Abstract
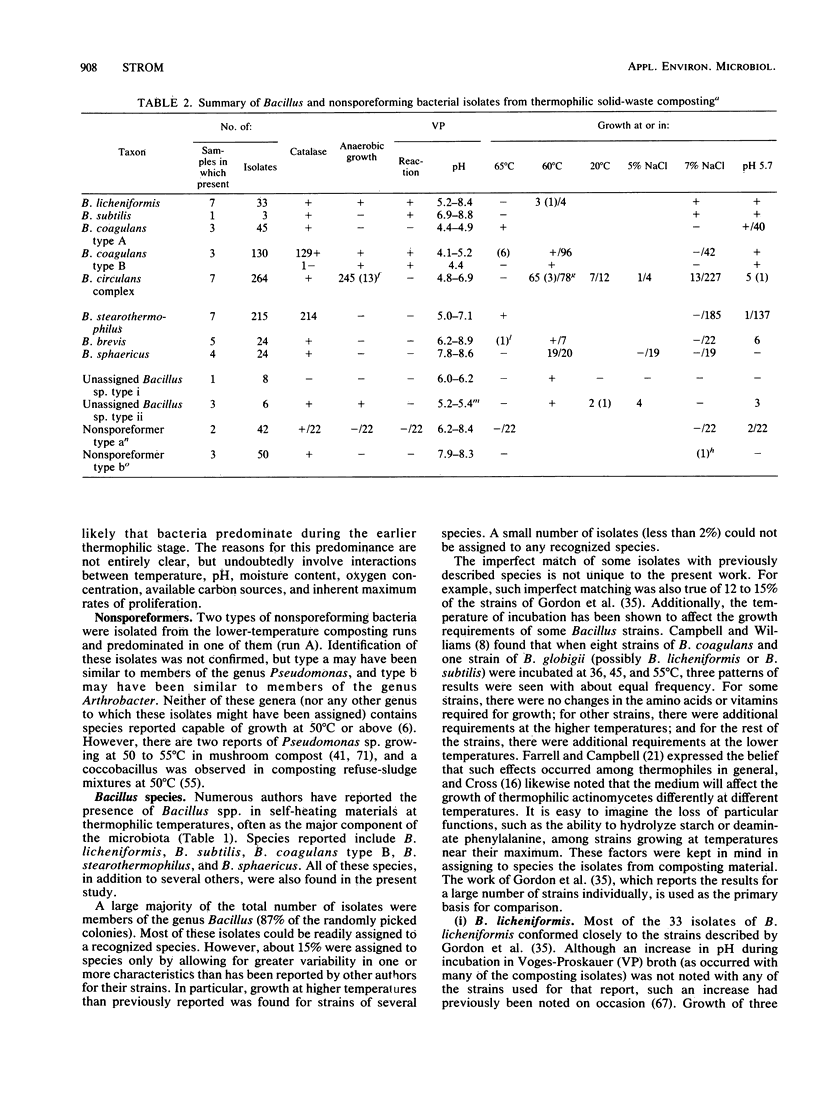
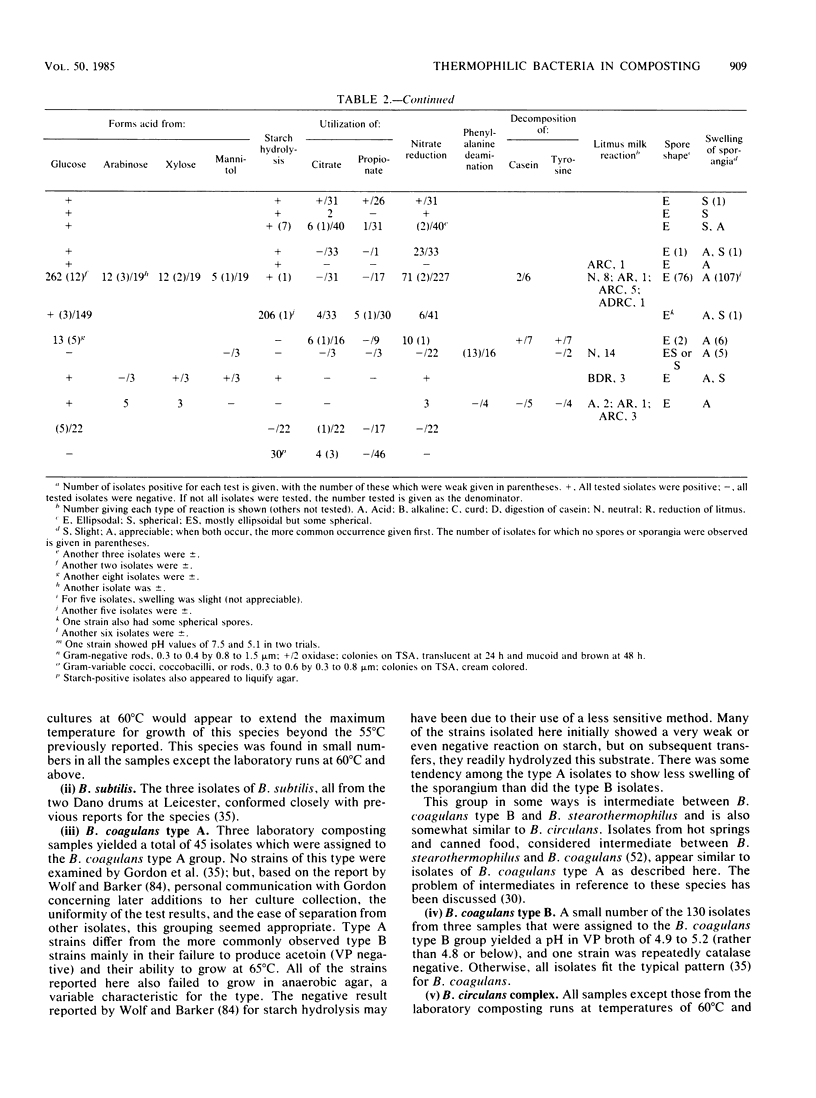
The thermophilic microbiota of solid-waste composting, with major emphasis on Bacillus spp., was examined with Trypticase soy broth (BBL Microbiology Systems) with 2% agar as the initial plating medium. Five 4.5-liter laboratory units at 49 to 69 degrees C were fed a mixture of dried table scraps and shredded newspaper. The composting plants treating refuse at Altoona, Pa., and refuse-sludge at Leicester, England, were also sampled. Of 652 randomly picked colonies, 87% were identified as Bacillus spp. Other isolates included two genera of unidentified nonsporeforming bacteria (one of gram-negative small rods and the other of gram-variable coccobacilli), the actinomycetes Streptomyces spp. and Thermoactinomyces sp., and the fungus Aspergillus fumigatus. Among the Bacillus isolates, the following, in order of decreasing frequency, were observed: B. circulans complex, B. stearothermophilus, B. coagulans types A and B, B. licheniformis, B. brevis, B. sphaericus, Bacillus spp. types i and ii, and B. subtilis. About 15% of the Bacillus isolates could be assigned to species only by allowing for greater variability in one or more characteristics than has been reported by other authors for their strains. In particular, growth at higher temperatures than previously reported was found for strains of several species. A small number of Bacillus isolates (less than 2%) could not be assigned to any recognized species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. The thermophilic aerobic sporeforming bacteria. Bacteriol Rev. 1953 Jun;17(2):125–173. doi: 10.1128/br.17.2.125-173.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY D. E., FRANKLIN J. G. Electron microscope survey of the surface configuration of spores of the genus Bacillus. J Bacteriol. 1958 Dec;76(6):618–630. doi: 10.1128/jb.76.6.618-630.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blyth W. Farmer's lung disease. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:261–276. [PubMed] [Google Scholar]
- CAMPBELL L. L., Jr, WILLIAMS O. B. The effect of temperature on the nutritional requirements of facultative and obligate thermophilic bacteria. J Bacteriol. 1953 Feb;65(2):141–145. doi: 10.1128/jb.65.2.141-145.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlyle R. E., Norman A. G. Microbial Thermogenesis in the Decomposition of Plant Materials: Part II. Factors Involved. J Bacteriol. 1941 Jun;41(6):699–724. doi: 10.1128/jb.41.6.699-724.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N. Transduction in Proteus morganii. Nature. 1966 Apr 9;210(5032):220–220. doi: 10.1038/210220a0. [DOI] [PubMed] [Google Scholar]
- Cross T., Goodfellow M. Taxonomy and classification of the actinomycetes. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:11–112. [PubMed] [Google Scholar]
- Cross T. Thermophilic actinomycetes. J Appl Bacteriol. 1968 Mar;31(1):36–53. doi: 10.1111/j.1365-2672.1968.tb00339.x. [DOI] [PubMed] [Google Scholar]
- ERIKSON D. Temperature/growth relationships of a thermophilic actinomycete, Micromonospora vulgaris. J Gen Microbiol. 1952 May;6(3-4):286–294. doi: 10.1099/00221287-6-3-4-286. [DOI] [PubMed] [Google Scholar]
- Festenstein G. N., Lacey J., Skinner F. A., Jenkins P. A., Pepys J. Self-heating of hay and grain in Dewar flasks and the development of farmer's lung antigens. J Gen Microbiol. 1965 Dec;41(3):389–407. doi: 10.1099/00221287-41-3-389. [DOI] [PubMed] [Google Scholar]
- Finstein M. S., Morris M. L. Microbiology of municipal solid waste composting. Adv Appl Microbiol. 1975;19:113–151. doi: 10.1016/s0065-2164(08)70427-1. [DOI] [PubMed] [Google Scholar]
- Gaughran E. R. THE THERMOPHILIC MICROORGANISMS. Bacteriol Rev. 1947 Sep;11(3):189–225. doi: 10.1128/br.11.3.189-225.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. E., Smith N. R. AEROBIC SPOREFORMING BACTERIA CAPABLE OF GROWTH AT HIGH TEMPERATURES. J Bacteriol. 1949 Sep;58(3):327–341. doi: 10.1128/jb.58.3.327-341.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSSEN A. Uber die Bedeutung der thermophilen Mikroorganismen für die Zersetzung des Stallmistes. Arch Mikrobiol. 1957;27(1):63–81. [PubMed] [Google Scholar]
- Kane B. E., Mullins J. T. Thermophilic fungi in a municipal waste compost system. Mycologia. 1973 Sep-Oct;65(5):1087–1100. [PubMed] [Google Scholar]
- Lacey J. Actinomycetes in soils, composts and fodders. Soc Appl Bacteriol Symp Ser. 1973 Jan;2:231–251. [PubMed] [Google Scholar]
- MARSH C. L., LARSEN D. H. Characterization of some thermophilic bacteria from the Hot Springs of Yellowstone National Park. J Bacteriol. 1953 Feb;65(2):193–197. doi: 10.1128/jb.65.2.193-197.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner P. D., Marsh P. B., Snowden R. B., Parr J. F. Occurrence of Aspergillus fumigatus during composting of sewage sludge. Appl Environ Microbiol. 1977 Dec;34(6):765–772. doi: 10.1128/aem.34.6.765-772.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIESE G. [Microbiological studies on the problem of selfheating of organic substances]. Arch Mikrobiol. 1959;34:285–318. [PubMed] [Google Scholar]
- PROOM H., KNIGHT B. C. The minimal nutritional requirements of some species in the genus Bacillus. J Gen Microbiol. 1955 Dec;13(3):474–480. doi: 10.1099/00221287-13-3-474. [DOI] [PubMed] [Google Scholar]
- ROTHBAUM H. P. Heat output of thermophiles occurring on wool. J Bacteriol. 1961 Feb;81:165–171. doi: 10.1128/jb.81.2.165-171.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIE E. H., SOBOTKA H., BAKER H. Factor converting mesophilic into thermophilic micro-organisms. Nature. 1961 Oct 7;192:86–87. doi: 10.1038/192086a0. [DOI] [PubMed] [Google Scholar]
- Strom P. F. Effect of temperature on bacterial species diversity in thermophilic solid-waste composting. Appl Environ Microbiol. 1985 Oct;50(4):899–905. doi: 10.1128/aem.50.4.899-905.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J. Cellulase production by Thermomonospora curvata isolated from municipal solid waste compost. Appl Microbiol. 1971 Aug;22(2):147–152. doi: 10.1128/am.22.2.147-152.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzenberger F. J., Kaufman A. J., Lossin R. D. Cellulolytic activity in municipal solid waste composting. Can J Microbiol. 1970 Jul;16(7):553–560. doi: 10.1139/m70-093. [DOI] [PubMed] [Google Scholar]
- TENDLER M. D., BURKHOLDER P. R. Studies on the thermophilic actinomycetes. I. Methods of cultivation. Appl Microbiol. 1961 Sep;9:394–399. doi: 10.1128/am.9.5.394-399.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von KLOPOTEK [On the occurrence and behavior of mold fungi in composts of urban w astes]. Antonie Van Leeuwenhoek. 1962;28:141–160. doi: 10.1007/BF02538731. [DOI] [PubMed] [Google Scholar]


