
- Johnson MK, Raye CL. Cognitive and brain mechanisms of false memories and beliefs. In: S IDL, Scarry E, editors. Memory, brain, and belief. Cambridge, MA: Harvard University Press; 2000. pp. 35–86. [Google Scholar]
- Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: a new assessment of autobiographical and personal semantic memory in amnesic patients. J Clin Exp Neuropsychol. 1989;11(5):724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Liu T, Slotnick SD, Serences JT, Yantis S. Cortical mechanisms of feature-based attentional control. Cerebral Cortex. 2003;13(12):1334–1343. doi: 10.1093/cercor/bhg080. [DOI] [PubMed] [Google Scholar]
- Melo B, Winocur G, Moscovitch M. False recall and false recognition: an examination of the effects of selective and combined lesions to the medial temporal lobe/diencephalon and frontal lobe structures. Cognitive Neuropsychology. 1999;16:343–359. [Google Scholar]
- Robertson L, Treisman A, Friedman-Hill S, Grabowecky M. The interaction of spatial and object pathways: Evidence from Balint's Syndrome. Journal of Cognitive Neuroscience. 1997;9(3):295–317. doi: 10.1162/jocn.1997.9.3.295. [DOI] [PubMed] [Google Scholar]
- Roediger HL, 3rd, Watson JM, McDermott KB, Gallo DA. Factors that determine false recall: a multiple regression analysis. Psychon Bull Rev. 2001;8(3):385–407. doi: 10.3758/bf03196177. [DOI] [PubMed] [Google Scholar]
- Roediger HLI, McDermott KB. Creating false memories: remembering words not presented in lists. Journal of Experimental Psycholgoy: Learning, Memory, and Cognition. 1995;21(4):803–814. [Google Scholar]
- Schacter DL, Israel L, Racine C. Suppressing false recognition in younger and older adults: the distinctiveness heuristic. Journal of Memory and Language. 1999;40(1):1–24. [Google Scholar]
- Schacter DL, Slotnick SD. The cognitive neuroscience of memory distortion. Neuron. 2004;44:149–160. doi: 10.1016/j.neuron.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Anes MD, Racine C. When true recognition suppresses false recognition: Evidence from amnesic patients. Journal of Cognitive Neuroscience. 1998;10:668–679. doi: 10.1162/089892998563086. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Verfaellie M, Koutstaal W. Memory illusions in amnesic patients: findings and impllicatinos. In: Squire L, Schacter DL, editors. Neuropsychology of Memory. New York: Guildford Press; 2002. [Google Scholar]
- Shomstein S, Yantis S. Control of attention shifts between vision and audition in human cortex. Journal of Neuroscience. 2004;24(47):10702–10706. doi: 10.1523/JNEUROSCI.2939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DY, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46(4):1185–1191. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Mazuz Y, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of Episodic Memory. USA: Oxford University Press; 1985. [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: Insights from fMRI subsequent memory effects and dual-attention theory. Neurobiology of Learning and Memory. 2009;91(2):139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from event-related fMRI. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RJ. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Neuroscience. 2005;9:9. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]


