Abstract
Pediatric neck masses should trigger a high index of suspicion for certain genetic disorders of connective tissue. To highlight this, we report on three infants with Menkes disease, an inherited disorder of copper transport, who developed large, unilateral neck masses at between 7 and 17 months of age. All were identified in imaging studies as internal jugular phlebectasia. The masses, which enlarged on crying or exertion, have remained clinically benign in these patients for 20, 17 and 2 months, respectively. While arterial tortuosity and aneurysms have been reported often in Menkes disease, venous phlebectasia has rarely been described. We speculate that low activity of the copper-dependent enzyme, lysyl oxidase, leading to reduced tensile strength in the deep cervical fascia comprising the carotid sheath may predispose to internal jugular phlebectasia in these individuals. Improved survival and neurological outcomes in infants with Menkes disease due to advances in early diagnosis and treatment may be associated with recognition of novel clinical stigmata of this condition such as internal jugular phlebectasia.
Keywords: internal jugular phlebectasia, venous aneurysm, neck mass, Menkes disease
Introduction
Menkes disease, also known as “kinky hair disease,” is a rare X-linked neurodegenerative disorder of copper metabolism caused by mutations in the gene ATP7A [1–4]. The missing or defective gene product in affected individuals is normally responsible for copper transport across cellular membranes, including the trans-Golgi network, the end result being cytosolic accumulation of copper and impaired incorporation of copper into copper-dependent enzymes [5]. The classic clinical phenotype associated with Menkes disease includes growth retardation, peculiar hair, and focal cerebral and cerebellar degeneration [6]. Some other clinical features are associated with deficiencies of specific copper enzymes [5]. For example, connective tissue abnormalities including skin and joint laxity, bladder diverticula, gastric polyps, vascular tortuosity, and brachial artery aneurysms are all ascribed to deficiency of the copper enzyme lysyl oxidase [6–8]. Lysyl oxidase normally plays a critical role in formation of extracellular matrix by oxidizing lysine residues in elastin and collagen, and thereby initiating formation of covalent crosslinks that stabilize these fibrous proteins and provide tensile strength to connective tissue [9].
Venous aneurysms or phlebectasias are rare vascular entities in adults and children [10,11], although the literature contains at least eighteen reports of affected pediatric patients [11–19], none of whom had signs or symptoms of Menkes disease. In all previously reported cases, phlebectasia was an isolated finding, apparently unrelated to any underlying systemic disorder or specific genetic syndrome.
Here, we report three infants with Menkes disease who developed massive internal jugular phlebectasia.
Case Reports
Patient #1
A 16-month-old male with Menkes disease was brought to a hospital emergency room with a history of a right lateral neck mass (Fig. 1A) that had enlarged over the previous one month. On physical examination, the mass was located at the anterior border of the right sternocleidomastoid muscle, and increased in size when the infant cried. At its largest size, it measured 7 cm × 4 cm. The mass was soft, fluctuant, and without a palpable thrill or audible murmur. It was non-pulsatile, and did not transilluminate. The overlying skin was not discolored, and not attached to the mass. There were no signs of respiratory distress. A magnetic resonance imaging scan of the neck (Fig. 1B) revealed a large saccular neck lesion, and ultra-sonography indicated right internal jugular phlebectasia. The patient is currently 36 months of age and has not had any adverse clinical effects from this venous abnormality in the 21 months since its discovery.
Figure 1.
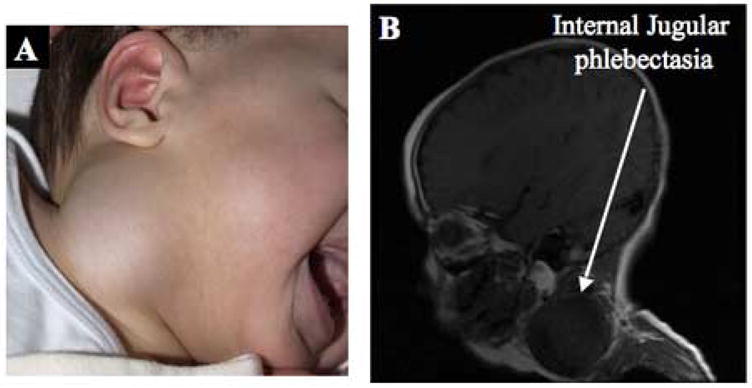
Patient 1. A. Right cervical neck mass. B. Sagittal magnetic resonance image reveals a large saccular neck lesion (arrow).
Patient #2
A 7 month old infant with Menkes disease was noted to have a left-sided neck mass (Fig. 2A) that displaced the pinna of the left ear and enlarged with crying (Fig. 2B). Ultrasonography indicated left internal jugular phlebectasia as the cause of the mass. At his current age of 24 months, this patient has not had any adverse clinical effects from this venous abnormality in the 17 months since its discovery.
Figure 2.
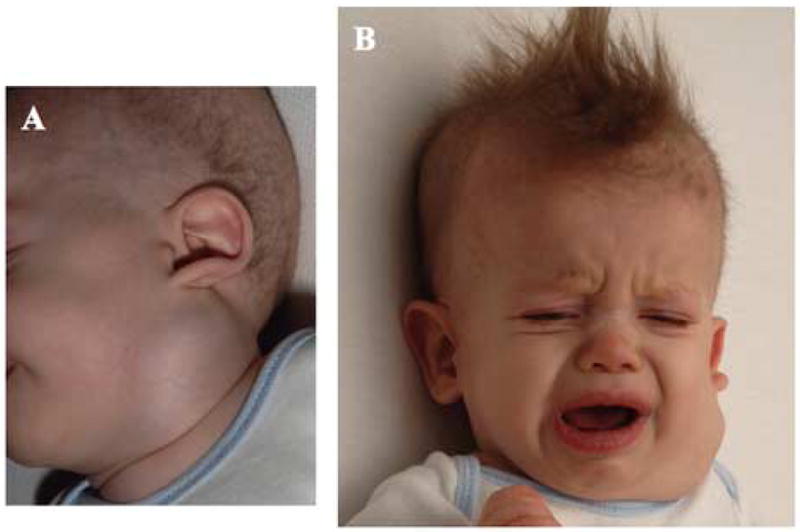
Patient 2. A. Left sided neck mass. Note displacement of the ear pinna. B. The mass increased in size when the infant cried.
Patient #3
A third infant with Menkes disease, age 17 months, was noted to have a left lateral neck mass. On physical examination, the mass was located on the anterior border of the left sternocleidomastoid muscle and enlarged upon crying. Computerized tomography of the neck indicated massive dilation of the internal jugular vein. Three-dimensional magnetic resonance angiography (Fig. 3B) indicated massive dilation of the left internal jugular vein that extended into the transverse sinus within the cranial cavity, and a smaller dilation at the origin of the right external jugular vein from the right subclavian vein. This patient is currently 19 months of age and has not had any adverse clinical effects from these venous abnormalities in the two months since their discovery.
Figure 3.
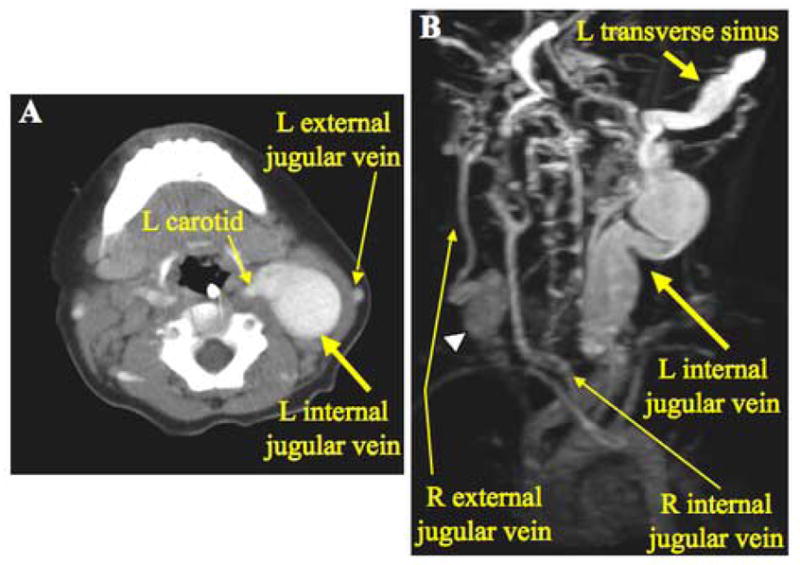
Patient 3. A. Axial computerized tomographic scan with contrast of the neck shows massive dilation of the left internal jugular vein. B. Rotated three-dimensional magnetic resonance angiography revealing extensive dilation of the left transverse sinus as well as the left internal jugular vein. On the right side, a dilated segment of the right external jugular vein (arrowhead) is evident.
Discussion
Menkes disease is associated with deficiency of several copper-dependent enzymes including cytochrome C oxidase, lysyl oxidase, dopamine beta-hydroxylase, copper/zinc superoxide dismutase, and ascorbic acid oxidase [5]. Lysyl oxidase is responsible for elastin and collagen cross-linking and is most likely the defect responsible for the phlebectasia found in these patients [9]. We previously described dilated submucosal veins and venules on histopathological analysis of gastric polyps from two Menkes disease infants [7]. A right internal jugular aneurysm was recently reported in another infant with Menkes disease who died from extensive pulmonary emphysema [20]. Taken together with these three cases, it appears that the internal jugular veins may be particularly predisposed to developing ectasia in this genetic syndrome. Of note, in our opinion, none of the 18 previous reported cases of isolated jugular phlebectasia in infants and children [11–19] were suggestive of Menkes disease.
Internal jugular veins collect blood from the brain and superficial parts of the face and neck and are continuous with the transverse sinuses of the brain. They originate in the posterior compartment of the jugular foramen at the base of the skull, and are enclosed within the neck by the carotid sheath, comprising three layers of deep cervical fascia. We speculate that reduced tensile strength in these fasciae (as well as in the blood vessel walls) of infants with Menkes disease is a specific risk factor for internal jugular phlebectasia.
Internal jugular phlebectasias are the most common venous malformations occurring in the head and neck region [10]. Jugular phlebectasias in childhood reportedly have a predilection to occur on the right side, while jugular phlebectasia in adulthood are more common on the left side [11]. In the now four cases of internal jugular phlebectasia in Menkes disease (the present report plus one other case [20]), we noted equal right and left side frequency. Possible causes of jugular phlebectasia in adults include mechanical compression or trauma, inflammation, and hypertension. In childhood, a differential diagnosis also includes cavernous hemangioma, cystic hygroma, thyroglossal duct cyst, branchial cleft cyst, laryngocele and cervical adenitis [13].
Management of jugular phlebectasia may be conservative. There are no reports of rupture or other serious sequellae [11,17], although cosmetic issues may warrant surgical repair in some instances [11–15,17,18]. The stable clinical courses, over 2 to 21 months, in the three Menkes disease patients reported here reinforce support for a conservative management strategy.
In summary, Menkes disease should be considered in the differential diagnosis of infantile neck masses. Ultrasonography and other imaging methods enable detection and delineation of internal jugular phlebectasia in Menkes disease, an inherited disorder of copper transport which affects connective tissue and may be a relatively common cause of this vascular abnormality.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nature Genet. 1993;3:7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- 2.Chelly J, Tumer Z, Tonnensen T, Petterson A, Ishikawa-Brush Y, Tommerup N, Horn N, Monaco AP. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature Genet. 1993;3:14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- 3.Mercer JFB, Livingston J, Hall B, Paynter JA, Begy C, Chandrasekharappa S, Lockhart P, Grimes A, Bhave M, Siemieniak D, Glover TW. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nature Genet. 1993;3:20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- 4.Kaler SG. Menkes disease mutations and response to early copper histidine treatment. Nature Genet. 1996;13:21–22. doi: 10.1038/ng0596-21. [DOI] [PubMed] [Google Scholar]
- 5.Kaler SG. Menkes disease. In: Barness LA, editor. Advances in Pediatrics. Vol. 41. C.V. Mosby; 1994. pp. 263–304. [PubMed] [Google Scholar]
- 6.Menkes JH, Alter M, Steigleder GK, Weakley DR, Sung JH. A sex-linked recessive disorder with retardation of growth, peculiar hair and focal cerebral and cerebellar degeneration. Pediatrics. 1962;29:764. [PubMed] [Google Scholar]
- 7.Kaler SG, Westman JA, Bernes SM, Elsayed AM, Bowe CM, Freeman KL, Wu CD, Wallach MT. Gastrointestinal hemorrhage associated with gastric polyps in Menkes disease. J Pediatr. 1993;122:93–95. doi: 10.1016/s0022-3476(05)83496-1. [DOI] [PubMed] [Google Scholar]
- 8.Godwin SC, Shawker T, Chang M, Kaler SG. Brachial artery aneurysms in Menkes disease. J Pediatr. 2006;149:412–415. doi: 10.1016/j.jpeds.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 9.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 10.Schartz IJ, Fine G. Venous aneurysms. N Engl J Med. 1962;266:1310–1312. doi: 10.1056/NEJM196206212662505. [DOI] [PubMed] [Google Scholar]
- 11.Gordon DH, Rose JS, Kotimeir P, Levin DC. Jugular venous ectasia in children: a report of three cases and review of the literature. Radiology. 1976;118:147–149. doi: 10.1148/118.1.147. [DOI] [PubMed] [Google Scholar]
- 12.Mallik R. C. A case of aneurysm of the internal jugular vein. J Laryngol Otol. 1977;91:893–895. doi: 10.1017/s0022215100084516. [DOI] [PubMed] [Google Scholar]
- 13.Passariello R, Cozzi F, Casalena G, Colarossi G, Rossi P, Simonetti G. Angiographic diagnosis of jugular venous system dilatation in Children. A report of five cases. Pediatr Radiol. 1979;8:247–250. doi: 10.1007/BF00974043. [DOI] [PubMed] [Google Scholar]
- 14.Danis RK. Isolated aneurysm of the internal jugular vein: a report of three cases. J Pediatr Surg. 1982;17:130–131. doi: 10.1016/s0022-3468(82)80195-4. [DOI] [PubMed] [Google Scholar]
- 15.Nekuda V, Kucera P. Aneuryzmaticka dilatace venae jugularis internae. Ceskoslovenska Otolaryngologie. 1983;32:54–57. [PubMed] [Google Scholar]
- 16.Leung A, Hampson SJ, Singh MP, Carr D. Ultrasonic diagnosis of bilateral congenital internal jugular venous aneurysms. Br J Radiol. 1983;56:588–591. doi: 10.1259/0007-1285-56-668-588. [DOI] [PubMed] [Google Scholar]
- 17.Hughes PL, Qureshi SA, Galloway RW. Jugular venous aneurysm in children. Br J Radiol. 1988;61:1082–1084. doi: 10.1259/0007-1285-61-731-1082. [DOI] [PubMed] [Google Scholar]
- 18.Al-Shaikhi A, Kay S, Laberge JM. External Jugular Venous Aneurysm: An Unusual Cause of a Neck Mass in a Young Child. J Pediatr Surg. 2003;38:1557–1559. doi: 10.1016/s0022-3468(03)00526-8. [DOI] [PubMed] [Google Scholar]
- 19.Rajendran VR, Vasu CK, Regi George AN, et al. Unilateral internal jugular phlebectasia. Ind J Pediatr. 2004;71:751–753. doi: 10.1007/BF02730668. [DOI] [PubMed] [Google Scholar]
- 20.Grange DK, Kaler SG, Albers GM, Petterchak JA, Thorpe CM, deMello DE. Severe bilateral panlobular emphysema and pulmonary arterial hypoplasia: unusual manifestations of Menkes disease. Am J Med Genet. 2005;139:151–155. doi: 10.1002/ajmg.a.31001. [DOI] [PubMed] [Google Scholar]


