Abstract
Objectives
β-Blockers are standard therapy for patients with heart failure (HF). This study compared the effects of chronic monotherapy with 2 different β1-selective adrenoceptor blockers, namely atenolol and metoprolol succinate, on left ventricular (LV) function and remodeling in dogs with coronary microembolization-induced HF [LV ejection fraction (EF) 30–40%].
Methods
Twenty HF dogs were randomized to 3 months of therapy with atenolol (50 mg once daily, n = 6), metoprolol succinate (100 mg, once daily, n = 7) or to no therapy (control, n = 7). LV EF and volumes were measured before initiating therapy and after 3 months of therapy. The change (Δ) in EF and volumes between measurements before and after therapy was calculated and compared among study groups.
Results
In controls, EF decreased and end-systolic volume increased. Atenolol prevented the decrease in EF and the increase in ESV. In contrast, metoprolol succinate significantly increased EF and decreased end-systolic volume. ΔEF was significantly higher and Δend-systolic volume significantly lower in metoprolol succinate-treated dogs compared to atenolol-treated dogs (EF: 6.0 ± 0.86% vs. 0.8 ± 0.85%, p < 0.05; end-systolic volume: −4.3 ± 0.81 ml vs. −1 ± 0.52 ml, p <0.05).
Conclusions
In HF dogs, chronic therapy with atenolol does not elicit the same LV function and remodeling benefits as those achieved with metoprolol succinate.
Key Words: Heart failure, Myocyte hypertrophy, Ventricular remodeling, Gene expression
Introduction
β-Adrenergic receptor blockers (β-blockers) are standard therapy for the medical management of chronic heart failure (HF) [1,2,3]. In patients with HF, chronic β-blockade therapy exerts beneficial effects on left ventricular (LV) function and global LV remodeling, and is associated with improved long-term survival in patients with HF of all etiologies [4, 5]. However, it is not clear whether all β-blockers share these benefits as a ‘class effect’ or whether different formulations exert different effects [6, 7]. To date, only 4 β-blockers, the nonselective β1-/β2-/α1-blocker carvedilol and the selective β1-blockers controlled release/extended release (CR/XL) metoprolol succinate, bisoprolol and, more recently, nebivolol, have been shown to be beneficial in large-scale controlled randomized clinical trials and consequently approved for the treatment of HF [1,2,3]. Atenolol, a selective β1-blocker, is currently approved as an antihypertensive [8] and anti-ischemic drug [9], but is commonly prescribed ‘off-label’ in patients with HF [10, 11], despite the absence of evidence-based efficacy data in this patient population based on the assumption that all β-blockers share the same salutary effects in the treatment of HF [12,13,14,15].
We previously showed that long-term monotherapy with metoprolol CR/XL improves LV ejection fraction (EF) and reduces LV end-systolic volume, indicators of long-term morbidity and mortality, in dogs with chronic HF [16]. Because atenolol is widely used in the treatment of patients with HF, it seemed reasonable to compare its effects on LV function and remodeling to those of metoprolol CR/XL in the same canine model of chronic HF. In the present study, we compared the effects of long-term monotherapy with atenolol to those of metoprolol CR/XL on the progression of LV dysfunction and LV global and cellular remodeling in dogs with intracoronary microembolization-induced HF.
Methods
Experimental Model
The canine model of chronic HF used in this study was previously described in detail [17]. In this preparation, LV dysfunction is produced by multiple sequential intracoronary microembolizations with polystyrene latex microspheres (70–102 μm in diameter), which results in loss of viable myocardium. The model manifests many of the hemodynamic and neurohormonal sequelae of HF observed in humans, including marked and progressive depression of LV systolic and diastolic function, reduced cardiac output and increased LV filling pressures, along with elevated levels of circulating norepinephrine. In the present study, 20 healthy mongrel dogs, weighing between 20 and 30 kg, underwent serial coronary microembolizations to produce HF. Embolizations were performed 1–3 weeks apart and discontinued when LV EF, determined angiographically, was between 30 and 40%. All procedures were performed during cardiac catheterization under general anesthesia and sterile conditions. Induction of anesthesia was initiated with intravenous administration of hydromorphone (0.22 mg/kg) and diazepam (0.17 mg/kg) and a plane of anesthesia was maintained with 1–2% isofluorane. The study was approved by the Henry Ford Health System Institutional Animal Care and Use Committee and conformed to the National Institute of Health Guide and Care for Use of Laboratory Animals.
Study Protocol
Two weeks after the last embolization, dogs underwent a prerandomization cardiac catheterization. One day later, dogs were randomized to 3 months of oral monotherapy with atenolol (50 mg once daily, n = 6), metoprolol CR/XL (100 mg once daily, n = 7) or no therapy at all (control, n = 7). At the end of the follow-up period, a final cardiac catheterization was performed. At the end of the cardiac catheterization and while under general anesthesia, the chest was opened and the heart rapidly removed for histological and biochemical examination. LV tissue samples were obtained from all 20 HF dogs as well as from 6 normal dogs (normal group, n = 6) for comparisons.
Hemodynamic and Angiographic Measurements
Hemodynamic and angiographic measurements were made at baseline, prior to any microembolizations, at the time of randomization, prior to initiation of therapy (pretreatment) and at the end of 3 months of therapy (posttreatment). Aortic and LV pressures were measured with catheter-tip micromanometers (Millar Instruments, Houston, Tex., USA). Pressures, and pressure-derived parameters, were recorded using a Mennen Medical Horizon 9000 A/D data analysis and recording system. All pressures and pressure-derived measures represented the average of consecutive pressure waveforms collected over 10–15 s. Left ventriculograms were obtained with the dog placed on its right side and recorded on 35-mm cinefilm at 30 frames/s during the injection of 20 ml of contrast material (RENO-M-60; Squibb, Princeton, N.J., USA). Correction for image magnification was made with a radiopaque calibrated grid placed at the level of the LV. LV end-systolic and end-diastolic volumes were calculated from LV silhouettes using the area-length method and LV EF was calculated as previously described [17]. Extrasystolic and postextrasystolic beats were excluded from any of the angiographic analysis. LV end-diastolic circumferential wall stress was calculated as described previously [18]; the time constant of isovolumic relaxation, τ, was calculated as described by Weiss et al. [19].
Histomorphometric Measurements
From each heart, 3 transverse slices (approximately 3 mm thick), 1 each from basal, middle and apical thirds of the LV, were obtained. From each slice, transmural tissue blocks were obtained and embedded in paraffin blocks. Transmural tissue blocks were also obtained from the free wall segment of the slice, mounted on cork using Tissue-Tek embedding medium, and rapidly frozen in isopentane precooled in liquid nitrogen and stored at −70°C until used. The volume fraction of replacement fibrosis (VFRF), volume fraction of interstitial fibrosis (VFIF), myocyte cross-sectional area (MCSA), a measure of cardiomyocyte hypertrophy, capillary density and oxygen diffusion distance (ODD) were measured as previously described [20, 21].
mRNA and Protein Expression
mRNA expression of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), adenylyl cyclase 5 (AC5), α-myosin heavy chain (α-MHC) and sarcoplasmic reticulum Ca2+-ATPase (SERCA-2a) was measured. Total RNA with an absorbance ratio (260 nm/280 nm) above 1.7 was isolated from frozen LV tissue, and approximately 4–10 μg RNA was reverse transcribed into cDNA in an assay volume of 80 μl as described previously [22]. mRNA expression of α-MHC was measured by amplification of cDNA by reverse transcriptase polymerase chain reaction followed by digestion with Pst1 restriction enzyme as described previously [23]. Fluorescent band intensity was quantified using a Bio-Rad GS-670 imaging densitometer and expressed in densitometric units. Protein levels of β1-adrenergic receptor (β1-AR), brain natriuretic peptide (BNP) and SERCA-2a were measured in sodium dodecyl sulfate LV homogenate prepared from LV powder by Western blots as described previously [22]. Band intensity was quantified using a Bio-Rad GS-670 imaging densitometer and expressed as densitometric units.
Statistical Analysis
All angiographic, histomorphometric and molecular biology analyses were conducted in a blinded manner. Within group, comparisons of hemodynamic and angiographic variables were made between measurements obtained at time of randomization and measurements made after completion of 3 months of therapy using the Student paired t test with a significance set at p < 0.05. To ensure that all study measures were similar at baseline before any embolizations and at the time of randomization before initiation of therapy (pretreatment), intergroup comparisons were made using a one-way analysis of variance (ANOVA) with α set at 0.05. If the overall ANOVA was significant, then pair-wise comparisons were performed using the Student-Newman-Keuls test. To assess treatment effect, the change (Δ) in each measure from pre- to posttreatment was calculated for each of the 3 study arms. To determine whether significant differences in Δ were present between the control group and each of the 2 treatment groups, ANOVA was performed with α set at 0.05. If the overall ANOVA was significant, then pair-wise comparisons were performed using the Student-Newman-Keuls test. Histomorphometric results as well as mRNA and protein expression results were examined among the 3 study groups and normal dogs using ANOVA with α set at 0.05. If significance was attained by overall ANOVA, pair-wise comparisons were performed using the Student-Newman-Keuls test. For all pair-wise comparisons, a probability value ≤0.05 was considered significant. All data are reported as the mean ± SEM.
Results
Baseline data for all study groups are shown in table 1. All dogs enrolled in the study had baseline hemodynamic and angiographic findings within the normal range for mongrel dogs in our laboratory. There were no significant differences in any of the baseline parameters between control dogs and dogs subsequently randomized to either atenolol or metoprolol CR/XL (table 1). Similarly, there were no significant differences between the 3 study groups in any of the measurements obtained at time of randomization (before treatment; table 2).
Table 1.
Baseline hemodynamic and angiographie measures
| Control (n = 7) | Atenolol (n = 6) | Metoprolol CR/XL (n = 7) | |
|---|---|---|---|
| HR, beats/min | 87 ± 1 | 80 ± 2 | 85 ± 3 |
| Mean AoP, mm Hg | 71 ± 2 | 72 ± 2 | 79 ± 4 |
| LV EDP, mm Hg | 11 ± 1 | 11 ± 1 | 11 ± 1 |
| LV EDV, ml | 53 ± 1 | 52 ± 2 | 54 ± 1 |
| LV ESV, ml | 25 ± 1 | 26 ± 2 | 26 ± 1 |
| LV EF, % | 51 ± 2 | 51 ± 2 | 51 ± 2 |
| Wall stress, g/cm2 | 40 ± 3 | 41 ± 2 | 43 ± 2 |
| τ, ms | 31 ± 1 | 32 ± 1 | 33 ± 1 |
HR = Heart rate; AoP = aortic pressure; EDP = end-diastolic pressure; EDV = end-diastolic volume; ESV = end-systolic volume.
Table 2.
Hemodynamic and angiographic measures before- and after treatment
| Before treatment |
After treatment |
p value |
|
|---|---|---|---|
| Control (n = 7) | |||
| HR, beats/min | 84 ± 2 | 84 ± 2 | 0.851 |
| Mean AoP, mm Hg | 73 ± 3 | 76 ± 4 | 0.668 |
| LV EDP, mm Hg | 14 ± 1 | 15 ± 1 | 0.356 |
| LV EDV, ml | 60 ± 1 | 63 ± 1 | 0.006 |
| LV ESV, ml | 38 ± 1 | 44 ± 1 | <0.001 |
| LV EF, % | 36 ± 1 | 31 ± 1 | <0.001 |
| Wall stress, g/cm2 | 54 ± 1 | 57 ± 3 | 0.341 |
| τ, ms | 44 ± 1 | 54 ± 1 | <0.001 |
| Atenolol (n = 6) | |||
| HR, beats/min | 81 ± 3 | 85 ± 2 | 0.234 |
| Mean AoP, mm Hg | 75 ± 4 | 73 ± 3 | 0.544 |
| LV EDP, mm Hg | 14 ± 1 | 13 ± 1 | 0.137 |
| LV EDV, ml | 60 ± 2 | 60 ± 2 | 0.771 |
| LV ESV, ml | 37 ± 1 | 36 ± 1 | 0.111 |
| LV EF, % | 38 ± 1 | 39 ± 1 | 0.137 |
| Wall stress, g/cm2 | 61 ± 6 | 55 ± 5 | 0.215 |
| τ, ms | 45 ± 1 | 42 ± 2 | 0.195 |
| Metoprolol CR/XL (n = 7) | |||
| HR, beats/min | 84 ± 3 | 82 ± 2 | 0.594 |
| Mean AoP, mm Hg | 75 ± 3 | 77 ± 4 | 0.567 |
| LV EDP, mm Hg | 15 ± 1 | 12 ± 1 | 0.007 |
| LV EDV, ml | 62 ± 2 | 61 ± 3 | 0.188 |
| LV ESV, ml | 39 ± 2 | 35 ± 2 | 0.002 |
| LV EF, % | 37 ± 1 | 43 ± 1 | <0.001 |
| Wall stress, g/cm2 | 58 ± 3 | 48 ± 3 | 0.005 |
| τ, ms | 45 ± 3 | 36 ± 1 | 0.008 |
HR = Heart rate; AoP = aortic pressure; EDP = end-diastolic pressure; EDV = end-diastolic volume; ESV = end-systolic volume.
Hemodynamic and Angiographic Findings
Heart rate and mean aortic pressure remained essentially unchanged in all 3 study groups after 3 months of therapy (table 2). LV end-diastolic pressure did not change significantly in control and atenolol dogs, but decreased significantly in dogs randomized to metoprolol CR/XL. Three months after randomization, control dogs showed a significant deterioration of LV EF accompanied by a significant increase in LV end-diastolic and end-systolic volume (table 2). No significant differences in LV EF and volumes were observed between before and after treatment in dogs treated with atenolol. In dogs treated with metoprolol CR/XL, LV EF increased significantly and LV end-systolic volume decreased significantly, whereas LV end-diastolic volume remained essentially unchanged (table 2). LV end-diastolic circumferential wall stress did not change in control and atenolol-treated dogs, but decreased significantly in the metoprolol CR/XL group. The time constant of isovolumic relaxation, τ, increased significantly in control dogs and decreased significantly in metoprolol CR/XL-treated dogs, but remained essentially unchanged in dogs randomized to atenolol.
Comparisons of Treatment Effect
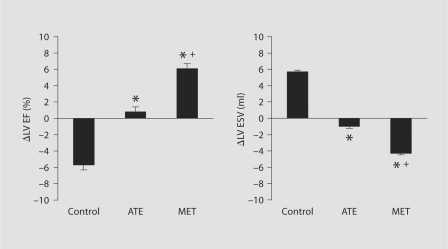
Between-group comparisons of the change (Δ) between pre- and posttreatment measurements are shown in table 3 and figure 1. There were no significant differences in heart rate and mean aortic pressure among the 3 study groups. Compared to control, both atenolol and metoprolol CR/XL prevented the increase in LV end-diastolic pressure and volume (table 3). Compared with controls, atenolol prevented the decline in LV EF and the increase in LV end-systolic volume (fig. 1). A similar trend, but to a greater extent, was seen in metoprolol CR/XL-treated dogs. Metoprolol CR/XL significantly reduced LV end-systolic volume and significantly increased LV EF compared with control dogs and dogs receiving atenolol (fig. 1). Furthermore, metoprolol CR/XL but not atenolol significantly decreased LV end-diastolic circumferential wall stress (table 3). Both atenolol and metoprolol CR/XL induced a significant shortening of the time constant of isovolumic relaxation, τ, albeit more evident in the metoprolol CR/XL group (table 3).
Table 3.
Treatment effect (Δ) comparison between the 3 study groups
| Control (n = 7) | Atenolol (n = 6) | Metoprolol CR/XL (n = 7) | |
|---|---|---|---|
| ΔHR, beats/min | −0.3 ± 1.5 | 4.5 ± 3.3 | −2.6 ± 4.6 |
| ΔMean AoP, mm Hg | 2.8 ± 6.3 | −1.2 ± 2.3 | 1.7 ± 5.2 |
| ΔLV EDP, mm Hg | 0.8 ± 0.9 | −1.5 ± 0.6∗ | −2.4 ± 0.6∗ |
| ΔLV EDV, ml | 3.3 ± 0.8 | −0.8 ± 0.5∗ | −1.3 ± 0.9∗ |
| ΔLV ESV, ml | 5.7 ± 0.7 | −1.0 ± 0.5∗ | −4.3 ± 0.8∗> + |
| ΔLV EF, % | −5.7 ± 0.7 | 0.8 ± 0.9∗ | 6.1 ± 0.9∗> + |
| ΔWall stress, g/cm2 | 3.2 ± 3.1 | −6.0 ± 4.5 | −10.0 ± 2.4∗ |
| Δτ, ms | 10.0 + 1.1 | −4.0 ± 2.6∗ | −9.0 ± 2.2∗ |
HR = Heart rate; AoP = aortic pressure; EDP = end-diastolic pressure; EDV = end-diastolic volume; ESV = end-systolic volume.
p < 0.05 vs. control; + p < 0.05 vs. atenolol.
Fig. 1.
Bar graphs depicting change (Δ) in LV EF and LV end-systolic volume (LV ESV) between pre- and posttreatment in control dogs, dogs randomized to atenolol (ATE) or dogs randomized to metoprolol CR/XL (MET). ∗ p < 0.05 vs. control; + p < 0.05 vs. ATE.
Histomorphometric Findings
Histomorphometric findings are shown in table 4. VFRF, VFIF, ODD and MCSA were significantly higher, while capillary densities were significantly lower in control than in normal animals. There was no significant difference in VFRF between control and dogs randomized to either atenolol or metoprolol CR/XL. Both treatments with atenolol and metoprolol CR/XL significantly reduced VFIF, ODD and MCSA, while increasing capillary density compared to control dogs (table 4). However, the extent of the normalization in all these parameters was significantly greater with metoprolol CR/XL than with atenolol (table 4).
Table 4.
Histomorphometric measures
| Normal (n = 6) | Control (n = 7) | Atenolol (n = 6) | Metoprolol CR/XL (n = 7) | |
|---|---|---|---|---|
| VFRF, % | 0.0 | 12.7 ± 1.3a | 11.2 ± 1.7a | 10.0 ± 1.4a |
| VFIF, % | 3.7 ± 0.1 | 14.2 ± 0.8a | 12.2 ± 0.3a,b | 9.7 ± 0.3abc |
| Cap/mm2 | 2,607 ± 80 | 1,786 ± 71a | 2,020 ± 24ab | 2,216 ± 22abc |
| Cap/cell | 1.00 ± 0.0 | 0.89 ± 0.04a | 0.99 ± 0.00b | 1.08 ± 0.00abc |
| ODD, μm | 8.9 ± 0.2 | 11.9 ± 0.2a | 11.1 ± 0.2ab | 10.3 ± 0.3abc |
| MCSA, μm2 | 409 ± 10 | 687 ± 26a | 629 ± 4a,b | 561 ± 4abc |
VFRF = Volume fraction of replacement fibrosis; VFIF = volume fraction of interstitial fibrosis; Cap/mm2 = capillary density for mm2; Cap/cell = capillary density for fiber; ODD = oxygen diffusion distance; MCSA = myocyte cross-sectional area.
p < 0.05 vs. normal
p < 0.05 vs. control
p < 0.05 vs. atenolol.
mRNA and Protein Expression
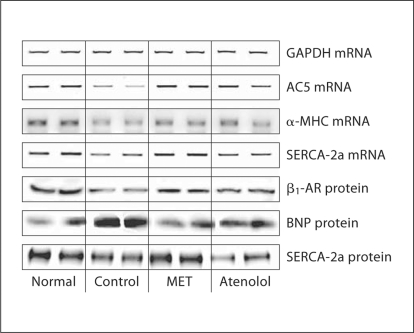
Results from mRNA and protein expression analyses are shown in tables 5 and 6 and in figure 2. As expected, expression of GAPDH was the same across all 4 study groups. mRNA expression of SERCA-2a and AC5 decreased significantly in control dogs compared to normal dogs. Atenolol had no effect on the expression of SERCA-2a compared to control, while metoprolol CR/XL normalized SERCA-2a mRNA expression. Both atenolol and metoprolol CR/XL, albeit to a greater extent with the latter, significantly increased the mRNA level of α-MHC when compared to control. Therapy with atenolol significantly increased the mRNA expression of AC5, whereas therapy with metoprolol CR/XL restored the mRNA expression of AC5 to near normal levels. Protein expression of β1-AR and SERCA-2a decreased and that of BNP increased significantly in control dogs compared to normal dogs. With both atenolol and metoprolol CR/XL we observed an increase, although not significant, in the expression of β1-AR. Therapy with metoprolol CR/XL but not with atenolol normalized the protein expression of SERCA-2a and BNP.
Table 5.
mRNA expression of housekeeping, fetal program and sarcoplasmic reticulum genes in LV free wall
| Normal (n = 6) | Control (n = 7) | Atenolol (n = 6) | Metoprolol CR/XL (n = 7) | |
|---|---|---|---|---|
| GAPDH | 143 ± 6 | 154 ± 5 | 151 ± 2 | 151 ± 2 |
| SERCA-2a | 119 ± 4 | 82 ± 6a | 85 ± 11a | 118 ± 3b-c |
| α-MHC | 55 ± 2 | 37 ± 2a | 43 ± 2a,b | 47 ± 2a,b |
| AC5 | 4,996 ± 163 | 2,306 ± 403a | 3,739 ± 685ab | 5,315 ± 230b-c |
All figures are densitometric units.
p < 0.05 vs. normal
p < 0.05 vs. control
p < 0.05 vs. atenolol.
Table 6.
Expression of fetal program and sarcoplasmic reticulurr. proteins in LV free wall
| Normal (n = 6) | Control (n = 7) | Atenolol (n = 6) | Metoprolol CR/XL (n = 7) | |
|---|---|---|---|---|
| β1-AR | 15.9 ± 2.9 | 5.5 ± 0.3 | 10.4 ± 0.9 | 10.0 ± 1.2 |
| BNP | 10.2 + 1.2 | 31.9+1.9 | 20.6+1.9 | 11.6 + 1.6 |
| SERCA-2a | 64.1 ± 1.8 | 49.0 ± 1.3 | 52.0 ± 2.1 | 63.4 ± 1.9 |
All figures are densitometric units.
Fig. 2.
Representative ethidium bromide agarose gel showing mRNA encoding GAPDH, AC5, α-MHC and SERCA-2a as well as protein encoding β1-AR, BNP and SERCA-2a in LV myocardium of 2 normal dogs, 2 dogs with HF randomized to vehicle (control), 2 dogs with HF treated with metoprolol CR/XL (MET) and 2 dogs with HF treated with atenolol.
Discussion
Neurohormonal activation occurs in HF initially as a compensatory response but ultimately contributes to progression of LV dysfunction and remodeling [24]. The enhanced and sustained sympathoadrenergic activation mediates many of the hemodynamic, functional and structural cardiac as well as circulatory maladaptations that occur with HF [24]. Inhibition of the sympathoadrenergic drive with β-blockers has been shown to prevent, halt or even reverse the vast majority of these negative changes in both experimental and clinical settings [24]. However, β-blockers are a heterogeneous group of drugs, with different pharmacodynamic and pharmacokinetic profiles, and may not be equivalent with respect to their efficacy for the treatment of chronic HF [25].
The results of the present study provide the first direct experimental evidence of a different effect of 2 β1-selective adrenergic receptor blockers, metoprolol CR/XL and atenolol, in the treatment of chronic HF. While long-term therapy with atenolol tended to prevent progressive LV dysfunction and remodeling, it failed to improve these hemodynamic and structural outcomes. In contrast, dogs randomized to metoprolol CR/XL showed a significant improvement of LV systolic and diastolic function as shown by increased EF, decreased end-systolic volume, decreased wall stress, decreased end-diastolic pressure and shortened time constant of isovolumic relaxation, τ. Both metoprolol CR/XL and atenolol prevented the increase in LV end-diastolic volume observed in the control dogs. At the cellular level, the observed greater beneficial effects of metoprolol CR/XL on LV function and global remodeling corresponded to reduced interstitial fibrosis and cardiomyocyte hypertrophy along with greater capillary density. In a similar fashion, dogs receiving metoprolol CR/XL manifested a more extensive reversal of the maladaptive LV cellular and biochemical remodeling than that achieved with atenolol, with normalization of many of the key components of the fetal gene program, such as SERCA-2a, α-MHC, AC5 and BNP. Taken together, these data suggest that long-term monotherapy with metoprolol CR/XL provides a more favorable effect than atenolol on LV functional and structural remodeling in dogs with HF.
No direct comparative studies on the effects of atenolol and metoprolol on LV dysfunction and remodeling in HF are available in the literature. In general, β1-selective adrenergic receptor blockers have been reported to exert positive effects on cardiac function and remodeling in patients with HF [4, 5, 7,12,13,14,15]. However, evidence from experimental studies suggests that these agents differ with respect to the mechanisms through which such improvements are achieved. Bisoprolol, for instance, the most β1-selective and β1-potent agent among β1-selective blockers [25], was shown to improve survival in rats with postinfarction HF without exerting any significant benefit on LV hemodynamic, function, global and structural remodeling [26, 27]. Findings from animal models of HF suggest that atenolol may positively affect LV structural remodeling by reducing fibrosis through modulation of matrix metalloproteinases [28], thus leading to decreased chamber stiffening and improved LV mechanical performance [28, 29].
In agreement with the findings of the present study, previous reports from our group showed that metoprolol CR/XL elicits protective activity on LV global remodeling and significantly improves LV hemodynamic, systolic and diastolic dysfunction in dogs with HF [16]. The mechanisms underlying these beneficial effects are thought to be related to the marked reduction of interstitial fibrosis and cardiomyocyte hypertrophy as well as increased capillary density at the tissue level. At the cellular level, the observed increase in LV EF following long-term therapy with metoprolol CR/XL is associated with improvement of calcium handling, activation of anti-apoptotic pathways and correction of myocardial energetic abnormalities [24]. In addition, therapy with metoprolol CR/XL inhibits the induction of fetal gene program, restoring a normal gene expression of myosin heavy chain isoforms, and downregulates mRNA gene expression of natriuretic peptides and matrix metalloproteinases in LV myocardium from dogs with HF [24].
The present study was designed to address an issue of clinical relevance, namely the potential equivalence of evidence-based and non-evidence-based β-blockers in the treatment of HF. For this reason, we selected metoprolol succinate as representative of β-blockers of proven efficacy in the treatment of HF from randomized controlled trials [1,2,3] and atenolol for β-blockers not recommended for treatment of HF. Atenolol was chosen because of the high prescription rate of this agent in large HF registries in clinical practice [10, 11]. In order to avoid potential bias related to choice of doses, we selected doses and frequency of administration of atenolol and metoprolol CR/XL commonly recommended in treatment guidelines [1,2,3, 8, 9], accepted to be equivalent [25] and previously used in comparative studies [30]. Current recommendations for the use of atenolol in hypertension and chronic stable angina is once daily [8, 9] and metoprolol CR/XL for HF is also once daily [1,2,3]. Comparisons in healthy volunteers and hypertensive or stable patients after myocardial infarction have been performed between metoprolol CR/XL 100 mg once daily and atenolol 50 mg once daily or metoprolol CR/XL 200 mg once daily and atenolol 100 mg once daily [30]. These studies provide compelling evidence to support the appropriateness of the selection of the doses of both metoprolol CR/XL and atenolol used in the present study. Our results, therefore, likely reflect actual differences in the intrinsic properties of these 2 agents. Atenolol and metoprolol possess substantially similar β-antagonist and pharmacokinetic properties [25]. Both drugs are highly selective β1-receptor blockers with equivalent β1-potency devoid of intrinsic sympathomimetic activity and membrane-stabilizing effect, and, at high doses, both may inhibit β2-receptors [25]. Selectivity of β1-receptor blockers has been studied in both animal models and living mammalian cells. Comparison of β1-receptor blockers in the ferret myocardium demonstrates that both atenolol and metoprolol have similar blocking and binding constants as well as similarity in their positive response to inotropic effects of isoprenaline [31]. In mammalian cells, both atenolol and metoprolol demonstrate similar degrees of β1-, β2- and β3-selectivity [32]. The effectiveness of these drugs depends not only on receptor affinity, but also on drug absorption, metabolism and tissue distribution. Bioavailability of atenolol and metoprolol is comparable, although atenolol presents a lower absorption and is not soluble in lipids [25]. Nonlipophilicity is the only difference in the ancillary properties of these agents and this may be of clinical relevance [33, 34]. It has been suggested that lipophilic β-blockers may exert a better effect on survival than hydrophilic agents in the secondary prevention of acute myocardial infarction [33, 34]. The theoretical advantage of lipophilic β-blockers is their ability to readily cross the blood-brain barrier, thus entering the central nervous system and thereby improving or restoring vagal tone and exerting anti-arrhythmic effects [33]. However, treatment with atenolol has also been found to increase indices of vagal tone, through peripheral rather than central β-blockade, with positive effects on the sympathovagal balance equivalent or even superior to those of metoprolol CR/XL [35]. Important differences in the pharmacodynamic profiles of atenolol and metoprolol may also account for the differences observed in our study. When compared to atenolol (50 mg once daily) over a 24-hour interval, metoprolol CR/XL (100 mg once daily) shows a more even plasma concentration, with lower fluctuation ratio and longer time period of concentration higher than 50% of maximum concentration [36]. In addition, the low peak plasma concentration produced by metoprolol CR/XL may lead to higher β1-selectivity than equivalent doses of atenolol [30], while cardioselectivity is maintained up to dosages of 200 mg of metoprolol CR/XL [37, 38].
Administration of β-blockers results in a reduction in heart rate and blood pressure in both experimental and clinical settings [25]. These effects are beneficial in many cardiovascular disease conditions such as hypertension, ischemic heart disease and HF. β-blockade therapy, when used, is often titrated to achieve a resting heart rate of approximately 60 beats/min and a blunting of the exercise-induced tachycardia response [1,2,3, 8, 9]. The apparent lack of change in resting heart rates observed in our study at the different study time points is most likely due to the fact that heart rates reported in the study refer to values recorded under general anesthesia and, therefore, do not truly reflect the actual resting ambulatory heart rates of the animals. Ambulatory ECG Holter monitoring, had it been done, would have provided additional insights into the mechanisms of action of atenolol and metoprolol in this animal preparation. Another potential limitation of the present study is lack of measurements of plasma norepinephrine or other markers of activation of the sympathetic nervous system. In previous studies of metoprolol tartrate using this canine model of HF, we have shown little or no reduction in plasma norepinephrine following long-term treatment [39]. Measurements of ambulatory heart rates as well as plasma norepinephrine concentration would have helped clarify whether the effects of both drugs at the structural, cellular and molecular level are mostly mediated through hemodynamic influences or mostly reflect direct myocardial actions.
In conclusion, the findings of the present study indicate that, in dogs with HF, long-term therapy with metoprolol CR/XL provides greater benefits than atenolol on LV dysfunction as well as global and cellular remodeling. These results do not support the use of atenolol as a replacement for metoprolol CR/XL to treat chronic HF.
Acknowledgements
Supported, in part, by research grants from AstraZeneca US and National Heart, Lung and Blood Institute (PO1 HL074237-04).
References
- 1.Adams KF, Lindenfeld J, Arnold JMO. Executive summary: HFSA 2006, comprehensive heart failure practice guidelines. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez-Sendon JL, Nieminen MS, Pierard L, Remme WJ. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 4.Frigerio M, Roubina E. Drugs for left ventricular remodeling in heart failure. Am J Cardiol. 2005;96:10L–18L. doi: 10.1016/j.amjcard.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Colucci WS, Swedberg K. β-Blockers in chronic heart failure. Circulation. 2003;107:1570–1575. doi: 10.1161/01.CIR.0000065187.80707.18. [DOI] [PubMed] [Google Scholar]
- 6.Bauman JL, Talbert RL. Pharmacodynamics of β-blockers in heart failure: lessons from the carvedilol or metoprolol European trial. J Cardiovasc Pharmacol Ther. 2004;9:117–128. doi: 10.1177/107424840400900207. [DOI] [PubMed] [Google Scholar]
- 7.Metra M, Dei Cas L, Poole-Wilson P. β-Blockers in heart failure: are pharmacological differences clinically important? Heart Fail Rev. 2004;9:131–137. doi: 10.1023/B:HREV.0000046367.99002.a4. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O'Rourke RA, Pasternak RC, Williams SV. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina – summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients with Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 10.Komajda M, Follath F, Swedberg K, Cleland J, Aguilar JC, Cohen-Solal A, Dietz R, Gavazzi A, Van Gilst WH, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, Widimsky J, Freemantle N, Eastaugh J, Mason J. The Euroheart failure survey programme – a survey on the quality of care among patients with heart failure in Europe. Part 2. Treatment. Eur Heart J. 2003;24:464–474. doi: 10.1016/s0195-668x(02)00700-5. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Radford MJ, Wang Y, Chen J, Heiat A, Marciniak TA. National use and effectiveness of beta-blockers for the treatment of elderly patients after acute myocardial infarction: National Cooperative Cardiovascular Project. JAMA. 1998;280:623–629. doi: 10.1001/jama.280.7.623. [DOI] [PubMed] [Google Scholar]
- 12.Hulsmann M, Sturm B, Pacher R, Berger R, Bojic A, Frey B, Stanek B. Long-term effect of atenolol on ejection fraction, symptoms, and exercise variables in patients with advanced left ventricular dysfunction. J Heart Lung Transplant. 2001;20:1174–1180. doi: 10.1016/s1053-2498(01)00341-2. [DOI] [PubMed] [Google Scholar]
- 13.Mattioli AV, Modena MG, Fantini G, Mattioli G. Atenolol in dilated cardiomyopathy: a clinical instrumental study. Cardiovasc Drugs Ther. 1990;4:505–507. doi: 10.1007/BF01857761. [DOI] [PubMed] [Google Scholar]
- 14.Rousseau MF, Chapelle F, Van Eyll C, Stoleru L, Hager D, Van Nueten L, Pouleur H. Medium-term effects of beta-blockade on left ventricular mechanics: a double-blind, placebo-controlled comparison of nebivolol and atenolol in patients with ischemic left ventricular dysfunction. J Card Fail. 1996;2:15–23. doi: 10.1016/s1071-9164(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 15.Sturm B, Pacher R, Strametz-Juranek J, Berger R, Frey B, Stanek B. Effect of β1 blockade with atenolol on progression of heart failure in patients pretreated with high-dose enalapril. Eur J Heart Fail. 2000;2:407–412. doi: 10.1016/s1388-9842(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 16.Morita H, Suzuki G, Mishima T, Chaudhry PA, Anagnostopoulos PV, Tanhehco EJ, Sharov VG, Goldstein S, Sabbah HN. Effects of long-term monotherapy with metoprolol CR/XL on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Cardiovasc Drugs Ther. 2002;16:443–449. doi: 10.1023/a:1022142620189. [DOI] [PubMed] [Google Scholar]
- 17.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am J Physiol. 1991;260:H1379–1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 18.Grossman W. Pressure measurements. In: Grossman W, Baim DS, editors. Cardiac Catheterization, Angiography, and Intervention. Philadelphia: Williams & Wilkins; 1991. p. 123. [Google Scholar]
- 19.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976;58:751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure: role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, Hegde S, Goldstein S. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation. 2000;102:1990–1995. doi: 10.1161/01.cir.102.16.1990. [DOI] [PubMed] [Google Scholar]
- 22.Rastogi S, Mishra S, Zacà V, Mika Y, Rousso B, Sabbah HN. Effects of chronic therapy with cardiac contractility modulation electrical signals on cytoskeletal proteins and matrix metalloproteinases in dogs with heart failure. Cardiology. 2007;110:230–237. doi: 10.1159/000112405. [DOI] [PubMed] [Google Scholar]
- 23.Feldman AM, Ray PE, Silan CM, Mercer JA, Minobe W, Bristow MR. Selective gene expression in failing human heart: quantification of steady-state levels of messenger rna in endomyocardial biopsies using the polymerase chain reaction. Circulation. 1991;83:1866–1872. doi: 10.1161/01.cir.83.6.1866. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah HN. Biologic rationale for the use of β-blockers in the treatment of heart failure. Heart Fail Rev. 2004;9:91–97. doi: 10.1023/B:HREV.0000046363.59374.23. [DOI] [PubMed] [Google Scholar]
- 25.Reiter MJ. Cardiovascular drug class specificity: β-blockers. Prog Cardiovasc Dis. 2004;47:11–33. doi: 10.1016/j.pcad.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Hu K, Gaudron P, Ertl G. Long-term effects of β-adrenergic blocking agent treatment on hemodynamic function and left ventricular remodeling in rats with experimental myocardial infarction: Importance of timing of treatment and infarct size. J Am Coll Cardiol. 1998;31:692–700. doi: 10.1016/s0735-1097(97)00527-5. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Ohta Y, Inoue M, Ma M, Wahed MI, Nakazawa M, Hasegawa G, Naito M, Fuse K, Ito M, Kato K, Hanawa H, Kodama M, Aizawa Y. Bisoprolol improves survival in rats with heart failure. J Cardiovasc Pharmacol. 2001;38(suppl 1):S55–S58. doi: 10.1097/00005344-200110001-00012. [DOI] [PubMed] [Google Scholar]
- 28.Senzaki H, Paolocci N, Gluzband YA, Lindsey ML, Janicki JS, Crow MT, Kass DA. β-Blockade prevents sustained metalloproteinase activation and diastolic stiffening induced by angiotensin II combined with evolving cardiac dysfunction. Circ Res. 2000;86:807–815. doi: 10.1161/01.res.86.7.807. [DOI] [PubMed] [Google Scholar]
- 29.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, Beaufils P, Delcayre C, Hatem SN. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 30.Agewall S, Kendall M. Treatment with β-blockers – the value of an even plasma concentration over 24 h. J Clin Pharm Ther. 1997;22:171–179. doi: 10.1046/j.1365-2710.1997.8775087.x. [DOI] [PubMed] [Google Scholar]
- 31.Lowe MD, Lynham JA, Grace AA, Kaumann AJ. Comparison of the affinity of beta-blockers for two states of the β1-adrenoceptor in ferret ventricular myocardium. Br J Pharmacol. 2002;135:451–461. doi: 10.1038/sj.bjp.0704450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kendall MJ. Clinical relevance of pharmacokinetic differences between β-blockers. Am J Cardiol. 1997;80:15J–19J. doi: 10.1016/s0002-9149(97)00833-3. [DOI] [PubMed] [Google Scholar]
- 34.Soriano JB, Hoes AW, Meems L, Grobbee DE. Increased survival with β-blockers: Importance of ancillary properties. Prog Cardiovasc Dis. 1997;39:445–456. doi: 10.1016/s0033-0620(97)80039-4. [DOI] [PubMed] [Google Scholar]
- 35.Kardos A, Long V, Bryant J, Singh J, Sleight P, Casadei B. Lipophilic versus hydrophilic β1 blockers and the cardiac sympatho-vagal balance during stress and daily activity in patients after acute myocardial infarction. Heart. 1998;79:153–160. doi: 10.1136/hrt.79.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blomqvist I, Westergren G, Sandberg A, Jonsson UE, Lundborg P. Pharmacokinetics and pharmacodynamics of controlled-release metoprolol: a comparison with atenolol. Eur J Clin Pharmacol. 1988;33(suppl):S19–S24. doi: 10.1007/BF00578408. [DOI] [PubMed] [Google Scholar]
- 37.Andersson B, Aberg J, Lindelow B, Tang MS, Wikstrand J. Dose-related effects of metoprolol on heart rate and pharmacokinetics in heart failure. J Card Fail. 2001;7:311–317. doi: 10.1054/jcaf.2001.28230. [DOI] [PubMed] [Google Scholar]
- 38.Wikstrand J. Achieving optimal β1-blockade with metoprolol CR/Zok. Basic Res Cardiol. 2000;95(suppl 1):I46–I51. doi: 10.1007/s003950070009. [DOI] [PubMed] [Google Scholar]
- 39.Sabbah HN, Shimoyama H, Kono T, Gupta RS, Sharov VG, Scicli G, Levine TB, Goldstein S. Effects of long-term monotherapy with enalapril, metoprolol and digoxin on the progression of left ventricular dysfunction and dilation in dogs with reduced ejection fraction. Circulation. 1994;89:2852–2859. doi: 10.1161/01.cir.89.6.2852. [DOI] [PubMed] [Google Scholar]