Abstract
Older adults often encounter difficulties in switching between tasks, perhaps because of age-related decreases in executive function. Executive function may largely depend on connections between brain areas—connections that may become structurally and functionally weaker in aging. Here we investigated functional and structural age-related changes in switching between a spatial and a verbal task. These tasks were chosen because they are expected to differentially use the two hemispheres. Brain measures included anatomical information about anterior corpus callosum size (CC; the major commissure linking the left and right hemisphere), and the event-related optical signal (EROS). Behavioral results indicated that older adults had greater task-switching difficulties, which, however, were largely restricted to switching to the spatial task and to individuals with smaller anterior CCs. The EROS data showed both general switching-related activity in the left middle frontal gyrus (with approximately 300-msec latency) and task-specific activity in the inferior frontal gyrus, lateralized to the left for the switch-to-verbal condition and to the right for the switch-to-spatial condition. This lateralization was most evident in younger adults. In older adults, activity in the switch-to-spatial condition was lateralized to the right hemisphere in individuals with large CC, and to the left in individuals with small CC. These data suggest that (a) task switching may involve both task-general and task-specific processes; and (b) white matter changes may underlie some of the age-related problems in switching. These effects are discussed in terms of the hypothesis that aging involves some degree of cortical disconnection, both functional and anatomical.
INTRODUCTION
Switching between tasks is required in many everyday situations, but it becomes progressively more difficult as we age (Kray & Lindenberger, 2000; Kramer, Hahn, & Gopher, 1999). Task switching can be considered a prototypical executive function, that is, a function required when there is a need for controlling attention and redirecting the information processing system (Bunge & Wallis, 2008). This may involve the dynamic activation of a set of processes and the suppression of others (Hasher, Lustig, & Zacks, 2008). Aging is typically accompanied by diminished efficiency of executive functions which, in turn, may be associated with both functional and structural changes in the relevant brain regions (Raz, 2000). Most neuroscientific studies of executive function have shown that the prefrontal cortex plays an important role (for a review, see Miller & Cohen, 2001; see also Bunge & Wallis, 2008). In this article, we investigate the spatio-temporal dynamics of the prefrontal cortex activity during task switching, and their changes with aging. We were also interested in the relationship between age-related changes in the structural connections between cortical areas and their ability to predict both specific patterns of brain activation and individual differences in behavioral performance. Results from this study suggest that some of the task-switching costs found in aging may be restricted to individuals exhibiting a decline in structural connectivity. As these older adults also showed altered functional connectivity, the data suggest that task switching and executive function may rely on the interaction between these two forms of connectivity (Rykhlevskaia, Fabiani, & Gratton, 2008).
The neurophysiological bases of executive function have become the focus of extensive investigation (e.g., Bunge & Wallis, 2008; Miller & Cohen, 2001). The task-switching paradigm has been used extensively in this research because it challenges executive function to a high degree. In this paradigm, subjects are confronted with stimuli that can be classified according to a variety of rules, and the rule to be used on a particular trial is frequently switched. There are, in fact, many versions of this task, in which the switching parameters (at every trial, on predictable occasions, etc.) and/or the number and types of alternative tasks are varied (e.g., Kray & Lindenberger, 2000). Data obtained from normal younger adults show that task switching may be associated with a significant cost, in terms of both response time (RT) and accuracy, even when subjects are given abundant time to prepare for the task (Monsell, 2003). This suggests that a significant proportion of the problems occurring in task switching are due to difficulties in inhibiting a frequently-used (but currently inappropriate) classification rule.
Lesion data have shown clear decrements in task-switching performance in people with damage to pre-frontal regions, in particular, those involving the left middle frontal gyrus (MFG, Aron, Monsell, Sahakian, & Robbins, 2004). The importance of the left MFG in task switching is also supported by brain imaging studies, which show that activity in this area is observed in conditions requiring switching between dimensions (e.g., Agran, Low, Ryklevskaia, Fabiani, & Gratton, submitted; Braver, Reynolds, & Donaldson, 2003; DiGirolamo et al., 2001; Dove, Pollmann, Schubert, Wiggins, & von Cramon, 2000). In addition, under these conditions, activations of other cortical areas have also been frequently observed, such as the inferior parietal lobule as well as superior and medial regions of the frontal cortex (Sohn, Ursu, Anderson, Stenger, & Carter, 2000). These data indicate that a set of brain areas, sometimes labeled the ‘‘fronto-parietal network’’ (FPN; Gilbert & Shallice, 2002; Mesulam, 1990; Posner & Petersen, 1990), may be involved in task switching and executive function in general. As these areas appear to be activated across a variety of tasks and conditions, we will label them ‘‘domain- or task-general’’ areas (e.g., Gratton, Low, & Fabiani, 2008; Gray, Chabris, & Braver, 2003; see also Köhler, Moscovitch, Winocur, Houle, & McIntosh, 1998 for a similar concept in the context of episodic memory).
In contrast, other regions of the brain (labeled domain-or task-specific) appear to be active when particular stimulus or response dimensions are used. For instance, in a memory task, Kelley et al. (1998) observed functional specialization in the middle/inferior frontal gyrus (MFG/IFG), with the left hemisphere more specialized for verbal stimuli, and the right hemisphere more specialized for face recognition (object recognition activated both the left and right hemispheres). It has also been frequently proposed that the left hemisphere may be more specialized for verbal material and the right hemisphere for spatial material (Smith, Jonides, & Koeppe, 1996), and this may be particularly true for inferior frontal regions (Reuter-Lorenz et al., 2000).
Within this framework, one of the roles of executive function during task switching is to coordinate task-general and task-specific processes. This may occur through a hierarchical, top–down mechanism, in which task-general control areas are activated whenever a switch is needed, leading to the subsequent activation of task-specific areas (so that the appropriate dimension can be used for stimulus classification). Lesion data appear to support this idea, as lesions of the left MFG (and other structures) may lead to a generalized weakening of task-switching abilities across a variety of tasks (Aron et al., 2004). However, even in this case, performance is not typically completely abolished.
Another possibility is that task switching can be accomplished via interactions among task-specific regions, even in the absence of direct top–down influence from task-general structures (see Agran et al., submitted; Gratton et al., 2008). For instance, the occurrence of a particular cue indicating the need for task switching may activate a task-specific region directly which, in turn, may inhibit other task-specific regions and bias the system toward using a particular dimension to process information. This may account for the ability of subjects to achieve some minimum level of task-switching behavior despite having lesions in the MFG and/or of other structures within the FPN. For the purpose of the current article, we will label this the ‘‘distributed hypothesis’’ to indicate the lack of dependence on a top–down control system. Evidence for the existence of this alternative system would be the observation of activity in task-specific areas in the absence or in advance of the activity in task-general areas.
A third possibility is that both systems may coexist, and that subjects may use both distributed and top–down mechanisms for achieving task-switching behavior. This finding may be consistent with the observation that lesions in the left MFG lead to reduced efficiency, but not complete failure of task-switching behavior. An outcome consistent with this logic would be activation of task-specific areas both before and after activation of task-general areas.
Note that the predictions we have made about brain activity during task switching refer not only to the activation of specific regions but also to their relative order of activation. A comparative analysis of these hypotheses requires a technology for studying brain activation that possesses both spatial and temporal resolution. In fact, the technology needs to be able not only to determine whether a particular region (such as the left MFG) is activated before another relatively close but more ventral area (such as the left or right IFG) but also whether the latter is activated only once or twice within a short interval. In the current study, we used the event-related optical signal (EROS), which provides a combination of subcentimeter spatial resolution and millisecond-level temporal resolution (Gratton & Fabiani, 2001, 2007; Gratton, Corballis, Cho, Fabiani, & Hood, 1995). Compared to more traditional measures with high temporal resolution, such as event-related brain potentials (ERPs), EROS possesses a greater spatial resolution and localization, does not require assumptions about the number of active areas, but has a more reduced penetration (it is sensitive only to activity in the most superficial 2 to 3 cm of the cortex) and a smaller signal-to-noise ratio.
In the current experiment, we were interested in evaluating some of the neurophysiological bases of impaired executive function in aging within a task-switching paradigm. Several tasks that are highly loaded on executive function (such as the Wisconsin Card Sorting Test; Milner, 1963) typically show a mild degree of impairment with aging. This effect, however, does not appear to be general but is restricted to a subset of older adults (Fabiani & Friedman, 1995). One possibility is that some older adults may suffer from some degree of structural brain impairment that does not necessarily reach clinical levels. In fact, a number of recent studies using advanced imaging analysis methods (such as voxel-based mor-phometry; Ashburner & Friston, 2000, 2001) have revealed consistent age-related anatomical changes in the brain (Gordon et al., 2008; Davatzikos & Resnick, 2002; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998). Specifically, among the areas showing the largest changes are those within the FPN. In addition, the anterior white matter, and in particular, the anterior portion of the corpus callosum (CC), also show clear volumetric decrements with advanced age (Gordon et al., 2008; Sullivan & Pfefferbaum, 2006; Gunning-Dixon & Raz, 2003; O’Sullivan et al., 2001). The anterior CC is responsible for carrying a large proportion of the fibers connecting the two frontal cortices. It is therefore possible that executive function impairments in aging may be associated with reductions in the size of both FPN cortical structures and the white matter tracts connecting them (for a discussion, see Reuter-Lorenz & Stanczak, 2000). In this article, we directly investigated the second of these possibilities by measuring the size of the anterior CC on anatomical MR images obtained for each subject. However, it is important to note that volumetric changes in the prefrontal cortex may also play a significant role, and that the changes in the CC may, in fact, be secondary to changes in cortical structures.
The role of white matter structures in the decline of executive function in aging underscores the importance of another concept—that executive function involves the coordination of different neurocognitive processes. As these processes may involve different brain regions, it appears quite plausible that a reduction in the efficiency of connections between areas may contribute to the age-related decline in executive function. As we mentioned, lesion and brain imaging studies suggest that the left MFG may be critical for executive function in general, and task switching in particular. We have also considered evidence that verbal tasks may involve preferentially left-hemisphere structures, whereas spatial tasks may involve preferentially right-hemisphere structures. These premises suggest the hypothesis that, for older adults with reduced CC, task switching should be more difficult when it involves switching to spatial tasks (right-dominant) than when it involves switching to verbal tasks (left-dominant), as control information from the left MFG needs to cross the CC in the former but not in the latter case.1 Indeed, there is some evidence that spatial tasks may be more affected by aging than verbal tasks, an observation which led to the formulation of the ‘‘hemiaging’’ hypothesis (Meudell & Greenhalgh, 1987; for a recent discussion, see Daselaar & Cabeza, 2005).
In the current study, we directly investigated several of these issues. We ran both younger and older adults in a spatial Stroop task (e.g., DeSoto, Fabiani, Geary, & Gratton, 2001). In this task, the words ‘‘above’’ or ‘‘below’’ were presented above or below a central fixation cross. A cue presented in advance of the word indicated whether the subject had to respond on the basis of the word meaning (verbal task) or its position (spatial task). In addition to accuracy and RT measures, we recorded EROS from a montage covering most of the frontal cortex. We focused on the brain activity associated with the cue, and in particular, when the cue signaled that a different dimension should be processed. In addition, for each subject, we obtained a structural MR scan. These data were obtained both for coregister-ing the optical data with the corresponding brain anatomy and for deriving anterior CC estimates for each individual (corrected for overall intracranial volume).
METHODS
Subjects
Participants were 16 younger (age = 18–30 years, 8 women) and 16 older adults (age = 65–82 years, 8 women). All signed informed consent, according to procedures established by the Campus IRB of the University of Illinois. Subjects were right-handed, native English speakers, with normal or corrected-to-normal vision, in good physical and mental health, and free from medications known to affect the central nervous system. All participants were administered the following screening tests: (1) the Modified Mini-Mental Status Exam (Mayeux, Stern, Rosen, & Leventhal, 1981) to screen for early signs of dementia; (2) the Beck’s Depression Inventory (Beck, Steer, & Brown, 1996) to screen for depression; and (3) the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised (Wechsler, 1981) to obtain an estimate of the subject’s verbal ability. Only subjects who had a score of 51 or above on the Modified Mini-Mental Status Exam (max: 57), who had a score of 12 or below on the Beck’s Depression Inventory, and who had an average or above score for their age group on the Vocabulary subtest of the Wechsler Adult Intelligence Scale—Revised were included in this study. Table 1 shows that the two groups were closely matched in vocabulary skills and years of education.
Table 1.
Demographic and Neuropsychological Characteristics of the Younger and Older Adult Groups (Standard Deviations in Parentheses)
| Younger (n = 16) |
Older (n = 16) |
|
|---|---|---|
| Measure | Mean (SD) | Mean (SD) |
| Age (years) | 23.11 (3.57) | 76.07 (5.23) |
| Education (years) | 15.94 (2.54) | 15.94 (3.21) |
| Modified MMS* | 56.69 (0.60) | 54.63 (1.50) |
| Vocabulary Scaled Score | 13.19 (2.01) | 12.13 (2.09) |
One-tailed t test, p < .01.
Stimuli and Tasks
Participants came to the Cognitive Neuroimaging Laboratory on three separate occasions. In the first session, they were administered neuropsychological tests after which they underwent practice on a spatial Stroop task in which the words ‘‘above’’ and ‘‘below’’ were presented above and below fixation. EROS data2 were recorded on the second and third sessions. Participants sat in a comfortable chair, one meter away from the computer display, with the response box held in their hands. Two conditions were used: (1) a ‘‘meaning’’ condition, where participants responded based on the meaning of the word, and (2) a ‘‘position’’ condition, where participants responded based on the position of the word. The two conditions were randomly intermixed within each block of trials with equal probability. Participants were instructed to fixate on a cross at the center of the screen. A cue consisting of two letter Ps (denoting position) or Ms (denoting meaning) flanking the fixation cross appeared for 500 msec, indicating which of the two dimensions was to be used to classify the upcoming imperative stimulus. Two seconds after the onset of the cue, the imperative stimulus was presented for 200 msec, approximately 2°above or below the fixation cross. On congruent (i.e., nonconflict) trials, meaning and position matched (e.g., the word ‘‘ABOVE’’ presented above the fixation cross); on incongruent (i.e., conflict) trials, the two dimensions did not match (e.g., the word ‘‘ABOVE’’ presented below the fixation cross). Participants were instructed to respond to the dimension of the imperative stimulus indicated by the cue. They were also instructed to make rapid and accurate judgments by pressing one of two buttons, one representing ‘‘above,’’ and the other ‘‘below.’’ Hand assignment was consistent across conditions for each participant and counterbalanced across participants. They were also trained by verbal feedback to respond at a speed that produced error rates ranging between 0.10 and 0.20, and were given a maximum of 1200 msec to respond. There were 16 conditions based on the combination of hand used to respond (left vs. right), cued stimulus dimension (meaning vs. position), congruency (congruent vs. incongruent), and task switching (switch vs. nonswitch). There were 64 trials for each of the 16 conditions, for a total of 1024 trials per participant.
Structural MR Recording and CC Analysis
A T1 structural MR scan was obtained for each subject in either a 3-T Siemens Allegra MR Scanner (Siemens, Munich, Germany) or a 1.5-T GE Signa Echo Speed MR Scanner (General Electric, Milwaukee, WI). On the Siemens scanner, MP-RAGE images were acquired with the following parameters: phase encoding direction = anterior to posterior; field of view = 240 × 240 × 172.8 mm; voxel size = 1.3 × 0.9 × 1.2 mm; matrix = 192 × 256 × 144; repeat time = 1800 msec; echo time = 4.38 msec; averages = 2; flip angle = 8°; time of inversion = 900 msec; bandwidth = 33.3 kHz. On the GE scanner, 3-D SPGR images were acquired with the following parameters: phase encoding direction = anterior to posterior; field of view = 240 × 240 × 161.2 mm; voxel size = 0.94 × 0.94 × 1.3 mm; matrix = 256 × 256 × 124; repeat time = 30 msec; echo time = 1 msec; averages = 1; flip angle = 45°; bandwidth = 15.63 kHz.
The size of the CC was derived from the sagittal slices of structural MR maps aligned on the anterior–posterior commissure axis. With the aid of FSL software (FMRIB, Oxford, UK, freeware), the cross-sectional area of the anterior third of the CC was automatically estimated. This procedure clearly demarcated the CC, which was then isolated from the rest of the brain. The area of its anterior third was calculated by averaging across five mid-sagittal slices. Further, a total brain volume estimate was obtained for each subject, and the ratio between the square root of the anterior CC area and the cubic root of the brain volume (henceforth referred to as adjusted CC size) was used for all the analyses. Most of the analyses were repeated using absolute anterior CC size measures, producing results that were qualitatively similar to those reported here, but these results will not be included for space reasons. Note that the type of scanner used for the measurement was not correlated with either CC size or adjusted CC size. For some of the analyses, subjects were assigned to small and large CC groups on the basis of a median split applied separately for each age group. Note that the correlation between volume-adjusted anterior CC size and gender was not significant (r = .05), and thus, gender was not included as a factor in any of the analyses.
EROS Recording and Analysis
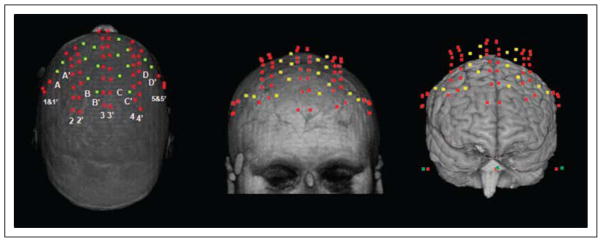
The data reported here are all time-locked to the onset of the cue. Therefore, they explore preparatory activities in the absence of response-related activities. Frequency–domain fast optical data were recorded using an eight-detector Imagent System (ISS, Champaign, IL) concurrently with ERP data. Each detector received light from 10 sources, which were time-multiplexed and arranged such that, during any given time division, only one source was within 6 cm of any given detector to avoid cross talk. The detectors (photomultiplier tubes) and sources (laser diodes emitting 690 nm light; average power × 1 mW) were connected to the subject’s head using optic fibers, which were held in place using a modified motorcycle helmet. Interdigitized montages were used in the two sessions to achieve a total of 160 channels (80 per session). Figure 1 shows an example of the combined montages, coregistered with the structural MR of a representative subject. The yellow and red dots in the figure indicate the locations of the detectors and sources, respectively. The rows marked by numbers and letters in the left panel indicate the montage for a given session (e.g., row 3 and row 3′ were used during different sessions). The order of montages was counterbalanced across subjects. The montages were chosen to jointly cover most of the frontal lobes, thus including the prefrontal regions of interest (ROI) in this study (i.e., superior and middle frontal gyri and the posterior portion of the IFG).
Figure 1.
The digitized locations of sources (red) and detectors (yellow) coregistered with a structural MRI viewed from the axial (left panel, nose at top) and coronal (middle panel) planes, and projected onto the brain (right panel). The numbered and lettered rows in the left panel indicate the montage for a given session (e.g., row B and row 3 would be used for one montage and row B′ and row 3′ would be used for the second montage, with the order of montages counterbalanced across subjects).
The light sources were modulated at 220 MHz, and the PMTs were fed a 220.00625-MHz frequency. This created a 6.25-kHz heterodyning frequency, which was used to compute phase delays (the optical-dependent variable used in the study to measure EROS). Optical data were obtained continuously over each block at a sampling frequency of 62.5 Hz.
Analysis of the optical data included the following steps: (a) phase range correction (to eliminate phase wrapping around the 360°mark); (b) pulse correction (using a procedure described in Gratton & Corballis, 1995); (c) filtering (using a 0–6 Hz bandpass); (d) segmentation into epochs time-locked to the cue presentation; (e) averaging separately for each subject, condition, and channel (only data related to the interval between cue and imperative stimulus are presented here); (f ) coregistration with MR data using three fiducial markers (based on digitization of the recording locations with a Polhemus 3-D digitizer); (g) transformation into Talairach space (Talairach & Tournoux, 1988); and (h) back projection of optical data onto the brain surface. Channels crossing the same voxel were averaged together (see Wolf et al., 2000, for a similar approach). Only channels with phase standard deviation <8°and source–detector distances between 15 and 50 mm were used for the analysis (see Gratton et al., 2006); (i) computation of individual subject averages; (j) computation of statistical maps across subjects, using an ROI approach. The boundaries of the ROI were left: x = −55; right: x = 55; front: y = 40; back: y = 15, which included a broad section of the prefrontal cortex (BA 6, BA 8, BA 9, and BA 46 on both hemispheres). Data were corrected for multiple comparisons based on random field theory (see Kiebel, Poline, Friston, Holmes, & Worsley, 1999). (For additional information about analysis of EROS data, see Gratton & Fabiani, 2007 and Gratton et al., 2006.)
RESULTS
Corpus Callosum Size
Figure 2 reports a scatterplot of the size of the anterior third of the CC (adjusted by overall brain volume) and age. On average, the older adults had smaller anterior CCs than the younger adults [t(30) = 2.13, p < .05]. The data indicate that a substantial proportion of the older adults had a small CC (i.e., anterior CC size < .0025 of total brain size; see Figure 2) when compared to the younger adults.
Figure 2.
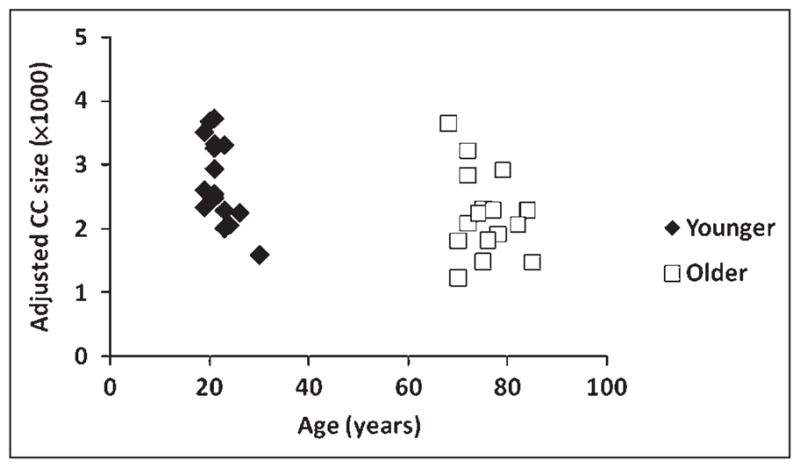
Scatterplot of volume-adjusted anterior corpus callosum (CC) size by age. CC size is expressed as a proportion of the total brain volume (multiplied by 1000).
Behavior
The average RT and accuracy for each group (younger and older), switch condition (switch vs. no-switch), and task (meaning vs. position) are presented in Table 2, together with their standard deviations. The data are collapsed across congruency levels, for reasons of clarity and because this variable is not critical to the current study. Switch costs, expressed as a proportional RT increase on switch compared to no-switch trials, are presented in Figure 3.
Table 2.
Average RT (msec) and Accuracy for Each Age Group and Condition
| No Switch |
Switch |
|||
|---|---|---|---|---|
| Position | Meaning | Position | Meaning | |
| RTs | ||||
| Younger | ||||
| Mean | 366 | 446 | 378 | 455 |
| SD | 108 | 104 | 112 | 113 |
| Older | ||||
| Mean | 590 | 666 | 640 | 695 |
| SD | 192 | 120 | 203 | 147 |
| Accuracy | ||||
| Younger | ||||
| Mean | 0.920 | 0.811 | 0.894 | 0.789 |
| SD | 0.051 | 0.090 | 0.060 | 0.083 |
| Older | ||||
| Mean | 0.941 | 0.926 | 0.914 | 0.927 |
| SD | 0.054 | 0.056 | 0.053 | 0.055 |
Figure 3.
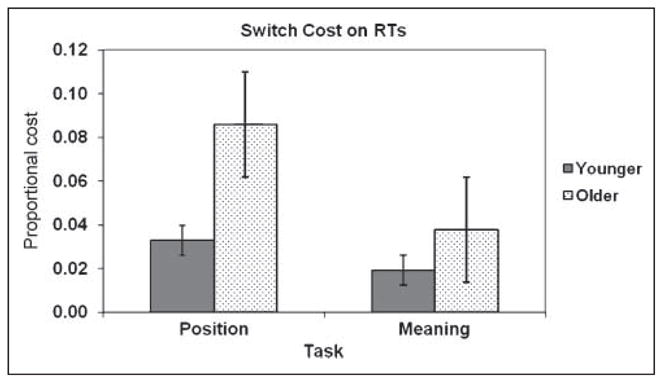
Average RT switch costs expressed as proportional increases in the RT for switch trials compared to that for no-switch trials, separately for each age group and task. Error bars refer to the standard error across subjects.
Both RT and accuracy data were submitted to four-way ANOVAs, with one between-subject factor (group) and three within-subject factors (task, congruency, and switch condition). For RT, the ANOVA revealed main effects of group [F(1, 30) = 23.59, p < .0001], task [F(1, 30) = 40.12, p < .0001], congruency [F(1, 30) = 63.94, p < .0001], and switch condition [F(1, 30) = 26.15, p < .0001]. It also revealed that age group interacted with switch condition [F(1, 30) = 8.36, p < .05] and congruency [F(1, 30) = 7.10, p < .05]. Congruency and task also interacted [F(1, 30) = 14.03, p < .001], and a three-way interaction between group, task, and congruency was also significant [F(1, 30) = 6.07, p < .05]. Thus, all the independent variables produced an effect on RTs. This included faster RTs for the position task compared to the meaning task. The effect of congruency was larger in the older than in the younger adults. The congruency effect was clearly attenuated in the younger adults in the position task, but this was not observed in the older adults.
The data presented above indicate that, as predicted, the switch costs for RTs were much greater in the older (39 ± 9 msec) than in the younger subjects (14 ± 4 msec). This difference held even when the proportional cost of task switching was computed [older adults: mean cost = 0.059 ± 0.014; younger adults: mean cost = 0.024 ± 0.008; t(30) = 2.13, p < .05]. This indicates that the cost of switching is disproportionate with respect to other RT costs observed in aging and cannot be simply attributed to generalized slowing (Salthouse, 1996).
To eliminate ceiling effects on the accuracy data, the data for each participant and condition were subjected to a probit transform before entering them in the ANOVA. The ANOVA revealed main effects of group [F(1, 30) = 22.89, p < .0001], task [F(1, 30) = 11.35, p < .01], congruency [F(1, 30) = 87.38, p < .0001], and switch condition [F(1, 30) = 10.34, p < .01]. It also revealed a number of two-way interactions, including Age group by Task [F(1, 30) = 9.02, p < .05], Task by Congruency [F(1, 30) = 20.61, p < .001], Task by Switch condition [F(1, 30) = 16.08, p < .001], and Switch condition by Congruency [F(1, 30) = 4.64, p < .05]. Further, significant three-way interactions included Group by Task by Switch condition [F(1, 30) = 6.08, p < .05], and Group by Congruency by Switch condition [F(1, 30) = 7.89, p < .01].
Although the pattern of accuracy results was quite complex, the data indicated that older adults were more accurate than younger adults, suggesting an overall speed–accuracy tradeoff between the two groups. However, this phenomenon had little or no influence on the various interactions of age with the other variables. For instance, the effects of task switching on accuracy were virtually identical in the older and younger adults, indicating that the greater RT cost for the older adults was not compensated by increased accuracy. The same was true for the congruency effect. Most important for the purpose of this article, the data revealed that younger adults were significantly more accurate in the position than in the meaning task; however, this difference was not apparent in the older adults. This leads to two observations: in general, the position task was easier (i.e., led to faster and more accurate responses than the meaning task); the advantage for the position task was greatly reduced or lost in the older adults. This finding, per se, is consistent with theories predicting impaired spatial processing or right hemisphere function in aging (such as the ‘‘right hemiaging’’ hypothesis). Interestingly, this age-related disadvantage in the spatial task only occurred for switch trials. This suggests that older adults may have particular problems redirecting their processing toward spatial information from verbal information.
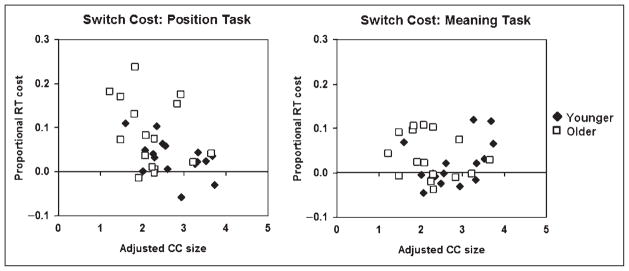
One of the main purposes of this study was to determine the extent to which age effects are mediated by anatomical changes, in particular, those related to structural connectivity. Our hypothesis was that the size of the anterior CC may be important in mediating switch costs, in particular, when the switch involves right-hemisphere tasks (in our case, the position task). We therefore hypothesized that switching-to-position costs may be inversely related to the size of the anterior CC (adjusted by brain volume). The relationship between CC size and the RT cost of switching to the meaning and position tasks is presented in Figure 4. These data indicate a clear relationship in the position task (r = −.446, p < .05), but not in the meaning task (r = .013, ns). As a reminder, the position task, and in particular, switching-to-position, showed the largest age-related deficit. To determine whether these deficits are, in fact, mediated by the reduced CC size, we performed a stepwise multiple regression procedure, in which adjusted CC size and age (in years) were entered as predictors of the switching-to-position costs. The raw correlation of age with switch-to-position costs was r = .388 ( p < .05). However, the results of the multiple regression indicated that only CC size provided a significant prediction of switch cost (beta = −.446, p < .05), and that once the effects of this variable were removed, age was not significantly correlated with switch costs (partial r = .24, ns). This indicates that structural impairment (as measured by CC size) may play a significant mediating role in certain behavioral switch costs associated with aging.
Figure 4.
Scatterplots depicting the relationship between proportional RT switch costs and the size of the anterior third of the corpus callosum (CC; adjusted by total brain volume), separately for the position task (left) and meaning task (right).
Overall, the behavioral data indicate that, as predicted, older adults found it more difficult to switch between tasks than younger adults. Interestingly, in particular for the older adults, switching to the spatial task was more difficult (in terms of RT cost) than switching to the verbal task, even though the verbal task was overall easier. To understand this apparently surprising finding, it may be useful to consider that task switching requires not only activating a new rule but also inhibiting a previously used rule (see Mayr & Keele, 2000, for a discussion of this issue). Further, on successive trials, the rule that had been previously inhibited may need to be reactivated. This may be particularly difficult for the most ‘‘dominant’’ or most ‘‘automatic’’ rules—in the current study, those associated with the (easier) spatial task. Mayr and his colleagues have argued that this is because the more automatic rule must be strongly inhibited. When this rule has been inhibited, switching back to it is particularly difficult (‘‘backward inhibition hypothesis’’).
This hypothesis leads to the prediction that the greater the performance advantage for the position task relative to the meaning task, the more difficult it will be for subjects to switch back to the position task. To test this hypothesis, we computed an index of ‘‘position-task advantage’’ by dividing the RT for the meaning task by the RT for the position task for each subject. We then examined the correlation between this index and the switch cost (separately for the switch-to-position and switch-to-meaning conditions). For the younger adults, as predicted by the backward inhibition hypothesis, the greater the advantage for the position task, the greater the switch-to-position cost (r = .39, p < .10 in a directional test) and the smaller the switch-to-meaning cost (r = −.67, p < .01). Interestingly, the ‘‘position-task advantage’’ was also negatively correlated with CC size (r = −.58, p < .01). These correlations where much smaller for the older adults (r = .05, r = −.50, and r = −.21, respectively). Thus, the data provide some support for the backward inhibition hypothesis. Further, the observations that the switch-to-position costs, as well as the relative advantage for the position task, are more pronounced in subjects with small CC provide some support for a ‘‘cortical disconnection hypothesis,’’ with respect to other theories such as the right hemiaging hypothesis, and suggest that cross-inhibition of homologous frontal areas may contribute to the observed effects.
One final analysis was performed to test the extent to which practice effect was present, given the fact that data were recorded from many trials over two sessions (see Rogers & Monsell, 1995). This was due to the necessity of collecting data from a denser montage than allowed by the hardware within one recording session, and to obtain a sufficient signal-to-noise ratio for EROS analyses. We compared the RT and accuracy from the first and second sessions. Session produced a main effect on RT [F(1, 30) = 6.45, p < .05], which was, however, only present in the older adults [Session × Age interaction: F(1, 30) = 6.55, p < .05]. However, the difference between age groups was still present in the second session. Session also interacted with the switch condition [F(1, 30) = 4.68, p < .05], but the effect of switch was present in both sessions (30 msec in Session 1 and 20 msec in Session 2). For accuracy, there was no main effect of session, but only a three-way interaction between age group, session, and congruency [F(1, 30) = 5.36, p < .05], indicating a reduced effect of congruency in older adults in Session 2. Overall, the effects of session were of marginally reducing (but never eliminating) some of the major effects found on behavior. Qualitatively, the same results were obtained in the second session as in the first session.
Event-related Optical Signal
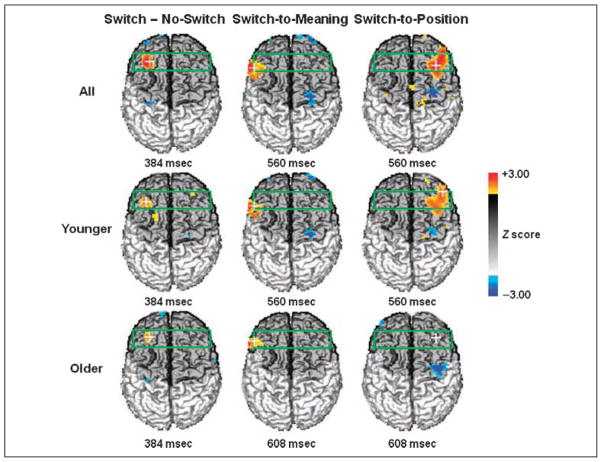
Average cue-related optical activity was obtained for each subject, switch condition, and task. These data were analyzed using three contrasts: (a) switch vs. no-switch; (b) switch-to-position vs. no-switch to position; and (c) switch-to-meaning vs. no-switch to meaning. These contrasts were first conducted across all subjects and then separately for each age group. Statistical maps are reported in Figure 5 for the switch versus no-switch contrast (left column) and for the breakdown by task (middle and right columns). These maps refer to the following latencies, which correspond to the peaks of EROS activity:3 between 300 and 400 msec for the general switch effect, and between 500 and 600 msec for the conditions broken down by task. The latency of the EROS response for the older adults for the conditions broken down by tasks was slightly longer (608 msec) than that for the younger subjects (560 msec).
Figure 5.
(A) Grand-average statistical event-related optical signal (EROS) maps (Z scores) for all subjects (top row), younger subjects (middle row), and older subjects (bottom row), for the difference between switch and no-switch trials combined across tasks (left column), for the meaning condition (middle column) and for the position condition (right column). The green box indicates the ROI used for analysis. The darker shade of gray indicates the areas from which optical data were recorded. The latency (msec) relative to the cue at which each map was derived is displayed beneath the corresponding map.
The EROS data analysis was conducted using an ROI encompassing both medial and lateral areas of prefron-tal regions, in both the left and right hemispheres (see Methods for boundaries, and green box in Figure 5 for a pictorial representation). The maps are based on average Z scores (computed across subjects) for each voxel.
The results indicate the presence of significantly greater brain activation at a latency of 384 msec in the left anterior MFG for the switch compared to the no-switch condition (peak Z = 3.13, criterion Z = 2.99, peak Talairach coordinates: x = −32, y = 27, z = 38, at the border between BA 8 and BA 9). This response was significant for an interval ranging from 368 to 400 msec after the cue, and was present for both the younger and older adults, and for the switch-to-meaning and switch-to-position tasks. The localization of this response corresponds closely to that observed in previous fMRI studies of task switching (Braver et al., 2003; DiGirolamo et al., 2001; Dove et al., 2000). The location of this optical activity and its peak latency also correspond closely to those we observed in a variety of other preparatory conditions (see Agran et al., submitted; Gratton et al., 2008). All these studies indicate that this left MFG activity is, in fact, one in a series of activities within the FPN during task preparation and attention control. We take the left MFG activity as evidence of a ‘‘task-general’’ response—which is operationally defined here as a response that is present for both the switch-to-meaning and switch-to-position conditions. The data suggest that this ‘‘task-general’’ activity is substantially similar for younger and older adults.
The EROS results also showed a second activity at longer latency (560–608 msec), which appeared in the left IFG for the switch-to-meaning condition (peak Z = 3.06, critical Z = 3.00, peak Talairach coordinates: x = −55, y = 19, z = 25, BA 9) and in the right MFG/IFG for the switch-to-position condition (peak Z = 2.98, critical Z = 2.98, peak Talairach coordinates: x = 32, y = 22, z = 45, BA 8, MFG, extending laterally and inferiorly to the IFG). We take this effect as evidence of ‘‘task-specific’’ activity—which is operationally defined as activity that is systematically lateralized as a function of task condition, and we label ‘‘canonical’’ the hemisphere that is expected to be more active in a given task (i.e., left hemisphere for the verbal/meaning task and right hemisphere for the spatial/position task) and ‘‘noncanonical’’ the opposite hemisphere (i.e., left hemisphere for the spatial/position task and right hemisphere for the verbal/meaning task). Differently from the left MFG activity, this more ventral activity (which from now on will be labeled MFG/IFG) showed different patterns in the younger and older subjects. Both groups showed clear left IFG activity in the switch-to-meaning compared to the no-switch-to-meaning contrast, but only the younger subjects showed clear right MFG/IFG activity in the switch-to-position versus no-switch-to-position contrast (see Figure 5).
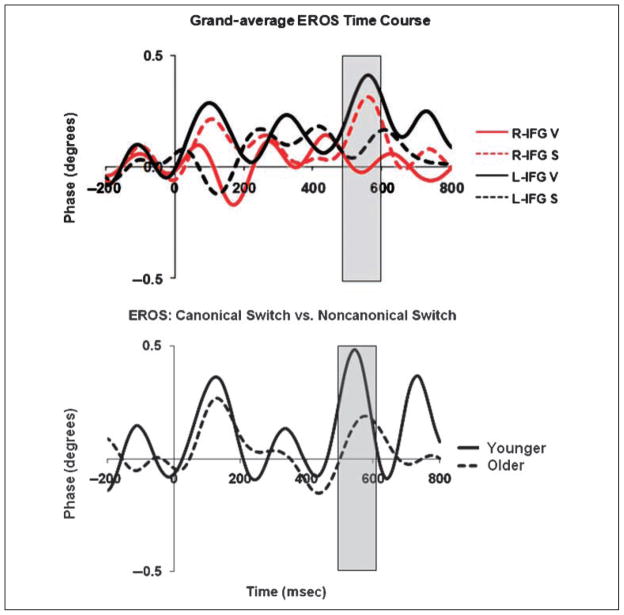
To clarify the time course of this activity, we further examined the EROS data from the ‘‘canonical’’ and ‘‘non-canonical’’ MFG/IFG. The grand-average waveforms from the left and right MFG/IFG (for both the spatial and verbal preparation contrasts) are presented in the top portion of Figure 6. The subtracted EROS waveforms (highlighting the differences between the left and right MFG/IFG for the verbal switch, and between the right and left MFG/IFG for the spatial switch) are presented at the bottom of Figure 6, separately for younger and older subjects. They show that this differential activity, which we can consider to be related to ‘‘task-specific’’ preparation during task switching, has two peaks: one occurring at a latency of approximately 128 msec after the cue, and the other occurring much later, at a latency of 560 msec (in younger adults) and 608 msec (in older adults). Both the early and late peaks were significantly different from baseline [t(31) = 2.06, p < .05, and t(31) = 3.32, p < .005, respectively]. However, the early MFG/IFG lateralization activity was not significantly correlated (r = .21) with CC size or with the subsequent MFG activity (r = −.04) and will therefore not be included in any of the correlation analyses presented in the next section.
Figure 6.
(Top) Grand-average time courses of event-related optical signal (EROS) activity (differences between switch and no-switch trials) from the peak locations of MFG/IFG activity, collapsed across age groups. R-IFG V = right MFG/IFG, switch-to-meaning (verbal) task; R-IFG S = right MFG/IFG, switch-to-position (spatial) task; L-IFG V = left MFG/IFG, switch-to-meaning task; L-IFG S = left MFG/IFG, switch-to-position task. (Bottom) Grand-average switch minus no-switch EROS lateralization waveforms obtained by subtracting the values recorded from the left MFG/IFG from those recorded from the right MFG/IFG for the position task, and vice versa for the meaning task. Separate waveforms were computed for younger (solid) and older (dashed) subjects. Time is expressed in milliseconds (msec), referred to the onset of the cue. In both the top and bottom panels, the transparent overlay indicates the interval where the largest ERP effect was observed.
Correlations between Measures
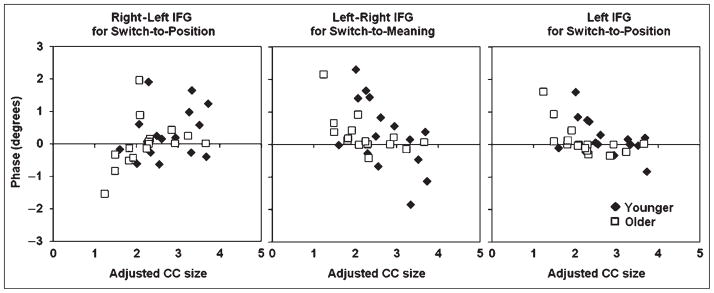
The EROS data presented above indicate that older adults do not appear to activate the right MFG/IFG when preparing to switch to a spatial task (Figure 5)—a type of activity that is instead found in the younger adults. As shown above, older subjects also exhibited specific behavioral deficits when they had to switch to the spatial task (see Figure 3). In addition, they had an overall reduced anterior CC size, even when adjusted by total brain volume. Finally, reduced anterior CC size was correlated with increased behavioral costs for the spatial task (see Figure 4). In this section, we test whether these phenomena are connected. Specifically, is the impaired right MFG/IFG activity restricted to (or more prominent in) subjects with small anterior CC size? To verify this hypothesis, anterior CC size (adjusted for total brain volume) was correlated with the amplitude of peak activity in the right MFG/IFG minus that in the left MFG/IFG at a latency of 560 msec (for the younger adults) and 608 msec (for the older adults) for the switch-to-spatial condition. The data are presented in the left portion of Figure 7. This figure indicates that the right MFG/IFG activity for the spatial switch was correlated with CC size (r = .39, p < .05);4 however (central panel of Figure 7), CC size was not as good of a predictor of left MFG/IFG activity for the verbal switch (r = −.32, p < .07). In fact, the presence of a negative, albeit not significant, correlation suggests that subjects with small CCs may use the left hemisphere to prepare for the meaning task at least equally, or perhaps even more, than subjects with large CCs (perhaps due to diminished cross-inhibitory control; Rykhlevskaia, Fabiani, & Gratton, 2006; see also Mayr & Keele, 2000). This was also supported by the lack of correlation between CC size and RT costs in the switch-to-meaning condition (Figure 4, right). Finally, the right portion of Figure 7 shows that only subjects with small CC exhibited left MFG/IFG activity in the switch-to-position condition (r = −.52, p < .01). All these CC-size effects were evident for both younger and older adults, although, overall, there were fewer younger adults with small CC sizes. The significance of the (non-canonical) left-hemisphere MFG/IFG activity in switching to the position task can also be corroborated by testing its correlation with the RT switch cost. In fact, for the switch-to-position condition, the amount of left MFG/IFG activity elicited by the cue was a good predictor of the RT switch cost in response to the upcoming imperative stimulus (r = .60, p < .001), suggesting that this activity has negative behavioral consequences.
Figure 7.
Scatterplots depicting the relationship between the size of the anterior third of the corpus callosum (CC; adjusted by total brain volume) and event-related optical signal (EROS) measures from the MFG/IFG (at a latency of 560 msec for the younger subjects and 608 msec for the older subjects). In all three plots, younger subjects are indicated by filled diamonds and older subjects by open squares. Left: relationship between CC size and amount of lateralization (difference between the activity in the right and left MFG/IFG for the switch/no-switch contrast) for the position task. Middle: relationship between CC size and amount of lateralization (difference between activity in the left and right MFG/IFG for the switch/no-switch contrast) for the meaning task. Right: relationship between CC size and activity in the left MFG/IFG only for the switch/no-switch contrast in the position task.
The data presented in Figure 7 suggest that the size of the anterior CC (or some other anatomical change of which it may be a proxy) may be important in mediating the activity in task-specific areas (MFG/IFG), but only when these areas are located in the right hemisphere. As the left MFG is active at an earlier time during task switching, it is possible that the anterior CC may play an important role in relaying information to the right hemisphere when switching-to-spatial task is required. If this is the case, then how do subjects with small CC perform the switch-to-position operation? One possibility is that they may perform it by activating left-hemisphere structures instead.
To verify this hypothesis, we separately analyzed the left and right MFG/IFG activity (EROS measures at a latency of 560 msec for the younger adults and 608 msec for the older adults) in subjects with large and small anterior CC. These results are presented in Figure 8, and indicate that (a) the large CC subjects showed predominantly left-hemisphere activity for the switch-to-verbal condition and predominantly right-hemisphere activity for the switch-to-spatial condition; conversely, (b) the subjects with small CC showed left-lateralized activity in both cases. In fact, the left MFG/IFG was significantly more active in the switch-to-spatial task for the small CC group than for the large CC group [t(24) = 2.26, p < .05].5 Further, in the small CC group, there was no significant difference between left and right MFG/IFG activity for the spatial switch [t(12) = 0.14], whereas a significant difference was observed in the large CC group [t(11) = 2.26, p < .05]. Finally, as presented above, the amount of left MFG/IFG activity during spatial switching was inversely correlated with adjusted CC size (right portion of Figure 7).
Figure 8.
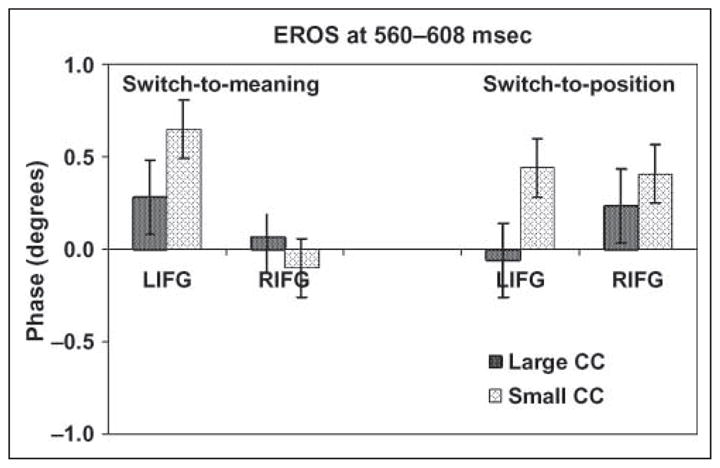
Grand-average activations in the left (LIFG) and right (RIFG) MFG/IFG at a latency of 560 msec (for younger subjects) and 608 msec (for older subjects) for each switch contrast (switch/ no-switch conditions). Separate averages are presented for subjects with large (darker bars) and small (lighter bars) adjusted corpus callosum (CC) size (using a median split to separate subjects into groups), and for each task. The error bars are based on standard error across subjects.
DISCUSSION
The EROS data indicate that the left6 MFG was activated when subjects were required to switch between tasks in preparation for processing an upcoming imperative stimulus. The latency of this activity was between 350 and 400 msec, and was evident for both younger and older subjects, and for both types of switch (switch-to-meaning and switch-to-position), suggesting that it may contribute to task-general preparatory processes (i.e., processes that do not depend on specific task dimensions). In contrast, more ventral prefrontal areas in the left and right MFG/IFG were each active only for one type of switch (at least in younger subjects): the left for the switch-to-meaning condition and the right for the switch-to-position condition. In fact, the older adults exhibited, on average, weaker activity in the right MFG/ IFG for the switch-to-position task. Analyses of the relationship between this activity and anterior CC size revealed that subjects with small CC (irrespective of age; see Figure 7, right) actually showed activity in the left MFG/IFG, rather than in the right MFG/IFG, after cues signaling that a switch-to-position was needed. These subjects also appeared to have disproportionate RT costs in the switch-to-position condition. Finally, the amplitude of the MFG/IFG activity was predictive of subsequent switch costs, thus suggesting that it reflects functionally important brain events. These results suggest that some of the problems that older adults exhibit in switching between tasks may be attributed to basic structural deficits, which in the current study were linked with CC size. It is important to note, however, that similar phenomena were also apparent in younger adults, albeit to a lesser degree due to the smaller number of subjects with small CCs in this age group.
Two hypotheses can be entertained to explain the connection between the structural and functional data reported here. One possibility is that reduced CC size may lead to reduced right MFG/IFG activation (as the earlier task-general MFG activity was lateralized to the left hemisphere, thus requiring callosal transfer when right MFG/ IFG activation was required)—a condition that might be labeled a ‘‘partial disconnection’’ syndrome (for a similar suggestion, see Reuter-Lorenz & Stanczak, 2000). Alternatively, both the reduced CC size and the absence of functional activation in the right MFG/IFG may be explained by a structural/functional right prefrontal deficit, consistent with the ‘‘right hemiaging’’ hypothesis (Dase-laar & Cabeza, 2005; Meudell & Greenhalgh, 1987; see also Rajah & D’Esposito, 2005). The latter hypothesis, however, fails to predict the existence of a negative correlation between the performance advantage in the position task and CC size.
Overall, these data highlight a network involved in task switching, which includes both task-general and task-specific structures, as well as variations in the operation of these structures as a function of aging and anatomical differences, with related behavioral costs. Some caveats, however, should be considered. To begin with, due to the recording system used in this study, we were limited to an examination of only the anterior portion of the FPN, and therefore, the role of parietal and other regions was not investigated. Second, efficient task switching should be considered an outcome to which much of the information processing system is likely to contribute. As such, it may involve a number of elementary operations (Crone, Wendelen, Donohue, & Bunge, 2006). The left MFG activity we observed is unlikely to represent all of the operations involved in task switching, and possibly not even all of those involved in its task-general phase (Gratton et al., 2008). In fact, the results presented here are consistent with various interpretations of left MFG activity, including that it may manifest change detection (e.g., Pessoa & Ungerleider, 2004) and/or the redirecting of attention (e.g., Gazzaley et al., 2007; Miller & Cohen, 2001). In fact, all of these processes may contribute to task switching (see Schneider & Logan, 2007). However, it is also possible that activation of the left MFG may not even be necessary for task switching to occur. Because task switching is an operation defined at the level of the entire system, it may be achieved in different ways under different conditions. Thus, although the left MFG may be important for task switching (as suggested by lesion studies, e.g., Aron et al., 2004), in some cases, task switching could be achieved before, or even in the absence of, left MFG activation. In fact, some of the data in our study are suggestive that this may occur, as MFG/ IFG activity separating task-specific switch from no-switch trials was observed even before the 350-msec latency landmark for peak MFG activation (Figure 6, bottom; see also Agran et al., submitted). The possibility that ‘‘task-specific’’ activation may precede ‘‘task-general’’ activation clearly indicates that task switching may occur not only through a top–down, hierarchically organized process but also through a distributed process. A possible interpretation of this finding is that, with time on task, participants may develop a ‘‘direct’’ link between the cue percept and task-specific areas, perhaps profiting from the consistent stimulus mapping over the large number of trials used in this study. If this were the case, then the early activation of the MFG/IFG should be observed only after extended training. Future research will investigate this issue,7 but in any case, this finding highlights the importance of a spatio-temporal analysis of brain activity. In summary, it is likely that task switching may be achieved with different strategies, including a hierarchical, centralized processing mode in which the left MFG exerts an important role, and a more distributed and automatic processing mode, which may depend instead on more ventral areas (MFG/IFG).
An interesting aspect of this study was the change in the overall pattern of brain activity as a function of aging, a finding commonly reported in neuroimaging aging research (for reviews, see Kramer, Fabiani, & Colcombe, 2006; Cabeza, 2002). For instance, a number of investigators using fMRI or PET have reported bilateral brain activations in aging during tasks that elicit largely unilateral activations in younger subjects (e.g., Colcombe, Kramer, Erickson, & Scalf, 2005; Cabeza, Anderson, Locantore, & McIntosh, 2002; Logan, Sanders, Snyder, Morris, & Buckner, 2002; Madden et al., 2002; Nielson, Langencker, & Garvan, 2002; Rosen et al., 2002; Grady, McIntosh, Horwitz, & Rapoport, 2000; Reuter-Lorenz et al., 2000; see also Fabiani & Friedman, 1995). In the current study, the change in brain activation pattern (which included bilateral activation of the MFG/IFG; see Figure 8) was restricted to individuals with specific structural characteristics (small anterior CC size), and, in fact, CC size was more strongly correlated with bilateral activation than age. This bilateral activation in subjects with small CC may be associated with reduced cross-hemispheric inhibition (Rykhlevskaia et al., 2006; see also Hasher et al., 2008).
The data reported here are consistent with the interpretation that individuals (and older adults in particular) adapt to the constraints imposed by the structural changes/characteristics of their brains, and ‘‘configure’’ their strategies (and patterns of brain activity) in order to perform the task. In the current study, subjects with small CC activated the left MFG/IFG, instead of the right, when a ‘‘switch-to-position’’ was required. It should be noted that, although these subjects also showed greater switch costs, their performance was still well above chance. Thus, whatever their strategies, it is clear that they were at least partially successful. Of course, because these data are only correlational, it is not possible to state conclusively that the functional reconfiguration we observed was caused by reduced CC size (or a structural deficit linked to it), and that, in turn, this caused the drop in performance.
Another hypothesis that could be proposed is that a small CC size is merely a proxy for an overall impaired brain, which would be challenged by more difficult tasks. According to this hypothesis, older adults with small CC might show a deficit in spatial switching simply because this is more difficult than verbal switching, following the ‘‘backward inhibition hypothesis’’ (Mayr & Keele, 2000). Two results are, in fact, not consistent with the interpretation that subjects with small CC have an overall less efficient brain. First, the RT advantage for the position task was even greater in the subjects with small CC than in the subject with large CC, suggesting that their drop in performance is not generalized. Second, this hypothesis does not explain why the subjects with smaller CC activated the left MFG/IFG while performing the spatial task (a finding that is instead predictable on the basis of the partial disconnection hypothesis).
The data reported here also present some limitations, which may be addressed by future studies. First, due to the design of the optical system used in this study, EROS data were only recorded from prefrontal areas. Some of the areas important in task switching and attentional control, such as deep thalamic structures, may be beyond the reach of optical methods, but other more superficial areas such as the parietal cortex are accessible with optical imaging. We have now developed a more extended EROS montage, which will allow us to cover the majority of the cortical surface. Second, only one type of task switching (between spatial and verbal dimensions) was studied. Other conditions need to be explored to demonstrate the generalizability of the results. We are currently exploring task-switching paradigms involving other dimensions (Gratton et al., 2008). Third, the task-switching conditions used here were confounded with other variables (such as cue change vs. cue repetition; see Schneider & Logan, 2007). This makes the interpretation of some of the findings ambiguous. For example, we cannot determine whether the left MFG activity was related to the detection of cue changes or to a change in the weight given to different rules. Using additional conditions (such as one in which multiple cues may signal the same dimension) may help resolve this ambiguity. Fourth, although the behavioral and functional correlations with CC size clearly indicated the role of anatomical and structural changes in mediating the effects of task switching, the interpretation of these changes is also ambiguous. It is possible that CC size (even when corrected by overall brain volume) may still be a proxy for a more diffused structural change, or for structural changes localized in specific cortical regions (such as the right MFG/IFG). Indeed, several recent studies indicate that aging is associated with both a reduction in anterior medial white matter (i.e., anterior CC size) and a reduction in gray matter in the dorsolat-eral prefrontal cortex as well in other brain regions (e.g., Gordon et al., 2008; Salat et al., 2005; Walhovd et al., 2005; Head et al., 2004; Davatzikos & Resnick, 2002; Raz et al., 1998). More refined anatomical measures, beyond the capabilities of the current study, may be needed to evaluate these alternative hypotheses. Coupling diffusion tensor imaging (for a review, see Le Bihan et al., 2001) and/or advanced morphological measures of cortical structures (e.g., voxel-based morphometry; Ashburner & Friston, 2000, 2001) with the types of measurements presented here may help us determine whether the changes in CC size are related to specific or diffuse changes in cortical anatomy and trophism. Fifth, a pure-run condition (i.e., one in which some blocks are entirely made up by switch or no-switch trials) was not used in the current study. This makes it difficult to evaluate the global costs of task switching (Kray & Lindenberger, 2000).
Notwithstanding these limitations, the data presented here do provide clear indications that a combined approach based on structural and time-resolved functional analysis of changes occurring with age can be useful in isolating components of cognitive aging. Specifically, we provided indications that some of the problems experienced by older adults in task switching may be associated with functional reprogramming, perhaps as a consequence of localized impairment in anatomical connectivity.
Acknowledgments
This work was funded by DARPA (via NSF EIA 00-79800 AFK) to G. Gratton and M. Fabiani, and by NIA grant AG 21887 to M. Fabiani. We thank Brian Gordon, Art Kramer, and Kathy Low for their comments on an earlier version of this manuscript.
Footnotes
This assumes the hierarchical, top–down model only. From a distributed processing perspective, subjects with small CCs should have problems switching to either dimension as inhibitory projections may be compromised bidirectionally. Of course, if both models were operating simultaneously, then one would still expect some level of disadvantage for the spatial switch.
ERP data were also recorded concurrently with the EROS data. The ERP results were, by and large, consistent with the EROS data reported here, but lacked the spatial specificity provided by EROS and were therefore omitted for reasons of space. They will be made available in summary form upon request.
They are also consistent with the latencies at which concurrently recorded ERP effects were present (results available upon request).
Note that age was not significantly correlated with this lateralized activity in preparation for a switch to the spatial task (r = −.21). This suggests that CC size rather than age is the critical factor.
The reduced n for this analysis is due to the fact that, due to different head shapes and sizes, not all subjects had coverage in this area.
Although it is not possible, on the basis of these data alone, to determine why the cue elicits activity preferentially in the left (rather the right) MFG, one can hypothesize that verbal cues (letters) may bias processing to the left hemisphere.
Such analysis is difficult in the current study due to the fact that different montages were used across sessions, with the montage order counterbalanced across subjects.
References
- Agran J, Low KA, Ryklevskaia EI, Fabiani M, Gratton G. When the rules keep changing: The timing of activation of task-general and task-specific brain regions involved in preparation submitted. [Google Scholar]
- Aron AR, Monsell S, Sahakian BJ, Robbins TW. A componential analysis of task-switching deficits associated with lesions of left and right frontal cortex. Brain. 2004;127:1561–1573. doi: 10.1093/brain/awh169. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Braver T, Reynolds J, Donaldson D. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39:713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wallis JD, editors. Perspectives on rule-guided behavior. New York: Oxford University Press; 2008. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in cognitive performance in aging humans. Psychology and Aging. 2005;20:363–375. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Crone EA, Wendelen C, Donohue SE, Bunge SA. Neural evidence for dissociable components of task-switching. Cerebral Cortex. 2006;16:475–486. doi: 10.1093/cercor/bhi127. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Cabeza R. Age-related changes in hemispheric organization. In: Cabeza R, Nyberg L, Park D, editors. Cognitive neuroscience of aging: Linking cognitive and cerebral aging. New York: Oxford University Press; 2005. pp. 325–353. [Google Scholar]
- Davatzikos C, Resnick SM. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cerebral Cortex. 2002;12:767–771. doi: 10.1093/cercor/12.7.767. [DOI] [PubMed] [Google Scholar]
- DeSoto MC, Fabiani M, Geary DC, Gratton G. When in doubt, do it both ways: Brain evidence of the simultaneous activation of conflicting responses in a spatial Stroop task. Journal of Cognitive Neuroscience. 2001;13:523–536. doi: 10.1162/08989290152001934. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, et al. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. NeuroReport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY. Prefrontal cortex activation in task switching: An event-related fMRI Study. Cognitive Brain Research. 2000;9:103–109. doi: 10.1016/s0926-6410(99)00029-4. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Friedman D. Changes in brain activity patterns in aging: The novelty oddball. Psychophysiology. 1995;32:579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Rissman J, Cooney J, Rutman A, Seibert T, Clapp W, et al. Functional interactions between prefrontal and visual association cortex contribute to top–down modulation of visual processing. Cerebral Cortex. 2007;17:125–135. doi: 10.1093/cercor/bhm113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Shallice T. Task switching: A PDP model. Cognitive Psychology. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- Gordon B, Rykhlevskaia E, Brumback CR, Lee Y, Elavsky S, Konopack JF, et al. Anatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45:825–838. doi: 10.1111/j.1469-8986.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degreaded and nondegraded face processing. Cognitive Neuropsychology. 2000;217:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Gratton G, Brumback CR, Gordon BA, Pearson MA, Low KA, Fabiani M. Effects of measurement method, wavelength, and source–detector distance on the fast optical signal. Neuroimage. 2006;32:1576–1590. doi: 10.1016/j.neuroimage.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Gratton G, Corballis PM. Removing the heart from the brain: Compensation for the pulse artifact in the photon migration signal. Psychophysiology. 1995;32:292–299. doi: 10.1111/j.1469-8986.1995.tb02958.x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Corballis PM, Cho E, Fabiani M, Hood D. Shades of gray matter: Noninvasive optical images of human brain responses during visual stimulation. Psychophysiology. 1995;32:505–509. doi: 10.1111/j.1469-8986.1995.tb02102.x. [DOI] [PubMed] [Google Scholar]
- Gratton G, Fabiani M. Shedding light on brain function: The event-related optical signal. Trends in Cognitive Sciences. 2001;5:357–363. doi: 10.1016/s1364-6613(00)01701-0. [DOI] [PubMed] [Google Scholar]
- Gratton G, Fabiani M. Optical imaging. In: Parasuraman R, Rizzo M, editors. Neuroergonomics: The brain at work. Cambridge: Oxford University Press; 2007. pp. 65–81. [Google Scholar]
- Gratton G, Low KA, Fabiani M. Time course of executive processes: Data from the event-related optical signal (EROS) In: Bunge SA, Wallis JD, editors. Perspectives on rule-guided behavior. New York: Oxford University Press; 2008. pp. 197–223. [Google Scholar]
- Gray CF, Chabris JR, Braver T. Neural mechanisms of general fluid intelligence. Nature Neuroscience. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive function in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hasher L, Lustig C, Zacks R. Inhibitory mechanisms and the control of attention. In: Conway A, Jarrold C, Kane M, Miyake A, Towse J, editors. Variation in working memory. New York: Oxford University Press; 2008. pp. 227–249. [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, et al. Dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Poline JB, Friston KJ, Holmes AP, Worsley KJ. Robust smoothness estimation in statistical parametric maps using standardized residuals from the general linear model. Neuroimage. 1999;10:756–766. doi: 10.1006/nimg.1999.0508. [DOI] [PubMed] [Google Scholar]
- Köhler S, Moscovitch M, Winocur G, Houle S, McIntosh AR. Networks of domain-specific and general regions involved in episodic memory for spatial location and object identity. Neuropsychologia. 1998;36:129–142. doi: 10.1016/s0028-3932(97)00098-5. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Fabiani M, Colcombe S. Contributions of cognitive neuroscience to the understanding of behavior and aging. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. 6. New York: Academic Press; 2006. pp. 57–83. [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychologica. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15:126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: Concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and non-selective recruitment: Dissociable neural mechanisms associated with cognitive decline in older adults. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Lamgley LK, Kawk TC, et al. Aging and attentional guidance during visual search. Psychology and Aging. 2002;17:24–43. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment and Parkinson’s disease. Neurology. 1981;31:645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- Mayr U, Keele SW. Changing internal constraints on action: The role of backward inhibition. Journal of Experimental Psychology: General. 2000;129:4–26. doi: 10.1037//0096-3445.129.1.4. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Meudell PR, Greenhalgh M. Age related differences in left and right hand skill and in visuo-spatial performance: Their possible relationships to the hypothesis that the right hemisphere ages more rapidly than the left. Cortex. 1987;23:431–445. doi: 10.1016/s0010-9452(87)80005-9. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Reviews of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:100–110. [Google Scholar]
- Monsell S. Task switching. Trends in Cognitive Sciences. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Langencker SA, Garvan HP. Differences in the functional neuroanatomy of inhibitory control across the adult lifespan. Psychology and Aging. 2002;17:56–71. doi: 10.1037//0882-7974.17.1.56. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summer PE, Morris RG, Williams SC, Markus HS. Evidence for cortical ‘‘disconnection’’ as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neural correlates of change detection and change blindness in a working memory. Cerebral Cortex. 2004;14:511–520. doi: 10.1093/cercor/bhh013. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Reviews of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FIM, Salthouse T, editors. The handbook of aging and cognition. Hillsdale, NJ: Erlbaum; 2000. pp. 1–90. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Stanczak L. Differential effects of aging on the functions of the corpus callosum. Developmental Neuropsychology. 2000;18:113–137. doi: 10.1207/S15326942DN1801_7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Monsell S. Costs of a predictable switch between simple cognitive tasks. Journal of Experimental Psychology: General. 1995;124:207–231. [Google Scholar]
- Rosen A, Prull M, O’Hara R, Race E, Desmond J, Glover G, et al. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Fabiani M, Gratton G. Lagged covariance structure models for studying functional connectivity in the brain. Neuroimage. 2006;30:1203–1218. doi: 10.1016/j.neuroimage.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia EI, Fabiani M, Gratton G. Combining structural and functional neuroimaging data for studying brain connectivity: A review. Psychophysiology. 2008;45:173–187. doi: 10.1111/j.1469-8986.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, et al. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Logan GD. Defining task-set reconfiguration: The case of reference point switching. Psychonomic Bulletin & Review. 2007;14:118–125. doi: 10.3758/bf03194038. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Koeppe RA. Dissociating verbal and spatial working memory using PET. Cerebral Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS. Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proceedings of the National Academy of Sciences, USA. 2000;97:13448–13453. doi: 10.1073/pnas.240460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Diffusion tensor imaging and aging. Neuroscience and Biobehavioral Reviews. 2006;30:749–761. doi: 10.1016/j.neubiorev.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: Three-dimensional proportion system: An approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wolf U, Wolf M, Toronov V, Michalos A, Paunescu LA, Gratton E. Detecting cerebral functional slow and fast signals by frequency–domain near-infrared spectroscopy using two different sensors. Paper presented at OSA Meeting in Optical Spectroscopy and Imaging and Photon Migration; Miami. April 2–5, 2000.2000. [Google Scholar]