Abstract
Chromoblastomycosis is a chronic fungal disease of the skin and subcutaneous tissues caused by a group of dematiaceous (black) fungi. The most common etiologic agents are Fonsecaea pedrosoi and Cladophialophora carrionii, both of which can be isolated from plant debris. The infection usually follows traumatic inoculation by a penetrating thorn or splinter wound. Several months after the injury, painless papules or nodules appear on the affected area; these papules then progress to scaly and verrucose plaques. We report a case of chromoblastomycosis caused by Phialophora richardsiae, which has been rarely associated with chromoblastomycosis. The case involved a 43-year-old male, who for the past 2 months had noted an erythematous, pustulous plaque that was somewhat dark brown in color on his right shin; the plaque also had intermittent purulent discharge and crust formation. On histopathological examination, chronic granulomatous inflammation and sclerotic cells were seen. The tissue fungus culture grew out the typical black fungi of P. richardsiae, which was confirmed by polymerase chain reaction. The patient has been treated with a combination of terbinafine and itraconazole for 3 months with a good clinical response.
Keywords: Chromoblastomycosis, Phialophora richardsiae
INTRODUCTION
Chromoblastomycosis, also called chromomycosis, is a subcutaneous chronic mycosis caused by a group of dematiaceous (black) fungi1,2. It occurs worldwide, but is most commonly seen in tropical and subtropical regions2-4. The skin lesions are polymorphic and must be differentiated from those associated with other clinical conditions5-7. Chromoblastomycosis has been noted and thus well documented in patients who are immunosuppressed after renal transplantation or who are otherwise on corticosteroid therapy5,7. An increased correlation of chromoblastomycosis with malignant diseases has also been noted6. Five causative fungi have been identified: Fonsecaea pedrosoi is the most prevalent, and Phialophora verrucosa, Cladosporium carrionii, Fonsecaea compacta, and Rhinocladiella aquaspersa also occur in descending order of frequency8-10. Less frequently, chromoblastomycosis is caused by Cladophialophora arxii, Exophiala spinifera, Exophiala dermatitidis, Exophiala jeanselmei and Wangiella dermatitidis6,7.
Phialophora richardsiae is one of the many dematiaceous fungi that can be isolated from plant material and soil9,11,12. It is a common contaminant of wood and is an industrial nuisance, causing bluing of wood pulp and paper13,14. P. richardsiae and other members of the genus Phialophora can cause disease in humans, most often manifested as an infectious subcutaneous granuloma or abscess15,16. Infection usually occurs on exposed body parts through traumatic skin inoculation with contaminated vegetable matter13-16.
Chromoblastomycosis is notoriously difficult to treat, with no one form of treatment being uniformly successful. Small lesions can be removed with wide and deep excision. Cryotherapy, topical heat therapy, systemic medications, and a combination of the above have been reported to be effective.
We report a 43-year-old man with chromoblastomycosis caused by P. richardsiae who was successfully treated with a combination of terbinafine and itraconazole.
CASE REPORT
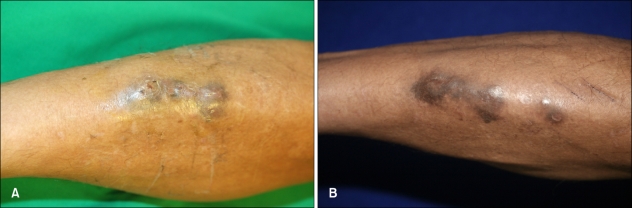
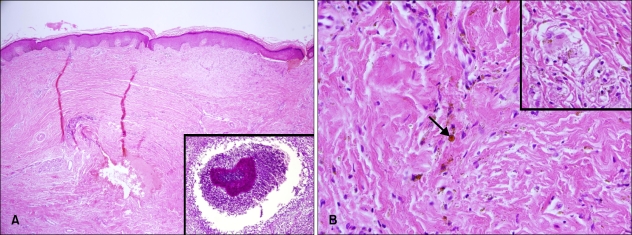
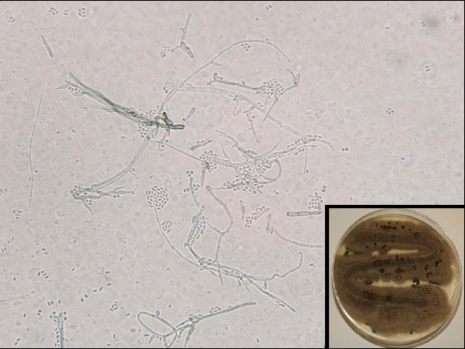
A 43-year-old male construction worker presented with a 2-month history of a slowly enlarging lesion on his right shin. The lesion had the appearance of an erythematosquamous plaque, with a slightly verrucous appearance and irregular shape. The patient's symptoms included mild itching. The lesion first appeared 2 months ago in the form of a papule that gradually extended to its current shape and had purulent drainage. Based on the clinical findings, the presumptive diagnoses of sporotrichosis and an atypical mycobacterial infection were made. On examination, the right shin showed a dark brownish, hyperkeratotic, erythematous, fluctuating plaque with focal purulent discharge, crust formation, and several conglomerated firm nodules (Fig. 1A). The patient's general condition was otherwise good. A skin biopsy was obtained from the verrucous plaque, and tissue fungus and open pus cultures were established. The histopathologic findings revealed hyperkeratosis, pseudoepitheliomatous hyperplasia, and a mixed granulomatous inflammatory cell infiltrate consisting of lymphocytes, histiocytes and multinucleated giant cells with sclerotic bodies in the dermis (Fig. 2). There was a focal micro-abscess in the deep dermis and periodic acid-Schiff (PAS) staining revealed a fungal colony in the micro-abscess (Fig. 2A). Tissue culture on Sabouraud's dextrose agar at room temperature showed growth of heaped and fuzzy colonies with a red-to-dark brown surface (Fig. 3). Staining with lactophenol-cotton blue after slide culture on corn meal agar showed a cluster of conidia accumulating at the apex of the phialides with flared collarettes, giving the appearance of a vase of flowers (Fig. 3). The significant characteristics were the distinct septate hyphae and many flask-shaped, polymorphic phialides with large, flaring collarettes producing 2 types of conidia (regularly-sized globose or irregularly-sized cylindrical phialoconidia). The 2 types of conidia were either separate or present in the same ball. Based on these morphologic features, the fungus was identified as P. richardsiae, which was confirmed by polymerase chain reaction (PCR). Ribosomal DNA internal transcribed spacer (ITS) domains were amplified using the following primers: ITS-1 (TCC GTA GGT GAA CCT GCG G) and ITS-4 (TCC TCC GCT TAT TGA TAT GC). PCR for M. tuberculosis and other atypical mycobacteria using DNA from tissue specimens were negative.
Fig. 1.
(A) Hyperkeratotic, erythematous black pustular plaque with crust formation on the right shin. (B) The skin lesion gradually improved after 3 months of combination treatment with itraconazole and terbinafine.
Fig. 2.
(A) Skin biopsy specimen showing hyperkeratosis, pseudoepitheliomatous hyperplasia, and a mixed dermal inflammatory cell infiltrate consisting of lymphocytes, and histiocytes (H&E, ×40). Inset: Periodic acid-Schiff stain reveals a fungal colony in a micro-abscess (×100). (B) Mixed inflammatory cell infiltrate with large, dark-brown, sclerotic bodies (arrow) in the dermis (H&E, ×400). Inset: Sclerotic bodies are found within giant cells (H&E, ×400).
Fig. 3.
Slide culture and lactophenol-cotton blue stain showed a cluster of conidia accumulating at the apex of the phialides with flared collarettes, giving the appearance of a vase of flowers. Inset: Culture on Sabouraud's dextrose agar at room temperature showed growth of heaped and fuzzy dark brown colored colonies.
Under the diagnosis of chromoblastomycosis caused by P. richardsiae, oral itraconazole (200 mg/day) was administered. Three months after monotherapy, the lesion showed a partial response but continued to have purulent discharge, so terbinafine (500 mg/day) was added. Three months after initiating combination treatment, the patient had a good clinical response (Fig. 1B).
DISCUSSION
Chromoblastomycosis is a dermal mycosis caused by a group of dematiaceous (black) fungi and characterized by the presence of verrucous-nodular lesions that are usually located on one of the limbs1,6.
The infection usually occurs through traumatic skin inoculation, with the majority of lesions occurring on the feet and legs of outdoor workers7,10. The lack of a history of trauma or inoculation injury in most patients may be due to a long latency period from the time of injury to the development of the lesion9,12. Under these circumstances, many patients may not recall a previous injury, especially if the injury was minor6-9. Our patient denied a history of trauma to the area. The only pertinent history was that he had worked on a construction site for several years.
The initial lesion of chromoblastomycosis is usually solitary and unilateral, presenting as a small, pink, smooth-surfaced, papular skin lesion4,6. The papules gradually increase over a few weeks and may have a scaly surface4,5. With time, the initial lesion may evolve into several types of skin lesions, leading to a polymorphic clinical appearance (nodular, tumorous, plaque, cicatricial and verrucous types)7,8.
Histologically, the tissue response in chromoblastomycosis is not specific and may be similar to other deep mycoses6,8. Hyperkeratosis and pseudoepitheliomatous hyperplasia are the main features within the stratum corneum and epidermis3,8. Several micro-abscesses, with or without pigmented fungal elements, may be present in the epidermis3,5. The tissue response to the fungi is typically mixed, consisting of micro-abscesses and a granulomatous response with giant cells11,12. The hallmark of the disease is the presence of muriform cells embedded in the granulomas, as well as a suppurative tissue reaction3,6. Muriform cells have been referred to as sclerotic cells, or as fumagoid, chromo, or Medlar bodies3,6,8. They are likened to "copper pennies" that may lie either singly or in chains or clusters3,4. The histopathologic specimen of our patient showed a mixed granulomatous inflammatory cell infiltrate and sclerotic cells with multinucleated giant cells.
On the mycologic examination, 3 morphologic patterns of sporulation or conidiation exist, which are referred to as Phialophora-like, Cladosporium-like, and Rhinocladiella-like6,9,11. The Phialophora type of sporulation gives rise to a flask-shaped phialide, which occurs either at the end or on the side of the hyphae10,11. The conidia are formed and accumulate at the flask's distal end and resemble 'flowers in a vase'9-11. Phialophora richardsiae in our case showed only Phialophora-like sporulation.
Reports on P. richardsiae occurring in humans are rather rare14,15. There are about 25 species in the genus Phialophora but only 5 species, i.e., P. verrucosa, P. richardsiae, P. repens, P. parasitica and P. cyanescens, that cause human infection11,12. These are classified according to the growth rate and color of the colonies, the shape of the phialide and collarette, and the color and shape of the conidia10-12. P. richardsiae most closely resembles P. verrucosa, but these species can be differentiated by careful microscopic morphologic examination10,14. The phialides of P. verrucosa are generally short and distinctly urn-shaped, with a broad base and a cup-like collarette10,12,16. In contrast, P. richardsiae has longer, more slender and tapering phialides that, when mature, terminate in a flat, saucer-shaped collarette14,16.
The differential diagnosis of chromoblastomycosis is broad and includes many infectious and non-infectious possibilities. There are 3 kinds of diseases caused by black fungi (chromoblastomycosis, phaeohyphomycosis, and mycetoma)6,7,10. Chromoblastomycosis and phaeohyphomycosis represent 2 poles of a spectrum of diseases caused by melanized fungi10,12. Clinically, the boundaries of this spectrum are unclear, and both infections may be caused by the same etiologic agents9-11. Although either immunocompetent and immunosuppressed hosts may present with both types of infections, chromoblastomycosis is usually observed in immunocompetent hosts, while phaeohyphomycosis is not9,10,12. The host immune status may play an important role in the clinical evolution of these diseases.
The present case of a P. richardsiae infection was identified as a phaeohyphomycosis rather than a chromoblastomycosis, because the infection remained localized and subepidermal14,16. Phaeohyphomycosis does not have sclerotic bodies, which represent a vegetative form of the fungus10,11 Furthermore, hyphal elements, rather than muriform and yeast cells, were found in the affected tissues14-16. Thus, our case is unique because the patient's chromoblastomycosis lesion was caused by a rarely isolated dematiaceous fungus, P. richardsiae. Invasive disease is unusual and occurs only in patients who are severely immunocompromised13,15. Conditions associated with deficient cell-mediated immunity appear to increase the risk of acquiring infection with P. richardsiae, but a normal host may also become infected as in our patient's case13-16.
Due to the marked chronicity of the lesions and the well-known resistance of many cases to simple therapies, it is difficult to treat chromoblastomycosis17,18. The preferred treatment is usually surgical excision of small and localized lesions with wide surgical margins to prevent local recurrence. For deep or extensive cutaneous involvement however, surgical treatment is often not feasible6,18. Prolonged treatment with systemic antifungal agents alone or in combination for deep or extensive lesions provides the best chance of a cure, but antifungal therapy for Phialophora infections has been generally disappointing8,9,12. It is important to know the clinical type of chromoblastomycosis in order to offer a more accurate prognosis for the patient presenting with this difficult-to-treat disease9,19. Despite many reports showing different clinical types with diverse cure rates, there is no study showing a correlation between a specific clinical type and the cure of chromoblastomycosis17-19.
Traditional therapy includes continuous dosing with oral itraconazole or terbinafine, and there have been reports that itraconazole is a favorable drug for treatment of chromoblastomycosis19,20. However, in some cases, monotherapy has been ineffective or is associated with a less than acceptable response; there have also been reports on the development of resistance to itraconazole17,18. To achieve a good response rapidly and prevent microbiologic resistance, combination therapy with itraconazole and terbinafine may be effective during the early stages of treatment because of the synergistic effect6,17,18. In our case, itraconazole monotherapy did not result in a clinical response; however, after administration of terbinafine in combination with itraconazole, the skin lesion was markedly improved.
The lesions of chromoblastomycosis usually respond to surgical excision, and surgery is currently considered the treatment of choice17,20. Our patient had a low income and made his living by working on a construction site, so he declined surgical excision. The clinical presentation, risk factors, and response to therapy of infections due to P. richardsiae are similar to those of other agents against chromoblastomycosis9,16,18. Further observations, especially in the area of antifungal susceptibility testing, are needed to determine whether a specific etiologic diagnosis is important in the management of these infections.
References
- 1.Hay RJ. Deep fungal infections. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's dermatology in general medicine. 7th ed. New York: McGraw-Hill; 2008. pp. 1833–1834. [Google Scholar]
- 2.James WD, Berger TG, Elston DM. Andrews' diseases of the skin: clinical dermatology. 10th ed. Philadelphia: Saunders Elsevier; 2006. pp. 323–324. [Google Scholar]
- 3.Elder DE, Elenitsas R, Johnson BL, Jr, Murphy GF, Xu X. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott-Raven; 2009. pp. 607–608. [Google Scholar]
- 4.Kim HU, Son GY, Ihm CW. A case of chromoblastomycosis showing a good response to itraconazole. Ann Dermatol. 1997;9:51–54. [Google Scholar]
- 5.Suh MK, Sung YO, Yoon KS, Ha GY, Kim JR. A case of chromoblastomycosis caused by Fonsecaea pedrosoi. Korean J Dermatol. 1996;34:832–836. [Google Scholar]
- 6.López Martínez R, Méndez Tovar LJ. Chromoblastomycosis. Clin Dermatol. 2007;25:188–194. doi: 10.1016/j.clindermatol.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Minotto R, Bernardi CD, Mallmann LF, Edelweiss MI, Scroferneker ML. Chromoblastomycosis: a review of 100 cases in the state of Rio Grande do Sul, Brazil. J Am Acad Dermatol. 2001;44:585–592. doi: 10.1067/mjd.2001.112220. [DOI] [PubMed] [Google Scholar]
- 8.Salgado CG, da Silva MB, Yamano SS, Salgado UI, Diniz JA, da Silva JP. Cutaneous localized annular chromoblastomycosis. J Cutan Pathol. 2009;36:257–261. doi: 10.1111/j.1600-0560.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 9.Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 2003;15(Suppl 2):36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 10.De Hoog GS, Queiroz-Telles F, Haase G, Fernandez-Zeppenfeldt G, Attili Angelis D, Gerrits Van Den Ende AH, et al. Black fungi: clinical and pathogenic approaches. Med Mycol. 2000;38(Suppl 1):243–250. [PubMed] [Google Scholar]
- 11.Vicente VA, Attili-Angelis D, Pie MR, Queiroz-Telles F, Cruz LM, Najafzadeh MJ, et al. Environmental isolation of black yeast-like fungi involved in human infection. Stud Mycol. 2008;61:137–144. doi: 10.3114/sim.2008.61.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura M, Goto A, Furuta T, Satou T, Hashimoto S, Nishimura K. Multifocal subcutaneous phaeohyphomycosis caused by Phialophora verrucosa. Arch Pathol Lab Med. 2003;127:91–93. doi: 10.5858/2003-127-91-MSPCBP. [DOI] [PubMed] [Google Scholar]
- 13.Pitrak DL, Koneman EW, Estupinan RC, Jackson J. Phialophora richardsiae infection in humans. Rev Infect Dis. 1988;10:1195–1203. doi: 10.1093/clinids/10.6.1195. [DOI] [PubMed] [Google Scholar]
- 14.Ikai K, Tomono H, Watanabe S. Phaeohyphomycosis caused by Phialophora richardsiae. J Am Acad Dermatol. 1988;19:478–481. doi: 10.1016/s0190-9622(88)70200-5. [DOI] [PubMed] [Google Scholar]
- 15.Lieb DF, Smiddy WE, Miller D, Cooperman EW. Case report: Fungal endophthalmitis caused by Phialophora richardsiae. Retina. 2003;23:406–407. doi: 10.1097/00006982-200306000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Guého E, Bonnefoy A, Luboinski J, Petit JC, de Hoog GS. Subcutaneous granuloma caused by Phialophora richardsiae: case report and review of the literature. Mycoses. 1989;32:219–223. doi: 10.1111/j.1439-0507.1989.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 17.Ranawaka RR, Amarasinghe N, Hewage D. Chromoblastomycosis: combined treatment with pulsed itraconazole therapy and liquid nitrogen cryotherapy. Int J Dermatol. 2009;48:397–400. doi: 10.1111/j.1365-4632.2009.03744.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonifaz A, Saúl A, Paredes-Solis V, Araiza J, Fierro-Arias L. Treatment of chromoblastomycosis with terbinafine: experience with four cases. J Dermatolog Treat. 2005;16:47–51. doi: 10.1080/09546630410024538. [DOI] [PubMed] [Google Scholar]
- 19.Xibao Z, Changxing L, Quan L, Yuqing H. Treatment of chromoblastomycosis with terbinafine: a report of four cases. J Dermatolog Treat. 2005;16:121–124. doi: 10.1080/09546630510033203. [DOI] [PubMed] [Google Scholar]
- 20.Kumarasinghe SP, Kumarasinghe MP. Itraconazole pulse therapy in chromoblastomycosis. Eur J Dermatol. 2000;10:220–222. [PubMed] [Google Scholar]