ABSTRACT
BACKGROUND
N-of-1 trials test treatment effectiveness within an individual patient.
OBJECTIVE
To assess (i) the impact of three different N-of-1 trials on both clinical and economic outcomes over 12 months and (ii) whether the use of N-of-1 trials to target patients’ access to high-cost drugs might be cost-effective in Australia.
DESIGN
Descriptive study of management change, persistence, and costs summarizing three N-of-1 trials.
PARTICIPANTS
Volunteer patients with osteoarthritis, chronic neuropathic pain or ADHD whose optimal choice of treatment was uncertain.
INTERVENTIONS
Double-blind cyclical alternative medications for the three conditions.
MEASURES
Detailed resource use, treatment and health outcomes (response) data collected by postal and telephone surveys immediately before and after the trial and at 3, 6 and 12 months. Estimated costs to the Australian healthcare system for the pre-trial vs. 12 months post-trial.
RESULTS
Participants persisting with the joint patient-doctor decision 12 months after trial completion were 32% for osteoarthritis, 45% for chronic neuropathic pain and 70% for the ADHD trials. Cost-offsets were obtained from reduced usage of non-optimal drugs, and reduced medical consultations. Drug costs increased for the chronic neuropathic pain and ADHD trials due to many patients being on either low-cost or no pharmaceuticals before the trial.
CONCLUSIONS
N-of-1 trials are an effective method to identify optimal treatment in patients in whom disease management is uncertain. Using this evidence-based approach, patients and doctors tend to persist with optimal treatment resulting in cost-savings. N-of-1 trials are clinically acceptable and may be an effective way of rationally prescribing some expensive long-term medicines.
KEY WORDS: N-of-1 trials, cost-effectiveness, follow-up study, rational prescribing
BACKGROUND
Chronic diseases are now among the most prevalent and costly of all health problems.1 A large proportion of health costs are attributable to pharmaceuticals.2 These costs would be reduced by targeting drugs just to patients who benefit from them, thereby freeing resources for others who may receive large incremental benefits from treatment. This is particularly important for conditions in which individual responses to a treatment are variable.
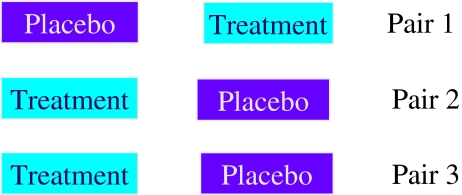
N-of-1 trials are multi-cycle within-patient, randomized, double-blind, cross-over comparisons of a drug and placebo (or another drug) using standardized measures of effect (Fig. 1). They provide evidence-based information on individual response to treatment and can be used to optimize the chronic disease management of the individual in the trial.
Figure 1.
Typical N-of-1 trial. The order of treatment and placebo are randomly assigned for each cycle.
The following are the essential characteristics of medicines suitable for an N-of-1 trial: 1) the condition for which the medication is being prescribed is chronic and [relatively] stable; 2) the half-life of the medication being tested is short; 3) there is rapid onset/offset of biological action of the medication; 4) the effect of the medication can be measured using a validated outcome measure; 5) the medication does not alter the underlying condition.3,4
Clinicians commonly conduct informal trials of therapy when they start a drug in a patient and judge the clinical response. However, compared with the more structured N-of-1 trials, these are methodologically inadequate to provide evidenced-based information for tailoring the individual’s chronic disease management. Although N-of-1 trials are not widely used, there is potential for these to become part of normal medical practice for targeted illnesses, drugs and participants.5,6 Moreover, N-of-1 trials may facilitate targeting of government subsidized medicines to patients for whom there is demonstrable benefit.7
Objectives
The objective of the study is to determine if the use N-of-1 trials reduces health care costs compared to “standard practice”. We summarize here the impact of three N-of-1 trials including a one-year follow-up. This follow-up was important to monitor adherence to the optimized therapy identified in the trial and observe the associated costs. We report the observed costs of management and the expected costs for scenarios where the higher-cost pharmaceuticals are restricted to responders only.
METHODS
Design
In 2003–2005, we conducted three N-of-1 trials:
Celecoxib (Celebrex) versus sustained release acetaminophen (SR-acetaminophen) (PanadolOsteo) for osteoarthritis performed in a community setting.
Gabapentin (Gantin) versus placebo for chronic neuropathic pain performed mostly in a hospital outpatient setting.
Dexamphetamine (dexamphetamine sulfate) versus methylphenidate (Ritalin/Ritalin LA) or placebo for ADHD performed in both a community and a hospital outpatient setting.
Ethics approval for the trials was obtained from The University of Queensland’s Medical Research Ethics Committee. Additionally, for the neuropathic pain trial, approval was obtained from the ethics committees of the participating institutions, Princess Alexandra Hospital, Brisbane and the Port Kembla Hospital, Port Kembla. For the ADHD trial, additional approval was obtained from the ethics committee of Mater Misericordiae Health Services, Brisbane. In late 2004, the Australian Therapeutic Goods Administration instructed all trials using celecoxib to cease due to possible adverse health events identified in other members of the selective COX-2 inhibitor class of drugs, and recruitment for this trial was ceased prematurely.
Participants
Participants were volunteer patients with osteoarthritis, chronic neuropathic pain or ADHD whose optimal choice of treatment was uncertain. The trial methods have been described elsewhere and are summarized in Table 1.8–10
Table 1.
Methods Summary for the Three N-of-1 Trials
| Chronic disease | Treatment and Comparator | Structure of trial | Outcome measures |
|---|---|---|---|
| Osteo-arthritis | Celecoxib and sustained-release (SR) acetaminophen | 12-week total | Pain, stiffness, functional limitation, frequency of adverse events and preferred pharmaceutical |
| 3 × 4-week cycles | Differential responses in pain, stiffness and functional limitation responses were determined by minimum clinically detectable differences; for adverse events, by a lower frequency on one pharmaceutical in at least two cycles; and preferred pharmaceutical by a preference for one pharmaceutical in at least two cycles. These variables were then equally weighted to determine the overall response status of each participant | ||
| Each cycle was 2 weeks on each pharmaceutical. | |||
| Chronic neuropathic pain | Gabapentin and placebo | 12-week total | P>ain, sleep interference, functional limitation, frequency of adverse events and preferred pharmaceutical. Differential responses in pain, sleep interference and functional limitation responses were determined by minimum clinically detectable differences; for adverse events, by a lower frequency on one pharmaceutical in at least two cycles; and preferred pharmaceutical by a preference for one pharmaceutical in at least two cycles. These variables were then equally weighted to determine the overall response status of each participant |
| 3 × 4-week cycles | |||
| Each cycle was 2 weeks on gabapentin or 2 weeks on placebo. | |||
| ADHD | Methylphenidate LA and dexamphetamine | 3-week total | Responders |
| 3 × 5-day cycles | Responders defined as those who had an improvement in symptoms, reported by their parent, with one treatment over the other in all three cycles | ||
| Two days on each pharmaceutical separated by one washout day, and a two-day washout period at the weekend. |
Outcomes Data
For this study, a pre-post trial design was used to compare participants’ use of medications before and after the N-of-1 trial. On completion of the trials participants were classified as “responders” and “non-responders”; at this time a management plan was agreed between the participant and their clinician (see Table 1). Follow-up semi-structured telephone interviews were conducted at three, six and 12 months for all participants who completed the trials. These interviews recorded information on the participant’s current therapeutic management, reasons for any changes in treatment strategy, and feedback about the impact of the trial on the management of the participant’s symptoms. This information was used to determine the effects of the N-of-1 trial on individuals’ disease management. Persistence with the treatment decision from the trial is defined as “the act of continuing the treatment for the prescribed duration”11 and was classified at 12 months.
Economic Analysis
The present study focuses on responders and non-responders as classified at the end of the trial. For this analysis, cases with complete records were used; cases identified as possible responders were classified as non-responders.
A cost analysis took the perspective of costs to the Australian healthcare system. These are costs associated with management of osteoarthritis, chronic neuropathic pain and ADHD largely incurred through visits to primary care physicians, specialists in hospital outpatients, and for pharmaceuticals. Allied health costs (e.g. physiotherapy) were not considered in the analysis.
Costs for these healthcare services are borne by the federal government through the PBS and Medicare for primary care physician visits, and by State governments for public hospital outpatient services. Co-payments and out-of-pocket expenses incurred by patients were not included.
Data collected from participants included details of treatments and disease management strategies for the three months before the N-of-1 trial (pre-trial data), data on resource use to 12 months follow-up and the fixed and variable costs of administering the trials. Participants were asked the number of visits to the doctor and details on pharmaceutical use at that time. These resources were assigned a standard cost of $31.45 per general practitioner (GP; i.e. family physician) visit and $65.40 per specialist visit (the amount rebated to the patient by Medicare for a primary care visit or a specialist follow-up visit;12 A$1 ≈ US$0.70); pharmaceuticals were assigned the full price from the Schedule of Pharmaceutical Benefits.13
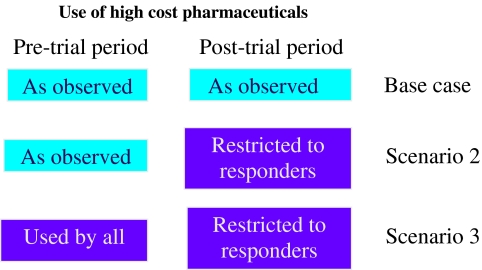
Three scenarios are analyzed (Fig. 2). First is the observed pre-and post-trial costs for the participants in the three trials, including costs for pharmaceuticals and medical consultations. In the second scenario, we assumed continuing access to the pharmaceutical in question is dependent on evidence of a response to the pharmaceutical from the N-of-1 trial (i.e. only responders to the higher cost pharmaceutical would be eligible to receive it). We used the observed pre-and post-trial medical consultation rates and calculated the expected costs for restricting the higher-cost pharmaceuticals to responders. In the third scenario, we assumed that all participants had been using the higher cost pharmaceutical for at least three months prior to entry into the trial, and only responders to the higher cost pharmaceutical would eligible to continue to receive that pharmaceutical. This scenario is an extension of the second scenario to model the expected costs and consequences for patients who are using the pharmaceutical prior to commencing the N-of-1 trial.
Figure 2.
Scenarios evaluated for use of higher cost pharmaceuticals.
The healthcare use and costs in the previous three months reported in the post-trial surveys at months three, six and 12 were used to estimate annual totals; the costs and healthcare use reported at the 12-month surveys for the previous three months was doubled to give a six month estimate. The reported pre-trial data were extrapolated to 12 months (by multiplying these costs by four). Differences in pre-and post-trial costs for the three scenarios were then calculated.
Data were analyzed using Excel and SPSS version 17. Means, medians, SDs and inter-quartile ranges (IQRs) are presented for descriptive statistics. In addition, the percentage of participants with a reduction in health care use, pharmaceuticals or costs in the post-trial period are reported.
To calculate the significance of mean differences between costs in the pre-and post-trial periods, t-tests were used. However, small sample sizes and differenced data are more likely to be non-normally distributed. Therefore, each series of differenced data were randomly resampled 10,000 times (i.e. bootstrapped) with the bias-corrected and accelerated (BCa) method.14,15 This enabled normalized 95% confidence intervals (95%CI) to be estimated and t-tests to be conducted. A statistically significant difference was defined as a probability of 5% or less that the result occurred by chance.
RESULTS
The demographic and clinical characteristics of the participants in each of the three trials have been described previously and are summarized in Table 2.
Table 2.
Summary Demographics and Participant Characteristics of the Three N-of-1 Trials
| Neuropathic pain trials 9a | Osteoarthritis trials 10a | ADHD trials 27a | |
|---|---|---|---|
| Number enrolled in trial, N (% completed N-of-1 trial) | 73 (75%) | 59 (69%) | 86 (88%) |
| Age in years-median (min, max) | 58 (24, 94) | 65 (47, 80) | 10 (5, 16) |
| Sex (% male) | 33% | 37% | 74% |
| Prior treatment | |||
| Years (mean, SD) | 6.6 (8.5) | 11.3 (10.3) | 5.1 (4.3) |
| range (min, max) | 0.8, 40 | 1, 46 | 0.7, 12 |
| Pre-trial main regular pharmaceuticals | Gabapentin (16%) | NSAID alone (56%) | Methylphenidate (42%) |
| Acetaminophen (15%) | NSAID with SR acetaminophen (17%) | Dexamphetamine (55%) | |
| Acetaminophen with codeine (11%) | No pharmaceuticals (15%) | No pharmaceuticals (3%) | |
| No pharmaceuticals (13%) | Other (12%) | ||
| NSAIDs (7%) | |||
| Pharmaceuticals in N-of-1 trials | Gabapentin vs. placebo | Celecoxib vs. SR-acetaminophen | Methylphenidate / Dexamphetamine vs. placebo |
| Key clinical results | Gabapentin was more effective than placebo in 29% of participants, in 2% placebo was better and in 69% there was no difference | Celecoxib was more effective than SR acetaminophen for 17% of participants; | Treatment with stimulants was definitely more effective than placebo for 42% of 66 completers, possibly in 17% and definitely not in 41%. |
| SR acetaminophen was the most effective for 2% and in 80% there was no difference | |||
| Number with complete pre-trial and 12-month follow-up data (n) b | 41 | 32 | 18 |
| Persistence with trial decision at 12 months (%) b | 31.7% | 44.9% | 69.8% |
| Mean cost of active pharmaceutical in trials (A$) c | $106.24 | $29.91 / $11.69 | $17.76 / $16.53 |
| Quantity dispensed | 100 × 300 mg tabs | 30 × 200 mg tabs / 192 × 665 mg tabs | 100 × 10 mg tabs / 100 × 5 mg tabs |
| Mean daily dose of active pharmaceuticals (mg) | 1317 mg | 244 mg / 3990 mg | 32.4 mg / 17.7 mg |
a Data reported for completers unless otherwise stated
b.Data pertaining to current analysis
c. Excludes dispensing fees
All participants in the osteoarthritis trials completed all pre-and post-trial surveys; however, the proportions completing all surveys in the neuropathic pain and ADHD trials were lower. We had no way of determining any bias for those who were lost to follow-up, and therefore restricted the analysis to only those completing all surveys. The ADHD trial had enrolled a substantial number of participants without obtaining pre-trial data because it had already commenced when funding for this current study was received.
The N-of-1 trials included identification of changes to patient management at the end of the trials and compliance with the clinical decision over the following 12 months. These results have been described in detail elsewhere and are summarized in Table 2.8–10,16 The rates of persistence with the treatment decision from the trial at 12 months were greatest for ADHD participants at approximately 70%, followed by 45% for celecoxib participants, and 32% of the participants from the neuropathic pain trial. The persistence rate in the celecoxib trial may have been reduced by well-publicized warnings published in the media and understandable anxiety.
The annual mean number of medical consultations was lower in the post-trial period than the pre-trial period for all three trials, with 5.9, 5.0 and 2.2 fewer consultations in the osteoarthritis, neuropathic pain, and ADHD trials, respectively (Table 3). These differences were statistically significant in the osteoarthritis trial (p < 0.001), but not in the neuropathic pain trial (p = 0.076) nor the ADHD trial.
Table 3.
Observed Costs and Potential Cost-Savings in the Three N-of-1 Trials
| Annual estimates | Mean difference | Lower 95%CI | Upper 95%CI | p-value | Participants with reduced healthcare use or cost-savings | ||
|---|---|---|---|---|---|---|---|
| Pre-trial | Post-trial | ||||||
| Osteoarthritis trials (Celecoxib vs. acetaminophen; N = 41) | |||||||
| Mean medical consultations (n) | 13.95 | 8.08 | −5.87 | −9.32 | −2.51 | 0.0007 | 70.7% |
| Mean prescription items (n) | 19.61 | 17.85 | −1.76 | −4.02 | 0.29 | 0.1108 | 43.9% |
| Mean cost medical consultations (A$) | 438.77 | 252.37 | −186.40 | −293.02 | −80.54 | 0.0006 | 70.7% |
| Mean cost prescription items (A$) | 428.87 | 356.62 | −72.26 | −144.03 | −2.68 | 0.0451 | 56.1% |
| Mean health system cost (A$) | 867.64 | 608.99 | −258.65 | −395.35 | −123.25 | 0.0002 | 75.6% |
| (SD) | (440.27) | (255.91) | (448.79) | ||||
| Median health system cost (A$) | 813.76 | 568.42 | −189.81 | ||||
| (Lower quartile, upper quartile) | (657.40; 952.32) | (434.45; 737.75) | (−494.78;−1.29) | ||||
| Neuropathic pain trials (Gabapentin vs. placebo; N = 32) | |||||||
| Mean medical consultations (n) | 19.41 | 14.44 | −4.97 | −10.44 | 0.53 | 0.0757 | 58.8% |
| Mean prescription items (n) | 7.53 | 7.15 | −0.38 | −2.32 | 1.47 | 0.6928 | 47.1% |
| Mean cost medical consultations (A$) | 1407.50 | 454.18 | −953.33 | −1166.08 | −745.20 | <0.0001 | 91.2% |
| Mean cost prescription items (A$) | 726.97 | 1145.52 | 418.55 | 99.75 | 742.85 | 0.0107 | 37.1% |
| Mean health system cost (A$) | 2143.35 | 1675.73 | −467.63 | −903.44 | −45.78 | 0.0326 | 60.6% |
| (SD) | (999.20) | (1117.30) | (1300.53) | ||||
| Median health system cost (A$) | 1950.58 | 1753.12 | −551.76 | ||||
| (Lower quartile, upper quartile) | (1494.62; 2513.87) | (707.30; 2304.92) | (−1211.18; 440.62) | ||||
| ADHD trials (stimulants vs. placebo; N = 18) | |||||||
| Mean medical consultations (n) | 6.00 | 3.78 | −2.22 | −5.61 | 0.94 | 0.1839 | 44.4% |
| Mean prescription items (n) | 3.11 | 2.11 | −1.00 | −2.67 | 0.44 | 0.2077 | 38.9% |
| Mean cost medical consultations (A$) | 188.70 | 118.81 | −69.89 | −174.72 | 31.45 | 0.1839 | 44.4% |
| Mean cost prescription items (A$) | 89.64 | 122.16 | 32.51 | −18.90 | 83.15 | 0.2117 | 33.3% |
| Mean health system cost (A$) | 278.34 | 240.97 | −37.38 | −166.03 | 83.85 | 0.5577 | 44.4% |
| (SD) | (343.65) | (199.07) | (282.08) | ||||
| Median health system cost (A$) | 217.44 | 220.63 | 7.65 | ||||
| (Lower quartile, upper quartile) | (100.18; 218.58) | (102.57; 303.84) | (−150.71; 86.40) | ||||
* includes specialist visits: pre-trial mean = 2.18, 3-month post-trial mean = 1.0; these specialist visits were excluded from the statistical tests
In all three trials, fewer pharmaceuticals were prescribed in the post-trial compared with the pre-trial period; these differences were not significant. However, the smaller number of items prescribed resulted in pharmaceutical costs that were significantly lower in the osteoarthritis trials only (A$72, p = 0.045). Significantly higher mean costs for pharmaceuticals were noted in the neuropathic pain trials (A$468; p = 0.011) and no significant difference in the ADHD trials (A$32; p = 0.212) (Table 3).
Following the osteoarthritis and neuropathic pain trials, the annual costs to the health system were significantly lower than the pre-trial period (i.e. cost-saving), and lower, but not significantly, (−A$37; p = 0.558) following the ADHD trials. These differences in total costs are largely due to the costs saved from lower medical consultation rates in the post-trial period. (Table 3).
The N-of-1 trials for osteoarthritis were most likely to result in cost-savings; (i.e. following these trials, cost-savings to the health system were obtained for 75.6% of the participants). In the neuropathic pain trials, cost-savings to the health system were obtained for 60.6% of participants whereas in the ADHD trials, cost-savings were obtained for 44.4% (Table 3).
Scenario Analyses-Trial Results Endorsed and Prior Use of Higher Costs Pharmaceuticals
Results for the scenarios where evidence is required for continued access to the pharmaceutical in question are reported in Table 4. When trial results are endorsed, the costs of pharmaceuticals are cost-saving for osteoarthritis (and of similar magnitude as those observed-see Table 3), but continue to result in higher costs for neuropathic pain and ADHD. However, compared with the observed costs (Table 3), the costs for pharmaceuticals in the neuropathic pain and ADHD trials are substantially lower when trial results are endorsed. Under this scenario, N-of-1 trials result in statistically significant cost-savings to the health system for osteoarthritis and neuropathic pain (p < 0.001 for both), but no statistically significant difference in the ADHD trials. When all participants were assumed to have used the higher cost pharmaceutical for the three months before the trials, significant cost-savings to the health system were evident for all three trials (Table 4).
Table 4.
Scenarios with Pharmaceutical Access Dependant on Results of N-of-1 Trials
| Annual estimates | Mean difference | Lower 95%CI | Upper 95%CI | p-value | Participants with reduced healthcare use or cost-savings | ||
|---|---|---|---|---|---|---|---|
| pre-trial | post-trial | ||||||
| Osteoarthritis trial (Celecoxib vs. placebo) | |||||||
| N-of-1 trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 438.77 | 252.37 | −186.40 | −293.02 | −80.54 | 0.0006 | 70.7% |
| Mean cost prescription items (A$) | 428.87 | 357.44 | −71.44 | −137.65 | −5.51 | 0.0341 | 48.8% |
| Mean (SD) health system cost (A$) | 867.64 (440.27) | 609.80 (256.78) | −257.84 (413.19) | −384.17 | −138.13 | <0.0001 | 75.6% |
| All patients on Celecoxib 3/12 pre-trial and trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 438.77 | 252.37 | −186.40 | −293.02 | −80.54 | 0.0006 | 70.7% |
| Mean cost prescription items (A$) | 459.24 | 357.44 | −101.81 | −167.90 | −36.27 | 0.0024 | 61.0% |
| Mean (SD) health system cost (A$) | 898.01 (419.61) | 609.80 (256.78) | −288.20 (418.03) | −412.04 | −163.58 | <0.0001 | 80.5% |
| Neuropathic pain trial (Gabapentin vs. placebo) | |||||||
| N-of-1 trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 1407.50 | 454.18 | −953.33 | −1166.08 | −745.20 | <0.0001 | 91.2% |
| Mean cost prescription items (A$) | 726.97 | 811.08 | 84.11 | −245.69 | 418.77 | 0.6198 | 51.4% |
| Mean (SD) health system cost (A$) | 2094.26 (911.80) | 1252.28 (859.19) | −841.98 (1129.94) | −1230.29 | −477.28 | <0.0001 | 79.4% |
| All patients on Gabapentin 3/12 pre-trial and trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 1407.50 | 454.18 | −953.33 | −1166.08 | −745.20 | <0.0001 | 91.2% |
| Mean cost prescription items (A$) | 999.44 | 811.08 | −188.36 | −516.51 | 137.56 | 0.2590 | 65.7% |
| Mean (SD) health system cost (A$) | 2366.73 (909.00) | 1252.28 (859.19) | −1114.45 (1124.66) | −1512.67 | −752.49 | <0.0001 | 85.3% |
| ADHD trial (Stimulants vs. placebo) | |||||||
| N-of-1 trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 188.70 | 118.81 | −69.89 | −174.72 | 31.45 | 0.1839 | 44.4% |
| Mean cost prescription items (A$) | 89.64 | 74.36 | −15.29 | −68.69 | 38.24 | 0.5753 | 55.6% |
| Mean (SD) health system cost (A$) | 278.34 (343.65) | 193.17 (170.81) | −85.17 (265.08) | −203.11 | 29.40 | 0.1510 | 50.0% |
| All patients on high-cost medications pre-trial and trial results endorsed | |||||||
| Mean cost medical consultations (A$) | 188.70 | 118.81 | −69.89 | −174.72 | 31.45 | 0.1839 | 44.4% |
| Mean cost prescription items (A$) | 123.05 | 74.36 | −48.70 | −93.91 | −2.37 | 0.0371 | 72.2% |
| Mean (SD) health system cost (A$) | 311.75 (324.09) | 193.17 (170.81) | −118.58 (248.68) | −234.19 | −12.99 | 0.0356 | 66.7% |
DISCUSSION
The N-of-1 trials for osteoarthritis, chronic neuropathic pain and ADHD appeared to have a high impact on prescribing with a high proportion of management changes compared to baseline over the 12 months of follow-up.9,10,16,17 In addition, persistence was high for the ADHD trial (70%). Indeed, another methylphenidate N-of-1 study has shown >80% persistence with the joint decision at similar time periods.17 However, persistence was lower with the osteoarthritis and chronic neuropathic pain trials (45% and 32%). For the celecoxib trial, persistence decreased substantially to about a third of participants after ceasing the trial prematurely due the warnings in the media about COX-2 inhibitors. This rate is similar to general compliance rates reported in the literature for participants who have not been involved in N-of-1 trials.18,19
Comparing the costs for pharmaceuticals and medical consultations for the 12 months pre-and post the N-of-1 trials, significant cost-savings to the health system were evident for the osteoarthritis and chronic neuropathic pain trials. The majority of cost-savings were from reductions in medical consultations rather than reduced pharmaceutical costs. In fact, the pharmaceutical costs for the chronic neuropathic pain trials and the ADHD trials increased following the trial due to many patients being on low-cost pharmaceuticals before the trial, and those showing a response to the higher cost pharmaceutical in the trials continued with the higher cost pharmaceutical after the trial. In the absence of an N-of-1 trial, these patients would have commenced higher cost pharmaceuticals with an uncertain response, and incurred higher costs than our estimates.
The costs reported above do not include the costs of running the trial. We estimate the marginal costs were approximately $600 per participant in each trial. The marginal cost includes recruitment, preparation and dispatch of pharmaceutical packs, data collection and analysis, generation of results, and feedback of results and follow-up for 12 months. In practice, active recruitment, 3, 6 and 12-month follow-up is not required outside of a research agenda; therefore, the marginal costs will be limited to dispatch and retrieval of medications, data entry and report generation with a marginal cost of approximately $100. However, there are substantial fixed costs including design of protocols, questionnaires, database and pharmaceutical packs that are independent of the number of participants. The total fixed cost for the three N-of-1 trials in the research setting was close to $70,000 ($350 per participant) but estimate these to be close to $9000 per trial in practice as greater efficiencies and economies of scale develop. Overall, the resources required for N-of-1 trials are relatively few to reduce therapeutic uncertainty.20
N-of-1 trials are most applicable when the use of more expensive agents are restricted to those patients who have tried and failed less expensive agents in the same therapeutic class. Treatment algorithms imposed by the funder (in Australia, the PBS) might specify that an N-of-1 trial must be undertaken to prove effectiveness in that patient; only when evidence of response is determined may the patient gain subsidized access to high cost therapy. Since all patients in this study had an uncertain response to treatment before the N-of-1 trial, the trial allowed identification of responders and non-responders. These N-of-1 trials indicate that many of these patients have no incremental benefit from the more expensive agent, and the cost-savings from identifying responders and non-responders can in some cases justify the cost of undertaking the N-of-1 trial. Furthermore, the potential to identify non-responders and avoid unnecessary exposure to both the risks and costs of ineffective treatment should not be underestimated. Some centers routinely use N-of-1 trials in clinical practice, for example some cystic fibrosis centers have developed N-of-1 trials of rhDNase.21
There are several limitations of these three studies. In this analysis, outcomes were dichotomized as responder or non-responder. This was a pragmatic approach given the relatively small numbers in the trials. However, some patients had a partial response where they may have reported a positive response in one or two of the three cycles. Therefore, prespecified thresholds for determining response should be developed prior to any N-of-1 trial. In this study, our responder threshold was a positive response in all three cycles. In practice, a clinician must make a binary decision about whether the patient should continue with the pharmaceutical or not. Therefore, if N-of-1 trials are to be used in practice, the definition of a responder should be developed that is specific to the pharmaceutical. In addition, the costs of the trials
It is possible that reductions in costs from the pre-trial to post-trial periods were exaggerated by the ‘regression to the mean’ phenomenon where people may be motivated to join trials when their problem is at its worst, making spontaneous improvement more likely.22 In the absence of a control group, we are not able to estimate the effect of this phenomenon, but the same phenomenon may be a factor with patients seeking care outside of trial settings.
The rate of loss to follow-up is acceptable,17,23 although it may be considered the greatest limitation of the study. The follow-up enabled persistence rates at 12 months with the treatment decision from the trial to be evaluated; these were 70% (ADHD trial), 45% (celecoxib trial) and 32% (neuropathic pain trial). Although not conclusive because of the lack of a control group, our results point towards a potential improvement in persistence after completing an N-of-1 trial, at least for ADHD. This finding is worth further study considering the difficulties with pharmaceutical-taking in many chronic conditions. Moreover, N-of-1 studies report compliance with the trial protocol for the period of the trial only without evaluating persistence with the decision following the trial. Therefore, our three studies provide some groundwork for future comparisons of persistence.
N-of-1 trials such as those reported here, represent the highest standards of establishing therapeutic benefits and harms in an individual.24,25 These trials not only account for patient perceptions and values, but patients are more likely to become compliant and adhere better to their treatment regimen, gain greater understanding of their disease and their treatment regimen, and improve the relationship with their healthcare professional.26
CONCLUSION
This study shows that using N-of-1 trials to identify optimal treatment led to patients and their doctors tending to persist with that treatment. Implementation of a large scale N-of-1 trials program would need to balance the perceived clinical benefits against the net costs of implementation, but may be worthwhile when there is uncertainty about individual response to high cost pharmaceuticals.
Acknowledgments
Thanks to, Jonathan Chan, and Fraser Mackenzie from Princess Alexandra Hospital for their help in recruiting participants; Jo Sturtevant from Princess Alexandra Hospital and Nancy Fish from Port Kembla Hospital for estimates of costs and assistance with trials; staff from the Mater Children’s Hospital, Brisbane for their help in implementing ADHD trials in the Mater Hospital; Eric Lee for technical advice and preparation of pharmaceuticals for the ADHD trial; the N-of-1 trial service staff for collecting the data; Virginia Priest for reviewing a previous draft of this manuscript and making valuable comments; and the doctors and participants who took part in the trials.
We did not receive any specific funding to write this article.
The Australian Health Ministers Advisory Council (http://www.ahmac.gov.au) provided funding but had no involvement in any facet of the research. For the celecoxib/acetominophen n-of-1 trial, GlaxoSmithKline Consumer Healthcare (http://www.gsk.com.au) supplied medication, placebos, some funding and invaluable support; participated in initial planning discussions; and viewed the manuscript prior to publication, but had no role in the conduct, collection, management, analysis or interpretation of data; preparation or approval of the manuscript or the decision to submit the final manuscript for publication. The Princess Alexandra Hospital (http://www.health.qld.gov.au/pahospital/) and the Port Kembla Hospital (http://www.sesiahs.health.nsw.gov.au/Hospitals/Port_Kembla_Hospital) contributed funding for the gabapentin N-of-1 trial. Apart from the investigators based at these hospitals, no other personnel had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Paul Scuffham had full access to the economic data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest statement GlaxoSmithKline Consumer Healthcare provided funding that supported the salaries of Jane Nikles and Norma McNairn on the project. Michael Yelland has been paid by GlaxoSmithKline to present the results of aspects of this research at two conferences.
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
- BCa
bias-corrected and accelerated
- GP
general practitioner i.e. family physician
- IQR
inter-quartile range
- OA
osteoarthritis
- PBS
pharmaceutical benefits scheme
- RCT
randomized controlled trial
- SD
standard deviation
- SR
slow-release
- 95% CI
95% confidence interval
References
- 1.Centre for Disease Control and Prevention. The Burden of Chronic Diseases and their Risk Factors: National and State Perspectives 2004. 2004; www.cdc.gov/NCCDPHP/burdenbook2004/. Accessed March 8, 2010.
- 2.Chodick G, Heymann A, Wood F, Kokia E. The direct medical costs of diabetes in Isreal. Eur J Health Econ. 2005;6(2):166–171. doi: 10.1007/s10198-004-0269-7. [DOI] [PubMed] [Google Scholar]
- 3.Guyatt G, Keller L, Jaeschke R, Rosenbloom D, Adachi J, Newhouse M. The N-of-1 randomized controlled trial: clinical usefulness. Our three-year experience. Ann Intern Med. 1990;112(4):293–299. doi: 10.7326/0003-4819-112-4-293. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt G, Sackett D, Taylor D. Determining optimal therapy. Randomized trials in individual patients. N Engl J Med. 1986;314(14):889–892. doi: 10.1056/NEJM198604033141406. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz R, Duan N, Niedzinski E, Hay M, Subramanian S, Weisner T. What ever happened to N-of-1 trials? Insiders’ perspectives and a look to the future. Milbank Q. 2008;86(4):533–555. doi: 10.1111/j.1468-0009.2008.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucker D, Schmid C, McIntosh M, D’Agostino R, Selker H, Lau J. Combining single patient (N-of-1) trials to estimate population treatment effects and to evaluate individual patient responses to treatment. J Clin Epidemiol. 1997;50(4):401–410. doi: 10.1016/S0895-4356(96)00429-5. [DOI] [PubMed] [Google Scholar]
- 7.Scuffham P, Yelland M, Nikles C, Pietrzak E, Wilkinson D. Are N-of-1 trials an economically viable option to improve access to selected high cost medications? The Australian experience. Value Health. 2008;11(1):97–109. doi: 10.1111/j.1524-4733.2007.00218.x. [DOI] [PubMed] [Google Scholar]
- 8.Nikles C, Mitchell G, Mar C, McNairn N, Clavarino Z. Long-term changes in management following n-of-1 trials of stimulants in attention-deficit/hyperactivity disorder. Eur J Clin Pharmacol. 2007;63(11):985–989. doi: 10.1007/s00228-007-0361-x. [DOI] [PubMed] [Google Scholar]
- 9.Yelland M, Poulos C, Pillans P, et al. N-of-1 randomized trials to assess the efficacy of gabapentin for chronic neuropathic pain. Pain Med. 2009;10(4):754–761. doi: 10.1111/j.1526-4637.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 10.Yelland M, Nikles C, McNairn N, Mar C, Schluter P, Brown R. Celecoxib compared with sustained-release paracetamol for osteoarthritis: a series of n-of-1 trials. Rheumatology. 2007;46:135–140. doi: 10.1093/rheumatology/kel195. [DOI] [PubMed] [Google Scholar]
- 11.Cramer J, Rosenheck R, Kirk G, Krol W, Krystal J, VA Naltrexone Study Group 425 Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health. 2003;6:566–573. doi: 10.1046/j.1524-4733.2003.65269.x. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health and Ageing. Medicare Benefits Schedule. 2006; http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/MBSOnline-2006. Accessed March 9, 2010.
- 13.Schedule of Pharmaceutical Benefits for Approved Paharmacists and Medical Practitioners. Canberra: Commonwealth of Australia; 2006. [Google Scholar]
- 14.Efron B, Tibshirani R. The Bootstrap Method for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):1–35. [Google Scholar]
- 15.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman Hall; 1993. [Google Scholar]
- 16.Nikles C, Yelland M, Glasziou P, Mar C. Do individualized medication effectiveness tests (N-of-1 trials) change joint patient-doctor decisions about which drug to use for osteoarthritis and chronic pain? Am J Ther. 2005;12:92–97. doi: 10.1097/00045391-200501000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Nikles C, Clavarino Z, Mar C. Using N-of-1 trials as a clinical tool to improve prescribing. Br J Gen Pract. 2005;55(512):175–180. [PMC free article] [PubMed] [Google Scholar]
- 18.Huser M, Evans T, Berger V. Medication adherence trends with statins. Adv Ther. 2005;22:163–171. doi: 10.1007/BF02849887. [DOI] [PubMed] [Google Scholar]
- 19.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 20.Tsapas A, Matthews D. Using N-of-1 trials in evidence-based clinical practice. JAMA. 2009;301(10):1022–1023. doi: 10.1001/jama.2009.276. [DOI] [PubMed] [Google Scholar]
- 21.Suri R. The use of human deoxyribonuclease (rhDNase) in the management of cystic fibrosis. BioDrugs. 2005;19(3):135–144. doi: 10.2165/00063030-200519030-00001. [DOI] [PubMed] [Google Scholar]
- 22.Pocock S. Current issues in the design and interpretation of clinical trials. Br Med J. 1985;290:39–42. doi: 10.1136/bmj.290.6461.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheather-Reid R, Cohen M. Efficacy of analgesics in chronic pain: A series of N-of-one studies. J Pain Symptom Manage. 1998;15:244–252. doi: 10.1016/S0885-3924(98)00369-8. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt G. Clinical care and clinical research: A false dichotomy. In: Daly J, editor. Ethical Intersections: Health Research, Methods and Research Responsibility. Sydney: Allen & Unwin; 1996. pp. 66–73. [Google Scholar]
- 25.Montori V, Guyatt G. Using N-of-1 trials in evidence-based clinical practice-Reply. JAMA. 2009;301(10):1023. doi: 10.1001/jama.2009.277. [DOI] [PubMed] [Google Scholar]
- 26.Tsapas A, Matthews D. N of 1 trials in diabetes: Making individual therapeutic decisions. Diabetologia. 2008;51(6):921–923. doi: 10.1007/s00125-008-0983-2. [DOI] [PubMed] [Google Scholar]
- 27.Nikles C, Mitchell G, Mar C, Clavarino Z, McNairn N. An N-of-1 trial service in clinical practice: Testing the effectiveness of stimulants for attention deficit hyperactivity disorder. Pediatrics. 2006;117(6):2040–2046. doi: 10.1542/peds.2005-1328. [DOI] [PubMed] [Google Scholar]