Abstract
Levicoleps biwae n. gen., n. sp. was discovered in organic mud on the shore of Lake Biwa, Japan. Its morphology and small subunit rRNA gene sequence were studied with standard methods. Further, we established a terminology for the colepid armour and selected four features for genus recognition: the number of armour tiers, the structure of the tier plates, the presence/absence of armour spines, and the number of adoral organelles (three or five). The Japanese colepid, a barrel-shaped ciliate with an average size of 75 × 45 μm, has six armour tiers and hirtus-type tier plates, but lacks armour spines, both in the environment and in laboratory culture. Thus, it is considered to represent a new genus. This rank is supported by the considerable genetic distance (7%) from the common Coleps hirtus. Although L. biwae looks quite similar to C. hirtus in vivo, it is very likely most closely related to Coleps amphacanthus, a species with conspicuous armour spines, as indicated by body size, the number of ciliary rows and, especially, the multiple caudal cilia. Lake Biwa is about four million years old and inhabited by many endemic organisms, ranging from algae to large fish. Thus, we suspect that L. biwae is restricted to Lake Biwa or, at least, to Asia. Based on literature data and the generic features established, we also propose the new genus Reticoleps for Coleps remanei Kahl, 1933, and resurrect the genus Pinacocoleps Diesing, 1865 to include Coleps incurvus Ehrenberg, 1833, Coleps pulcher Spiegel, 1926, Coleps tessalatus Kahl, 1930 and, probably, Baikalocoleps quadratus Obolkina, 1995a. Nine colepid genera are diagnosed and dichotomously keyed.
Keywords: Biogeography, Coleps, endemic protists, Pinacocoleps, Reticoleps n. gen.
CILIATES of the family Colepidae Ehrenberg, 1838 are characterized by an armour composed of complex, calcified plates (Corliss 1979). They occur in a conspicuous variety of habitats: in freshwater and the sea, in the benthos and plankton, and even in the limnetic and marine psammon (Dragesco and Dragesco-Kernéis 1991; Kahl 1930, 1935; Obolkina 1995a, b). Probably, many species wait to be discovered, especially in the poorly studied interstitial of large freshwater lakes and sandy marine shores.
Kahl (1930, 1935), the last reviser of the colepids, recognized 14 valid and two doubtful Coleps species. Since then, several new colepid genera and about 20 new species have been described, according to the Zoological Record, for instance, by Foissner (1983, 1984), Lepsi (1962), Noland (1937), and Vacelet (1961). Unfortunately, most descriptions are very incomplete, both in the past and present. For instance, plate details were studied mainly by Kahl (1930, 1935), and the reported lack of a circumoral kinety in Planicoleps, Baikalocoleps, Kotinia, Macrocoleps, and Tiarinella (Dragesco and Dragesco-Kernéis 1991; Obolkina 1995a) is very likely caused by insufficient preparations. Detailed investigations and redescriptions, including scanning electron microscopy (SEM) of individual armour plates, are available for only a few common freshwater species (Foissner 1984; Foissner, Berger, and Kohmann 1994; Foissner, Berger, and Schaumburg 1999; Huttenlauch 1985, 1986, 1987; Wilbert and Schmall 1976).
The poor knowledge of colepids is unfortunate because they are, due to the complex armour plates, ideal for investigating the hotly discussed question of whether or not endemic, free-living micro-organisms exist (for a review, see Foissner 2006). At the present state of knowledge, Planicoleps occurs only in Lake Tanganyika (Africa), and the genera Baikalocoleps, Kotinia, Macrocoleps, and Tiarinella seem to be restricted to Lake Baikal. We investigated a third ancient freshwater lake, the 4-million-year-old Lake Biwa in Japan, and immediately recognized a special colepid in the shore mud. This new species, which also represents a new genus, is described here in great detail so that later researchers can reliably compare it with species from other biogeographic regions.
MATERIALS AND METHODS
Materials
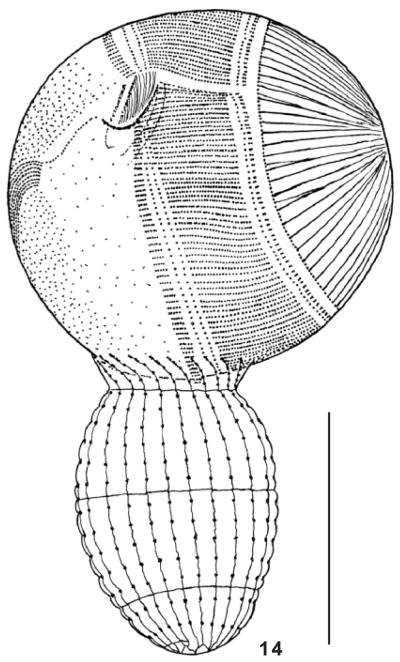
Levicoleps biwae n. gen., n. sp. was discovered in a manually taken mud sample from the flat shore of Lake Biwa at the end of November, 2006. The site was very near to the Lake Biwa Museum and contained various filamentous algae and decaying water plants, especially Nelumbo nucifera. See Rossiter (2000) for a detailed description of the lake. Levicoleps biwae, which was moderately abundant above and in the mud, could not be found in two plankton samples taken with a fine-meshed net from the north and south basin of the lake. In the environmental sample, L. biwae fed on coccoid green algae, Trachelomonas, dinoflagellates, and even on rather large and highly mobile ciliates, such as Urocentrum turbo (Fig. 14). In the laboratory, L. biwae could be cultivated on Eau de Volvic enriched with some squashed wheat grains and a few milliliters of natural mud. Here, it engulfed heterotrophic flagellates, Euplotes, and even large starch grains from the squashed wheat kernels. Levicoleps biwae grew well in such raw cultures for some months, but then became smaller and smaller and declined, even in fresh medium. Pure cultures with some middle-sized ciliates and flagellates as a food source were not successful. When feeding on large prey, the circumoral tier and the anterior tier opened widely, and one has the impression that L. biwae nibbles at the prey (Fig. 14).
Fig. 14.
Levicoleps biwae n. gen., n. sp. feeds, inter alia, on the large Urocentrum turbo, by widely opening the mouth and the circumoral and anterior secondary armour tier. Scale bar: 50 μm.
Morphological methods
Specimens from the environmental sample and the raw cultures were used for the investigations. Living cells were studied using a high-power oil immersion objective and differential interference contrast. Various silver impregnation methods and SEM of washed, air-dried cells were used to reveal the ciliary pattern, cytological details, and the calcified armour. All these methods are described in Foissner (1991). Counts and measurements on silvered specimens were performed at a magnification of 1,000X. In vivo measurements were conducted at magnifications of 100–1,000X. Illustrations of live specimens were based on free-hand sketches and micrographs; those of impregnated cells were made with a drawing device. Coleps hirtus, which occurred in the same sample, was studied for comparison.
Molecular methods
Single live cells of L. biwae were isolated under a light microscope and transferred to sterile Mili-Q water droplets 2 times to facilitate the removal of contaminants, suspended in 2 μl of sterile Mili-Q water, and placed in 0.2-ml thin-walled polymerase chain reaction (PCR) tubes. Samples were then frozen at −20 °C until analysis.
PCR amplification
The first round of PCR amplification was carried out on single cells, using two sets of external primers, SR1 and SR12 (Nakayama et al. 1996) and the PCR protocol of Puitika et al. (2007).
Cloning, sequencing, and tree construction
PCR products were purified with Wizard® SV Gel and a PCR Clean-Up System (Promega, Madison, WI). Sequencing of three L. biwae specimens was performed on an ABI PRISM® 310 Genetic Analyzer (Applied Biosystems, Inc., Foster City, CA) for both DNA strands, using the primers SR1, SR3, SR6, and SR8–SR12 of Nakayama et al. (1996) and the ciliate-specific primer set CS 322 F and EU929R of Puitika et al. (2007).
The L. biwae sequences and reference sequences from the nucleotide sequence library (NCBI) were aligned with CLUSTAL X 1.83 (Thompson et al. 1997). Phylogenetic trees were generated using neighbour-joining (NJ), maximum parsimony (MP), and maximum likelihood (ML). Neighbour-joining analysis was conducted using the program package MEGA 4.0 (Tamura et al. 2007). Distances were estimated by the NJ method with the TrN model of substitution (Tamura and Nei 1993) and with the assumption of rate heterogeneity among sites. The γ-shaped parameters (α) were estimated with eight categories from PUZZLE version 5.2 (Strimmer and von Haeseler 1996). The proportion of invariable sites was 2.237 for the data sets. The transition/transversion ratio of the Hasegawa, Kishino, and Yano (1985) model was estimated by maximizing the likelihood value for the NJ topology. The statistical significance of the tree branches was assessed by 1,000 bootstrap resamplings (Felsenstein 1985). Maximum parsimony analysis was conducted using default settings in MEGA (Tamura et al. 2007). Maximum likelihood analysis was conducted using version 4.0 of PAUP (Swofford 2002). The shape parameter of the γ distribution was the same as used in the NJ analysis.
Accession numbers
The accession numbers of the SSU rDNA nucleotide sequences used for the phylogenetic analysis are given in the phylogenetic tree.
Terminology
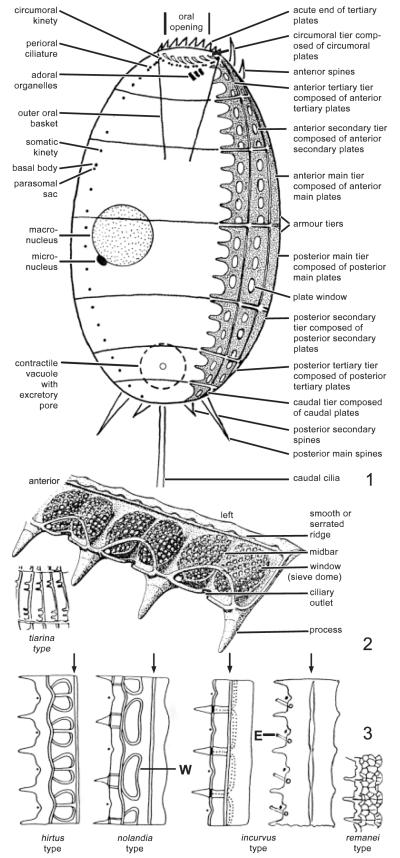
General ciliate terminology follows Corliss (1979). The colepid terminology, which is rather confused and shown in Fig. 1–3, is based on Kahl (1930) and Huttenlauch and Bardele (1987). Of particular importance is the number of armour tiers: frequently, only those that can be easily seen are counted, while the circumoral and caudal tiers are neglected. Thus, tier number is often incorrect in the literature.
Fig. 1–3.
. Terminology and basis for generic classification of the Colepidae. 1. Ventral view showing main terminology. 2. Terminology for the armour plates, using Coleps amphacanthus as an example (from Huttenlauch 1986). 3. Genera are distinguished according to the structure of the calcified armour plates (from Huttenlauch 1985 and Kahl 1930, 1935); arrows indicate plate ridge, dots mark cilia. E, extrusomes; W, window.
RESULTS
Levicoleps biwae n. gen., n. sp.
(Table 1 and Fig. 4–16, 18–20, 22–24, 26–47, 49, 51)
Table 1.
Morphometric data on Levicoleps biwae n. gen., n. sp.
| Characteristicsa | M | SD | SE | CV | Minimum | Maximum | n | |
|---|---|---|---|---|---|---|---|---|
| Body, length, μm | 72.4 | 71.0 | 5.3 | 1.2 | 7.3 | 65.0 | 81.0 | 21 |
| Body, width, μm | 49.1 | 49.0 | 4.9 | 1.1 | 10.0 | 42.0 | 60.0 | 21 |
| Body, length:width ratio | 1.5 | 1.5 | 0.1 | 0.1 | 5.2 | 1.3 | 1.6 | 21 |
| Body, length:width ratio in vivo | 1.8 | 1.8 | 0.2 | 0.1 | 9.3 | 1.6 | 2.2 | 16 |
| Anterior body end to macronucleus, distance, μm | 36.4 | 36.0 | 5.7 | 1.2 | 15.5 | 24.0 | 48.0 | 21 |
| Macronucleus, length, μm | 12.4 | 12.0 | 1.7 | 0.4 | 13.4 | 10.0 | 15.0 | 21 |
| Macronucleus, width, μm | 9.7 | 10.0 | 1.3 | 0.3 | 13.1 | 8.0 | 13.0 | 21 |
| Macronucleus, number | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| Micronucleus, number (protargol impregnation) | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 1.0 | 1.0 | 21 |
| Circumoral kinety, diameterb, μm | 5.9 | 6.0 | 1.1 | 0.3 | 18.7 | 4.5 | 8.0 | 13 |
| Internal oral basket, length, μm | 8.3 | 8.0 | 0.9 | 0.2 | 10.9 | 7.0 | 10.0 | 21 |
| Anterior main plate, width from ridge to ridge, μm | 5.0 | 5.0 | 0.4 | 0.1 | 7.8 | 4.0 | 6.0 | 21 |
| Anterior main plate, number of windowsc | 7.1 | 7.0 | 1.2 | 0.3 | 17.7 | 5.0 | 9.0 | 21 |
| Anterior secondary plate, number of windowsc | 2.5 | 3.0 | — | — | — | 2.0 | 3.0 | 21 |
| Posterior main plate, number of windowsc | 5.9 | 6.0 | 0.9 | 0.2 | 15.1 | 5.0 | 7.0 | 21 |
| Posterior secondary plate, number of windowsc | 2.2 | 2.0 | — | — | — | 2.0 | 3.0 | 21 |
| Somatic kineties, number | 24.3 | 25.0 | 1.7 | 0.4 | 6.9 | 20.0 | 27.0 | 21 |
| Caudal cilia, number | 7.6 | 7.0 | 0.7 | 0.2 | 11.4 | 6.0 | 9.0 | 21 |
Data based, if not mentioned otherwise, on mounted, silver nitrate-impregnated, randomly selected specimens from the environmental sample.
From specimens with closed mouth.
Identical with number of basal bodies.
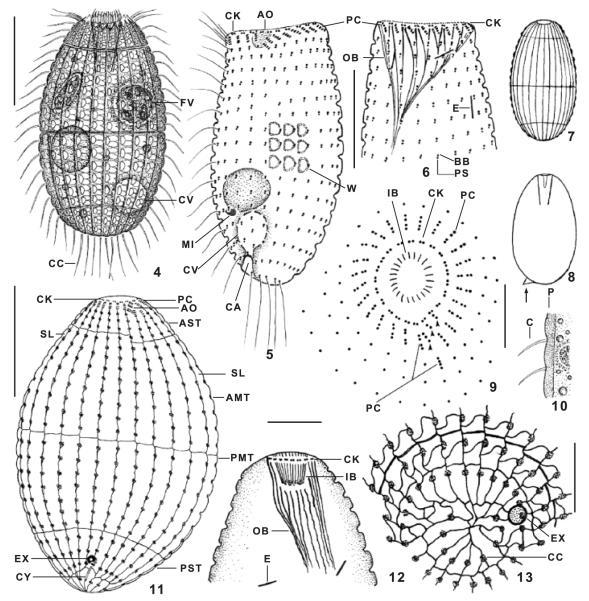
Fig. 4–13.
Levicoleps biwae n. gen., n. sp. from life (4, 7, 8, 10), after protargol impregnation (5, 6, 9, 12), and after silver nitrate impregnation (11, 13). 4. Broad side view of a representative specimen, length 75μm. 5, 6. Ciliary pattern of ventral and dorsal side of holotype specimen, which has the mouth opened. 7. Schematic view at low magnification, showing the main armour tiers and the crenulate surface. 8. A 90-μm-long specimen with a minute spine (arrow). 9. Oral ciliary pattern of a squashed specimen; the outer oral basket is not shown; arrowheads mark the minute adoral organelles. 10. Optical section showing the cortex which is about 3l mm thick due to the endoskeletal, calcified armour plates. 11. Ventral view of holotype specimen, showing the silverline and ciliary pattern as well as the contractile vacuole apparatus. 12. Oral basket of a specimen with the mouth closed. 13. Posterior polar view, showing the arrangement of the basal bodies and silverlines. AMT, anterior main tier; AO, adoral organelles; AST, anterior secondary tier; BB, basal body; C, cilium; CA, canal of contractile vacuole; CC, caudal cilia; CK, circumoral kinety; CV, contractile vacuole; CY, cytopyge; E, extrusomes; EX, excretory pore of contractile vacuole; FV, food vacuole; IB, inner oral basket; MI, micronucleus; OB, outer oral basket; P, cortex with armour plates; PC, perioral ciliature; PMT, posterior main tier; PS, parasomal sac; PST, posterior secondary tier; SL, silverlines; W, windows. Scale bars: 30 μm (Fig. 4, 11), 20 μm (Fig. 5, 6), 10 μm (Fig. 9, 12, 13).
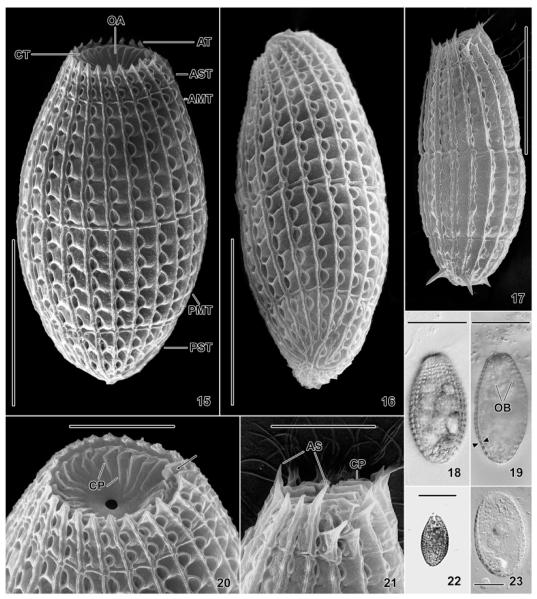
Fig. 15–23.
Levicoleps biwae n. gen., n. sp. (15, 16, 18–20, 22, 23) and Coleps hirtus (17, 21) from life (18, 19, 22, 23) and in the scanning electron microscope (15–17, 20, 21). 15, 16. Lateral and dorsal view, showing the flattening of the cell and the main components of the calcified armour; note the absence of anterior and posterior spines. 17, 21. Lateral view of C. hirtus, which occurred together with L. biwae. These species are easily distinguished by the presence vs. absence of spines and the number of plate (ciliary) rows, which is significantly higher in L. biwae (cp. Fig. 15). 20. Oblique anterior polar view of a specimen with the mouth partially open. Note the central mouth opening and the site of the adoral organelles (arrow). 18, 22. Surface views at medium (250×) and low (100×) magnification. 19, 23. Optical sections showing the body shape and thick cortex (apposed triangles). AMT, anterior main tier; AS, anterior spines; AST, anterior secondary tier; AT, acute end of a plate of the anterior secondary tier; CP, circumoral plates; CT, circumoral tier; OA, oral apparatus; OB, oral basket; PMT, posterior main tier; PST, posterior secondary tier. Scale bars: 40 mm (Fig. 18, 19, 22), 30 μm (Fig. 15–17, 23), 10 μm (Fig. 20, 21).
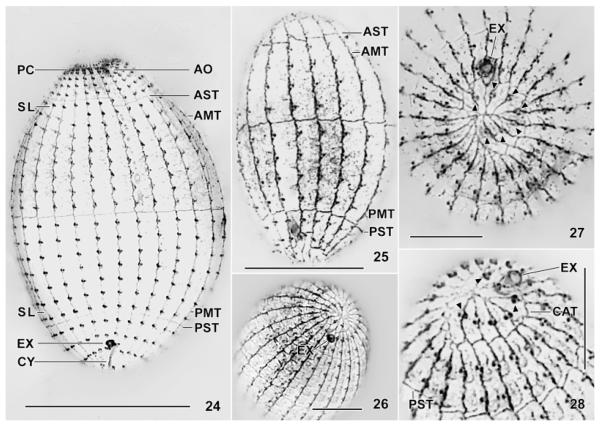
Fig. 24–28.
Levicoleps biwae n. gen., n. sp. (24, 26–28) and Coleps hirtus (25) after silver nitrate impregnation. All specimens are from the environmental sample, i.e. not from laboratory cultures. 24. Ventral view of holotype specimen, showing the silverline and ciliary pattern as well as the main armour tiers. 25. Coleps hirtus is easily distinguished from L. biwae by the significantly lower number of ciliary (plate) rows. 26–28. Posterior polar views showing the ciliary and silverline pattern as well as the circle of caudal cilia (triangles) and the pore of the contractile vacuole. AMT, anterior main tier; AO, adoral organelles; AST, anterior secondary tier; CAT, caudal tier; CY, cytopyge; EX, excretory pore of contractile vacuole; PC, perioral ciliature; PMT, posterior main tier; PST, posterior secondary tier; SL, silverlines. Scale bars: 40 μm (Fig. 24), 30 μm (Fig. 25), 15 μm (Fig. 26–28).
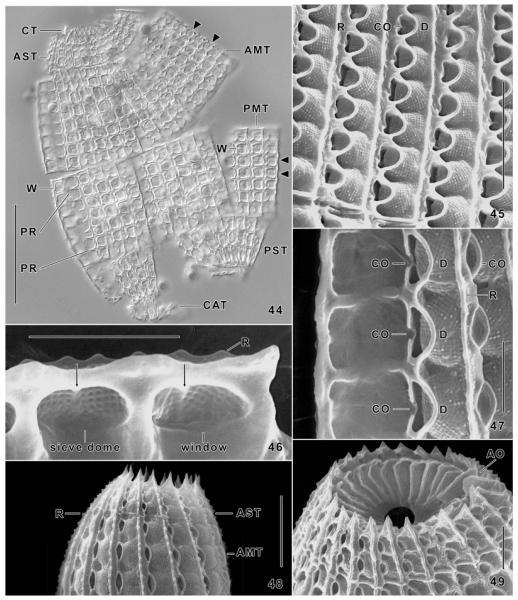
Fig. 44–49.
Levicoleps biwae n. gen., n. sp. (44–47, 49) and Coleps hirtus (48) from life (44) and in the scanning electron microscope (45–49). 44. A squashed specimen showing the six armour tiers and individual plates in interference contrast; triangles denote sites where the typical, pretzel-shaped structure of the windows can be seen. 45–47. Plates at high magnification; arrows (46) mark the inconspicuous midbar. 48. The armour of C. hirtus has the same fine structure as that of L. biwae. 49. Oral area showing the pharyngeal opening and the circumoral tier, which has a slight irregularity at the site of the adoral organelles. AMT, anterior main tier; AO, adoral organelles; AST, anterior secondary tier; CAT, caudal tier; CO, ciliary outlet; CT, circumoral tier; D, sieve dome; PMT, posterior main tier; PR, plate processes; PST, posterior secondary tier; R, plate ridges; W, plate windows. Scale bars: 30 μm (Fig. 44), 10 μm (Fig. 45, 48), 5 μm (Fig. 46, 47, 49).
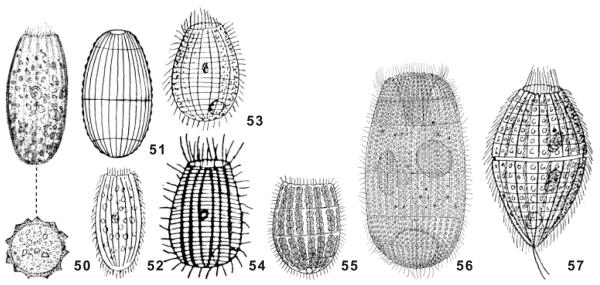
Fig. 50–57.
Colepids without spines; all from life. 50. Coleps inermis, longitudinal surface view and transverse view; length about 50 μm (from Perty 1858). 51. Levicoleps biwae as seen at low (~ × 100) magnification, length 80 μm (original). 52. Coleps inermis, length about 50 μm (from Perty 1858, redrawn by Kahl 1930). 53. Coleps striatus, length about 50 μm (from Smith 1897, redrawn by Kahl 1930). 54. Coleps striatus, length about 50 μm (from Smith 1897). 55. Coleps kenti, length 52 μm (from Bhatia 1936). 56. Planicoleps psammophilus, length 90 μm (from Dragesco and Dragesco-Kernéis 1991). 57. Coleps trichotus, length 52–55 μm (from Savi 1913, redrawn by Kahl 1930).
Morphological description
The size in vivo is 60–90 × 30–50 μm, usually about 75 × 45 μm in the environmental sample, while about 60 × 40 μm after prolonged laboratory cultivation. Body width increases after silver nitrate impregnation by about 15%, so that the length:width ratio is 1.5 in preparations and 1.8 in vivo (Table 1). It is barrel shaped and inconspicuously asymmetrical, occasionally slightly narrowed in mid-body where the main armour plates abut. The anterior end is transversely truncate and crown-like due to the acute ends of the secondary tier plates, while the posterior end is moderately broadly rounded. Poorly nourished specimens are flattened laterally by up to 30%, while well-fed individuals are more or less distinctly ellipsoidal (Fig. 4, 5, 7, 8, 15, 16, 18, 19, 22–24). The macronucleus is in or near mid-body close to the cell’s periphery and is globular to broadly ellipsoidal with an average length:width ratio of 1.3; it is about 15 μm across in vivo. The discoidal micronucleus, about 3 × 1 μm in vivo, is attached to the macronucleus (Fig. 4, 5, 29, 31). The contractile vacuole is ventrally, in line with the adoral organelles, and is distinctly subterminal with a conspicuous, posteriorly directed canal about 5 μm long, whose opening is recognizable both in silver and SEM preparations (Fig. 4, 5, 11, 13, 24, 26–28, 40, 41, 43). The cytoproct, which occurs posterior to the contractile vacuole pore, appears as a granular, thick silverline (Fig. 11, 24). Extrusomes, which stain darkly with silver carbonate and protargol, are mainly in the oral opening, but are difficult to recognize in vivo because they are only 4 × ~ 0.3 μm in size (Fig. 6, 12, 32, 34). The cortex is conspicuous because it is about 3 μm thick due to the armour plates, which are studded with small but distinct convexities caused by the sieve domes (Fig. 4, 10, 18, 19, 22, 23). The cytoplasm is colourless, usually containing some food vacuoles up to 20 μm wide and highly refractile lipid droplets 1–5 μm across (Fig. 4, 18, 22, 23). This ciliate swims moderately fast, and never rests, except when engulfing large prey (Fig. 14).
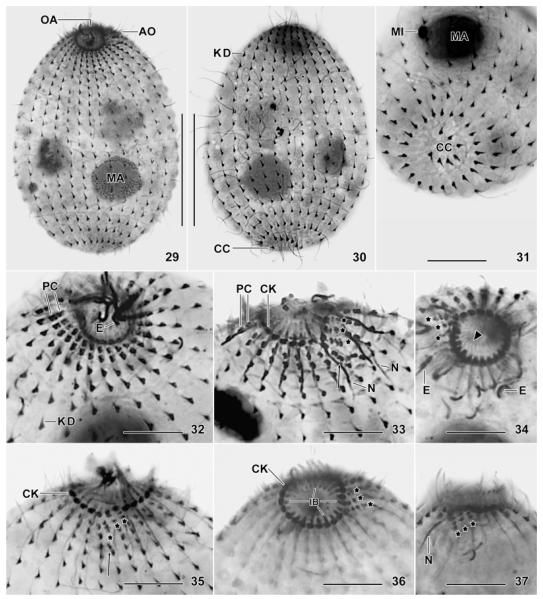
Fig. 29–37.
Levicoleps biwae n. gen., n. sp., somatic and oral ciliary pattern and other organelles after silver carbonate impregnation. All specimens more or less flattened by coverslip pressure. 29, 30. Overview showing left and right side of same specimen; note the short kinetodesmal fibre at the right side of the kinetids. 31. Oblique, posterior polar view showing seven caudal cilia. 32–37. Oral area showing the perioral ciliature and the 3 min adoral organelles (asterisks) from various perspectives; the first and second adoral organelle each consist of two (di?) kinetids, while organelle 3 is composed of three (di?) kinetids; note that the circumoral kinety is not interrupted at the site of the adoral organelles. The arrows (33, 35) denote the two perioral dikinetids underneath adoral organelle 1 and the triangle (34) marks the fibres forming the inner oral basket. AO, adoral organelles; CC, caudal cilia; CK, circumoral kinety; E, extrusomes; IB, inner oral basket; KD, kinetodesmal fibres; MA, macronucleus; MI, micronucleus; N, nematodesmata forming the outer oral basket; OA, oral apparatus; PC, perioral dikinetids. Scale bars: 30 μm (Fig. 29, 30), 20 μm (Fig. 31), 10 μm (Fig. 32–37).
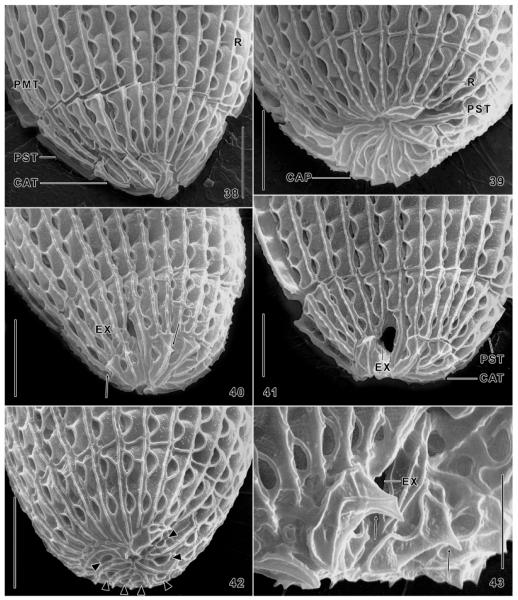
Fig. 38–43.
Levicoleps biwae n. gen., n. sp., posterior body region in the scanning electron microscope. All specimens seen have an individually distinct plate and ridge pattern in the caudal tier; a few specimens have minute spines ( ≤ 2 μm) associated with some caudal plates (40, 43, arrows). The plate ridge may be smooth (38, 41, 42) or slightly serrate (39, 40). Triangles (42) mark openings for the caudal cilia. CAP, caudal plates; CAT, caudal tier; EX, plate opening for the contractile vacuole; PMT, posterior main tier; PST, posterior secondary tier; R, plate ridge. Scale bars: 10 μm (Fig. 38–42), 5 μm (Fig. 43).
The armour is of the hirtus type and composed of six tiers: circumoral tier, anterior secondary tier, anterior main tier, posterior main tier, posterior secondary tier, and caudal tier, each consisting of an average of 24 rectangular plates with paired sieve domes (windows). The number of plates and windows is rather variable (CV 7%–18%, Table 1). The plates are colourless to brownish, likely depending on age, about 2.5 μm thick and fairly flexible because they do not split under coverslip pressure (Fig. 4, 15, 19, 44). The circumoral tier, which is hardly recognizable in vivo, is composed of windowless, triangular, slightly convex plates with the narrower end directed pharyngeally. The plates are slightly disordered and quadrangular at the site of the adoral organelles (Fig. 15, 20, 44, 49). The anterior secondary plates have an acute anterior end, thus forming a beautiful crown (Fig. 15, 20, 49). The anterior main tier, the posterior main tier, and the posterior secondary tier are without peculiarities. The caudal tier is recognizable only in oblique or posterior polar view, and the arrangement, shape, and fine structure of the caudal plates is slightly different in all specimens analysed. They may even form a stopper-like accumulation (Fig. 38). Rarely, and only in the largest specimens, are there some posterior spines up to 2 μm long (Fig. 8, 38–44). The fine structure of the armour plates is as shown in Fig. 2: the plate ridge, which is at the left margin, is smooth to slightly serrate, but never wing-like; the midbar, which separates the windows of a pair, is usually difficult to recognize both in vivo and with the SEM (Fig. 3–5, 15, 16, 20, 44–47).
The somatic cilia are about 9 μm long in vivo and are very regularly arranged, forming an average of 18 transverse circles and 24 longitudinal rows (Table 1); small irregularities occur frequently. Each ciliary row commences with two dikinetids, forming the perioral ciliature; the anterior cilium of the perioral dikinetids is distinctly shortened. The kineties are slightly shortened and their perioral portion is curved rightwards underneath the adoral organelles (Fig. 4, 5, 11, 24, 29, 30, 35, 42). Six to nine, but usually seven, caudal cilia about 15 μm long are arranged in a circle, which is more or less open to the right side of the cell (Fig. 4, 5, 13, 27, 28, 30, 31, 42). The somatic kinetids are composed of an anterior, ciliated basal body (large granule in silver preparations) and a posterior, slightly obliquely oriented parasomal sac (small granule in silver preparations). The kinetodesmal fibre is short. The ciliary outlet in the armour plates is hemispherical and broadly fusiform when seen obliquely (Fig. 5, 6, 24, 30, 32, 44–47).
The silverline pattern is of the “stria” type: the silverlines connect basal bodies longitudinally and converge on the posterior pole, forming a wide, rather irregular reticulum. The ends of the main tiers are marked by a thick, granular silverline (Fig. 11, 13, 24, 26–28).
The oral opening occupies the central half of the anterior pole. In vivo it is about 8 μm across, but may open widely when engulfing large prey (Fig. 4–9, 11, 14, 15, 19, 24, 32–37, 49). The circumoral kinety is circular and slightly disordered, but not interrupted at the site of the adoral organelles. It is composed of 20–25 dikinetids associated with 5-μm-long, conical, bristle-like processes, each probably containing an extrusome (Fig. 4, 5, 6, 9, 11, 12, 33–36). The internal oral basket in the centre of the mouth is narrow obconical and about 10 μm long in vivo; it is formed by fibres originating from the circumoral dikinetids and is impregnated with silver nitrate and protargol. At the proximal end, there is an accumulation of argyrophilic granules or, in SEM preparations, a small opening (Fig. 8, 9, 12, 20, 34, 36, 49). The external oral basket, which is distinct in vivo and protargol preparations, is made of bundles of nematodesmata originating from the circumoral dikinetids and extending to the second third of the body (Fig. 4, 6, 8, 12, 19, 33, 37). Three minute, obliquely arranged adoral organelles lie in a small concavity posterior to the circumoral kinety; organelles 1 and 2 are each composed of two (di?) kinetids, while organelle 3 is composed of three (di?) kinetids. All adoral organelles are associated with about 7-μm-long fibres that contribute to the external oral basket (Fig. 5, 9, 11, 20, 24, 33–37, 49).
Gene sequence
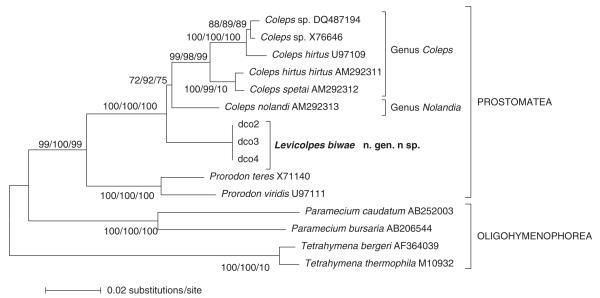
The 18SSU rDNA sequence of L. biwae is 1,749 bp long and available under Accession number AB354737 of the DDBJ database. Comparing the L. biwae sequence with other ciliate SSU rDNA sequences identifies Coleps spp. as the closest relatives in all analyses. We present the NJ tree with bootstrap support values for the MP and ML trees (Fig. 58). The three specimens analysed have identical sequences. The base substitutions between L. biwae and C. hirtus is comparatively large, viz., 122 bp ( ~ 7%). Interestingly, C. hirtus seems to be a mixture of at least two species. Possibly, the U97109 population, which has been submitted by Hirt, Dyal, Embley, Esteban, and Finlay (unpubl.), represents the “green”, symbiotic algae containing C. hirtus viridis Ehrenberg, 1831 (see Foissner et al. 1999 for a detailed redescription) because it is likely from Priest Pot, where Esteban and Finlay have been working and where a green C. hirtus is abundant in the hypolimnion (Guhl, Finlay, and Schink 1994).
Fig. 58.
Neighbour-joining phylogenetic tree based on the small subunit (SSU) rDNA sequence of Levicoleps biwae n. gen., n. sp. and other ciliates. The scale bar indicates the genetic distance. Numbers at branching points show bootstrap values of 1,000 replicates each for three methods: neighbour-jouning (NJ), maximum parsimony (MP), and maximum likelihood (ML). GenBank numbers follow species names.
DISCUSSION
Family Colepidae
The Colepidae Ehrenberg, 1838 include ciliates with a calcified armour and a prostome ciliary organization (Corliss 1979; Foissner 1984; Foissner et al. 1994, 1999; Huttenlauch 1986, 1987; Huttenlauch and Bardele 1987; Kahl 1930; Small and Lynn 1985; Wilbert and Schmall 1976). Although details vary considerably, the basic organization is the same in all genera and species (Fig. 1). Thus, the family is well circumscribed, except for the genus Plagiopogon, which Kahl (1930) and Corliss (1979) assigned to the haptorids while Small and Lynn (1985) classified it in the Colepidae. Unfortunately, details of the scales and the ciliary pattern of Plagiopogon are not known, and thus both classifications remain doubtful.
Briefly, the colepid armour is composed of several plate tiers that are distinct in most but not all genera (Macrocoleps). The tiers consist of oblong, calcified plates with a complex fine structure (Fig. 2). Depending on the species, there are about 15–60 monokinetidal ciliary rows that extend meridionally, rarely spirally. Each ciliary row commences with two or more dikinetids, forming the perioral ciliature (Fig. 1, 9, 32). The oral apparatus consists of the oral basket, a dikinetidal circumoral kinety, and 3–5 min adoral organelles in a more or less deep cavity close to the circumoral kinety (Fig. 1, 5, 6, 9, 12, 15, 20, 35).
We recognize nine genera with a total of about 40 species. An updated revision of the species is not available.
Recognition of genera
Diesing (1865), who synonymized Coleps Nitzsch, 1827 with Plagiopogon Stein, 1859, founded three colepid genera, using armour details as distinguishing features: Dictyocoleps, Pinacocoleps (resurrected in the diagnostic section), and Cricocoleps (for Coleps amphacanthus), all considered as junior synonyms of Coleps by later authors (Corliss 1979; Kahl 1930; Kent 1881). Kahl (1930) and Corliss (1979) recognized only two genera, also using armour details as the main generic features: Coleps Nitzsch, 1827 and Tiarina Bergh, 1881. They considered Stappersia Meunier, 1910 as a junior synonym of Tiarina. No new colepid genus was established between 1882 and 1985, when Small and Lynn created Nolandia, using the different orientation of the adoral organelles to distinguish Nolandia from Coleps. A few years later, Dragesco and Dragesco-Kernéis (1991) discovered a new colepid in the psammon of Lake Tanganyika and established the genus Planicoleps, using the number of armour tiers, the lack of armour spines, and the presence of special extrusomes as generic features. Finally, Obolkina (1995a) established four new genera for colepids discovered in the coastal psammon of Lake Baikal, using body shape, the number of armour tiers, the presence vs. absence of armour spines and a circumoral kinety, the number of adoral organelles, and the habitat (freshwater vs. marine) as the main generic features: Baikalocoleps, Kotinia (for the homonym Alexandria, see Aescht 2001), Macrocoleps, and Tiarinella. Thus, altogether 12 generic names have been created for colepids, to which we add the new genera Levicoleps and Reticoleps (see “Diagnostic”).
Based on the data reviewed above and the present investigations, we selected four features for genus recognition in the family Colepidae: the number of armour tiers, the presence vs. absence of armour spines, the type of tier plates, and the number of adoral organelles.
The number of armour tiers is six in the smaller species ( <90 μm) and eight in most of the larger ones. Thus, the number appears size related and a weak generic feature. However, Macrocoleps shows that the situation is more complex: the large ( > 100 μm) species of this genus have about 12 irregular tiers and thus differ significantly from other colepids (Obolkina 1995a). Some authors included only the main tiers in the diagnosis, excluding the narrow circumoral and caudal tier. This is obviously the case in Baikalocoleps and Planicoleps, which thus do not have four (Obolkina 1995a) or six (Dragesco and Dragesco-Kernéis 1991), but six and eight tiers, respectively.
The presence vs. absence of armour spines is often considered as a variable phenotypic feature. However, there are also many examples that such processes are stable, for instance, the odontostomadids which perfectly match the original descriptions (Kreutz and Foissner 2006). In the colepids, they are obviously also very stable because few spineless populations have been described (see “Discussion”) and no changes occur on even prolonged cultivation. Further, the 7% sequence divergence between Coleps and Levicoleps, which differ mainly by the presence vs. absence of armour spines, indicate that spines are an important character in colepids. Unfortunately, the adaptive value of the spines is not known, but they persist in laboratory cultures, at least in C. hirtus and L. biwae.
The structure and shape of the tier plates has been considered a species-specific feature by Kahl (1930) and others. However, scales are now widely considered both genus- and species-specific in many protists. Thus, we suggest to follow this trend, that is, to use the main colepid plate types for genus distinction (Fig. 3).
The fourth generic feature, the number of adoral organelles is at first glance also quantitative and thus not very strong; further-more, the organelles are small, and their details thus difficult to recognize. However, the number of adoral organelles is not size-related, suggesting some generic significance. For instance, several large colepids (Planicoleps, Macrocoleps) have only three adoral organelles, as is typical for small species (Foissner et al. 1994, 1999), while the large Kotinia species have five (Obolkina 1995a).
Certainly, our selection of generic features is a matter of intuitive feeling rather than of solid knowledge, but it provides a testable hypothesis for further investigations, especially molecular phylogenies. Other features that might be of significance include body shape and flattening, the number of perioral dikinetids and caudal cilia, and the habitat (freshwater vs. marine). In contrast to Obolkina (1995a), we do not include the circumoral kinety because this is an ordinal character and its supposed absence in several genera is very likely the result of insufficient preparations.
Using the nomenclatural rules and the four generic features discussed above, the 14 colepid genera decrease to nine: Dictyocoleps is an objective synonym of Coleps because it includes C. hirtus Nitzsch, 1827; Cricocoleps and Stappersia are very probably junior synonyms of Coleps and Tiarina, respectively; Baikalocoleps is a subjective synonym of (likely) Pinacocoleps; and Tiarinella is a subjective synonym of Tiarina. As concerns Baikalocoleps and Tiarinella, there is a fair chance that their genetic distance from, respectively, Pinacocoleps and Tiarina is sufficiently high to be classified as distinct genera, similar to the case of Coleps and Levicoleps. See the diagnostic section for the characterization of the other genera.
The adoral organelles and the genus Nolandia
The adoral organelles of the colepids are, with few exceptions, <3 μm long, that is, are very small and close to the circumoral kinety. Thus, they are difficult to investigate and prone to misinterpretations. Fortunately, there are now detailed electron microscopic investigations available (Huttenlauch 1986, 1987) that give us the possibility to reinterpret earlier light microscopic descriptions. Such data show that the arrangement and structure of the adoral organelles is very similar in all colepids. The organelles are in a minute depression close to the circumoral kinety and obliquely arranged with respect to the meridional somatic ciliary rows, just as are the adoral membranelles of Tetrahymena (Fig. 1). Usually the individual organelles consist of <10 dikinetids forming short rows that slightly increase in length from anterior (organelle 1) to posterior (organelle 3). Thus, each organelle is composed of two rows of basal bodies of which those of the left row bear 2-μm-long cilia while those of the right row are barren. This might explain why the adoral organelles may appear to be composed of mono- or dikinetids in the light microscope, depending on the silver method used and the interpretation of the observer (Fig. 1, 9, 33–37). Huttenlauch (1986, 1987) showed that the circumoral kinety is not interrupted by the adoral organelles, which matches the present data (Fig. 9, 33–37), but disagrees with previous light microcopic observations (Foissner 1984; Wilbert and Schmall 1976); obviously, the circumoral dikinetids neighbouring the adoral organelles were interpreted as belonging to the latter.
Most of these data were not known when Small and Lynn (1985) established the genus Nolandia: “genus differs from Coleps with brosse files ( = adoral organelles) parallel, rather than perpendicular, to body kineties”. Small and Lynn (1985) concluded this from a reinvestigation of Coleps nolandi by Wilbert and Schmall (1976) and original data from protargol-impregnated C. elongatus. However, the figure they provided is rather schematic and the observations were not confirmed in a more detailed study which shows, by clear micrographs, an oblique arrangement of the organelles in C. elongatus, highly similar to that described in C. hirtus and C. nolandi (Foissner et al. 1999; Wilbert and Schmall 1976). Thus, Nolandia is obviously based on a misobservation and thus should be synonymized with Coleps. On the other hand, C. nolandi, type and sole species of the genus Nolandia, has unique tier plates (Foissner et al. 1994; Huttenlauch 1985; Kahl 1930), requiring generic separation. The International Code of Zoological Nomenclature does not rule on such “taxonomic” problems, but requires that the name and its author(s) are conserved if the genus has been typified correctly. Thus, an emended (improved) diagnosis of Nolandia is proposed here to overcome this problem, even if it is entirely different from the original diagnosis.
Levicoleps as a new genus
As discussed above, we base Levicoleps n. gen. on the absence of armour spines. Although this feature tends to vary in general, it is apparently very stable in colepids because few spineless populations have been described. More importantly, no spines developed in cultures held for up to 6 months. Further, there is 7% genetic divergence to C. hirtus and C. spetai, in spite of the identical fine structure of the armour plates, showing that L. biwae is a rather different species (Fig. 58). Indeed N. nolandi, which has different armour plates, clusters in between the C. hirtus group and L. biwae, supporting our classification as a distinct genus. Further molecular studies are required to clarify whether the considerable genetic distance of Levicoleps is due to ancient isolation (endemism) or due to a rather different ancestor. Unfortunately, the sequence of the supposed closest relative, C. amphacanthus, is not known (Table 2).
Table 2.
Comparison of well-known colepids.
| Species | Methodsa | Number of specimens studied |
Length: width ratio |
Number of tiers |
Number of ciliary rows (extremes and median) |
Number of caudal cilia |
References |
|---|---|---|---|---|---|---|---|
| Coleps hirtus hirtus | CHL | 15 | 1.9 | 6 | 15–16; 15 | 1 | Foissner (1984) |
| Coleps hirtus viridis b | IV, SC | ≤ 10 | 1.8 | 6 | 14–19; 15 | 1 | Foissner et al. (1999) |
| Coleps spetai | P | 12 | 1.6 | 6 | 16–18; 17 | 1 | Foissner (1984) |
| Coleps elongatus b | IV, SC | 17 | 2.3 | 6 | 16–18; 16 | 2 | Foissner et al. (1999) |
| Coleps amphacanthus b | P | 10 | 1.8 | 6 | 22–28; 27 | ~ 10 | Foissner and O’Donoghue (1990) |
| Levicoleps biwae | CHL | 21 | 1.5 | 6 | 20–27; 25 | 7 | Present paper |
| Nolandia nolandi b | CHL, P | ≤ 10 | 2.5 | 6 | 12–17; 13 | 1 |
Wilbert and Small (1976), Foissner et al. (1999) |
| Planicoleps psammophilus | P | ≥15 | 2.2 | 8 | 32–38; 36 | 0 | Dragesco and Dragesco-Kernéis (1991) |
CHL, Chatton-Lwoff silver nitrate impregnation, as modified by Corliss (1953); IV, in vivo; P, protargol impregnation; SC, silver carbonate impregnation.
Include some unpublished data.
Comparison of L. biwae with related species
Five colepids without armour spines have been described (Fig. 50–57): Coleps inermis Perty, 1852, a doubtful species, which could be a haptorid ciliate; C. striatus Smith, 1897; C. kenti Bhatia, 1936; C. trichotus Savi, 1913, which possibly represents a distinct genus; and Planicoleps psammophilus Dragesco and Dragesco-Kernéis, 1991. Of these, only the first three species resemble L. biwae, while C. trichotus is conspicuously fusiform (Fig. 57) and P. psammophilus is much larger (Table 2). The three species resembling L. biwae are smaller than 55 μm (vs. 75 μm) and have, according to the figures, fewer than 20 ciliary rows (vs. 25). Thus, they are more closely affiliated to C. hirtus than to L. biwae, which resembles a spineless C. amphacanthus in these respects (Table 2).
The armour and armour plates of L. biwae are virtually identical to those of C. hirtus and C. amphacanthus (Foissner et al. 1999; Huttenlauch 1986), except for the spines, which are lacking in L. biwae (cp. Fig. 1, 15, 38–45, 49 with Fig. 17, 21, 48). Spineless “C. hirtus” have been found in England (Kent 1881), Switzerland (Perty 1852), the United States (Smith 1897), and in India (Bhatia 1936). Whether these populations belong to the same or different species is not known because the descriptions are too incomplete. However, these descriptions show the existence of one or more spineless Levicoleps in these regions, resembling C. hirtus in size and number of ciliary rows.
Biogeographic aspects
Ancient lakes are those with an uninterrupted history that dates back more than 100,000 yr (Gorthner 1994). They are famous for their high biodiversity, including many endemic species. For instance, about 50% of the species known from Lake Baikal (Russia) and Lake Tanganyika (Africa) are endemic (Martens 1997). Such numbers mainly refer to multicellular organisms because protists are poorly explored in all ancient lakes. Some endemic amoebae and ciliates have been reported: for instance, Liliimorpha viridis from Lake Baikal (Obolkina 1995b) and Difflugia biwae from Lake Biwa (Mori and Miura 1980; Nishino and Watanabe 2000).
Ancient lakes could decide the hotly discussed problem of whether or not endemic protists exist (for a review, see Foissner 2006). The data available support restricted distribution, for instance, the many endemic diatoms in Lake Tanganyika (Cocquyt 2000), a considerable number of endemic ciliates in Lake Baikal (Obolkina 1995b), and some endemic algae in Lake Biwa (Mori and Miura 1980; Nishino and Watanabe 2000).
Colepids with their complex armour plates are biogeographic flagships par excellence. Indeed, several new colepid genera and species were discovered in the psammon of Lake Baikal (Obolkina 1995a) and Lake Tanganyika (Dragesco and Dragesco-Kernéis 1991). Now, a further new colepid has been discovered in Lake Biwa (Fig. 4). Are all these discoveries only a fortunate chance? We do not believe this but interpret it as an indication for restricted distribution. Otherwise they would not have been found in the few samples taken. Whether they are local, regional, or continental endemics requires further investigation. Probably they are rather widely distributed, like D. biwae, formerly considered as a local endemic of Lake Biwa (Mori and Miura 1980), but which has been reported recently from three lakes in central China (Yang and Shen 2005). In Europe, North America, and India obviously occurs a different Levicoleps, which is closely similar to the common C. hirtus (see “Comparison of L. biwae with related species” ), while L. biwae resembles a spineless C. amphacanthus. Indeed, we found such a species recently in Germany. It highly resembles C. hirtus, except for the lack of distinct spines.
DIAGNOSES
In the following compilation, mainly those species which have been carefully described or reinvestigated are assigned to a certain genus. All others remain in Coleps for now.
Coleps Nitzsch 1827
Emended diagnosis
Colepidae with spiny armour composed of six tiers with plates of the hirtus type (Fig. 3). Three adoral organelles.
Type species (by monotypy)
Coleps hirtus (O. F. Müller 1786) Nitzsch, 1827. Basionym: Cercaria hirta O. F. Müller, 1786.
Etymology
Unfortunately, Nitzsch (1827) did not provide the etymology of Coleps. At first glance, it seems to be a Greek noun with the meaning “bend of the knee”. Obviously, this is senseless, which is supported by the gender: the Greek Coleps is feminine, while all authors have treated the Coleps of Nitzsch as masculine. Possibly, Coleps is derived from the Latin neuter noun “colum” (sieve; indeed, Coleps looks like a sieve at moderate magnification!). Nitzsch (1827) may have added “eps” as an artificial combination of letters. As long as the etymology has not been clarified, we suggest treating Coleps as masculine.
Species assignable
Coleps amphacanthus Ehrenberg, 1833 (redescribed by Huttenlauch 1986, 1987; Huttenlauch and Bardele 1987; Foissner and O’Donoghue 1990; Kreutz and Foissner 2006); C. elongatus Ehrenberg, 1831 (redescribed by Foissner et al. 1999; Small and Lynn 1985); C. hirtus hirtus (O. F. Müller 1786) Nitzsch, 1827 (redescribed by Foissner 1984; Foissner et al. 1999); C. hirtus viridis Ehrenberg, 1831 (redescribed by Foissner et al. 1999); C. spetai Foissner, 1984 (reviewed in Foissner et al. 1999).
Kotinia Obolkina in Aescht, 2001
Emended diagnosis
Colepidae with spiny armour composed of eight tiers with not yet specified plates. Five adoral organelles.
Type species (by original designation)
Kotinia arcuata (Obolkina 1995a) Obolkina in Aescht, 2001. Basionym: Alexandria arcuata Obolkina, 1995a.
Etymology
Named after Bolshie Koty, the locus classicus in southern Baikal. Feminine gender.
Species assignable
Kotinia arcuata (Obolkina 1995a) Obolkina in Aescht, 2001; Kotinia affinis (Obolkina 1995a) Obolkina in Aescht, 2001; Kotinia heterolobata (Obolkina 1995a) Obolkina in Aescht, 2001.
Remarks
Originally, this genus was named Alexandria which is, however, a homonym; for details, see Aescht (2001). All species need redescription, especially of the fine structure of the armour plates.
Levicoleps n. gen.
Diagnosis
Colepidae with smooth armour composed of six tiers with plates of the hirtus type (Fig. 3). Three adoral organelles.
Type species
Levicoleps biwae n. sp.
Etymology
Composite of the Latin adjective “levis” (smooth) and the generic name Coleps. Masculine gender.
Species assignable
So far only the type species, L. biwae, can be assigned to the new genus (but see Discussion).
Levicoleps biwae n. sp
Diagnosis
Size in vivo about 75 × 45 μm, barrel shaped. On average 24 ciliary rows, each composed of about 18 monokinetids and two perioral dikinetids; six to nine caudal cilia. Anterior and posterior main plates each with an average of seven and six windows, respectively; anterior and posterior secondary plates each with two to three windows.
Type locality
Japan, shore of Lake Biwa near the Lake Biwa Museum, Karasuma Peninsula, Shiga Prefecture 35°04′20.64″N 135°56′21.74″E.
Type material
Two holotype slides (hapantotypes; one protargol impregnated, the other silver nitrate impregnated) have been deposited in the Oberösterreichische Landesmuseum in Linz (LI). The paratype slides have been deposited in the Lake Biwa Museum.
Gene sequence
Accession number of the SSU rDNA of L. biwae is AB354737 in the DDBJ database.
Etymology
Named after the site found; “biwae” is a noun in genitive case.
Macrocoleps Obolkina, 1995a
Emended diagnosis
Colepidae with spiny armour composed of about 12 irregular tiers with not yet specified plates. Three adoral organelles.
Type species (by original designation)
Macrocoleps caudatus Obolkina, 1995a.
Etymology
Not given in the original description. Obviously, it is composite of the Greek adjective “macro” (large) and the generic name Coleps. Masculine gender.
Species assignable
Macrocoleps caudatus Obolkina, 1995a; Macrocoleps aculeatus Obolkina, 1995a.
Remarks
Both species need redescription, especially of the fine structure of the armour plates.
Nolandia Small and Lynn, 1985
Emended diagnosis
Colepidae with spiny armour composed of six tiers with plates of the nolandi type (Fig. 3). Three adoral organelles.
Type species (by monotypy)
Nolandia nolandi (Kahl 1930) Small and Lynn, 1985. Basionym: Coleps nolandi Kahl, 1930.
Etymology
Named in honour of Lowell Evan Noland (1896–1972), who provided a revision of the genus Coleps in 1925. Feminine gender.
Species assignable
So far only the type species can be assigned to this genus. Nolandia nolandi was redescribed by Foissner et al. (1994), Huttenlauch (1985), and Wilbert and Schmall (1976).
Remarks
For taxonomy, see Discussion.
Pinacocoleps Diesing, 1865
Emended diagnosis
Colepidae with spiny armour composed of six tiers with plates of the incurvus type (Fig. 3). Number of adoral organelles not known.
Type species (by monotypy)
Pinacocoleps incurvus (Ehrenberg 1833) Diesing, 1865. Basionym: Coleps incurvus Ehrenberg, 1833.
Etymology
Not given in the original description. Possibly, it is a composite of the Greek “pina” (tile) and of the generic name Coleps, referring to the appearance of the cell’s armour. Masculine gender.
Species assignable
The incurvus plate type occurs in a fresh-water and two marine species (Kahl 1930), which are thus combined with Diesing’s genus: Pinacocoleps incurvus (Ehrenberg 1833) n. comb., basionym: Coleps incurvus Ehrenberg, 1833; P. tessalatus (Kahl 1930) n. comb., basionym: Coleps tessalatus Kahl, 1930; P. pulcher (Spiegel 1926) n. comb., basionym: Coleps pulcher Spiegel, 1926. None of these species has been investigated with modern methods. Possibly, each represents a distinct genus or subgenus because plate details look fairly different (Fig. 3). Likely, B. quadratus Obolkina, 1995a also belongs to Pinacocoleps. Obolkina (1995a) defined this genus mainly by the lack of a circumoral kinety; all other features match Coleps or Pinacocoleps. Unfortunately, plate details of B. quadratus were described too incompletely to be sure about the generic assignment. Thus, and because the species might be genetically highly distinct from other colepids—considering the special habitat, we neither formally synonymize the genus nor combine the species with another genus.
Remarks
We resurrect, with improved diagnosis, this almost forgotten genus created by Diesing in 1865. Interestingly, he already used armour/plate details to define the genus.
Planicoleps Dragesco and Dragesco-Kernéis, 1991
Emended diagnosis
Colepidae with smooth armour composed of eight tiers with not yet specified plates. Three adoral organelles.
Type species (by original designation)
Planicoleps psammophilus Dragesco and Dragesco-Kernéis, 1991.
Etymology
Not given in the original description. Very likely, the name refers to the strong flattening (plane) of the type species. Masculine gender.
Species assignable
So far only the type species can be assigned to this genus (Fig. 56).
Remarks
Planicoleps differs from Levicoleps in having eight instead of six plate tiers and in adoral organelle 3, which is an irregular field of basal bodies in Planicoleps. Possibly, there are also differences in the fine structure of the plates which, unfortunately, Dragesco and Dragesco-Kernéis (1991) described rather superficially.
Reticoleps n. gen.
Diagnosis
Colepidae with spiny armour composed of six tiers with plates of the remanei type (Fig. 3). Number of adoral organelles not known.
Type species
Reticoleps remanei (Kahl 1933) n. comb. Basionym: Coleps remanei Kahl, 1933.
Etymology
Composite of the Latin noun “reticulum” (fine net) and the generic name Coleps. Masculine gender.
Species assignable
So far only the type species can be assigned to the new genus. Other marine and psammophilic colepids do not have a reticular plate fine structure (Fig. 3).
Remarks
Like Nolandia, this new genus is based on the unique structure of the armour plates, as explained in the section “Recognition of genera”.
Tiarina Bergh, 1881
Emended diagnosis
Fusiform Colepidae with spiny or smooth armour composed of six tiers with plates of the tiarina type (Fig. 3). Three adoral organelles.
Type species (by monotypy)
Tiarina fusa (Claparéde and Lachmann 1859) Bergh, 1881. Basionym: Coleps fusus Claparéde and Lachmann, 1859.
Etymology
Not given in the original description. Obviously, the name is derived from the Latin tiārās (tiara = crown of oriental kings), referring to the complex fine structure of the armour. Feminine gender.
Species assignable and remarks
Possibly only the type species belongs to that genus because T. meunieri Kahl, 1930 has a smooth, rather different armour, suggesting resurrection of the genus Stapperisa. The type species, briefly investigated by Small and Lynn (1985), has a colepid organization with, however, rather conspicuous adoral organelles, while Tiarinella gracilis Obolkina, 1995a has minute adoral organelles and conspicuous oral spines, suggesting that it could represent a distinct genus (see also “Discussion”). Unfortunately, the fine structure of the armour plates was insufficiently described (“like fuzzy squares”). Considering these problems, we do not formally synonymize Tiarinella with Tiarina.
KEY TO THE COLEPID GENERA
The key uses simple features recognizable without silver impregnation. Nonetheless, silver impregnation should be applied because not yet discovered genera might differ in features recognizable only in silver preparations.
| 1. Armour tiers regular, i.e. forming rings … | 2 |
| – Armour tiers irregular, i.e. not forming rings … | Macrocoleps |
| 2. Six armour tiers … | 4 |
| – Eight armour tiers … | 3 |
| 3. Armour with spines … | Kotinia |
| – Armour without spines … | Planicoleps |
| 4. Body fusiform … | Tiarina |
| – Body barrel- or pillow-shaped … | 5 |
| 5. Armour without spines … | Levicoleps |
| – Armour with spines … | 6 |
| 6. Tier plates of remanei type, i.e. with reticular fine structure (Fig. 3) … |
Reticoleps |
| – Tier plates different … | 7 |
| 7. Plate ridge at left margin, windows of hirtus type … | Coleps |
| – Plate ridge in or left of plate midline … | 8 |
| 8. Plate window of nolandi type (Fig. 3) … | Nolandia |
| – Plate windows of incurvus type (Fig. 3) … | Pinacocoleps |
ACKNOWLEDGMENTS
Financial support was provided to W. Foissner by Prof. Dr. Tadao Takahashi, Nishikyusyu University, on behalf of the Japanese Society of Protozoologists; the Lake Biwa Museum, Comprehensive Research Project 06-02; the MEXT, Kakenhi (Grant 18760431 to S. Shimano); and the Austrian Science Foundation (Grant P19699-B17 to W. Foissner). We greatly acknowledge the technical assistance of Mag. Birgit Peukert, Mag. Gudrun Fuss, and Andreas Zankl. Special thanks to Dr. Mark Grygier, organizer of the Lake Biwa biodiversity studies, for having a critical look on the manuscript.
LITERATURE CITED
- Aescht E. Catalogue of the generic names of ciliates (Protozoa, Ciliophora) Denisia. 2001;1:1–350. [Google Scholar]
- Bergh RS. Bidrag til Cilioflagellaternes Naturhistorie. Videnskabel. Meddel. fr. D. Naturhist. Forening Kjøbenhavn. 1881;3:60–76. [Google Scholar]
- Bhatia BL. Protozoa: Ciliophora. In: Sewell RBS, editor. The Fauna of British India, including Ceylon and Burma. Taylor and Francis; London: 1936. pp. 1–493. [Google Scholar]
- Claparéde E, Lachmann J. Études sur les infusoires et les rhizopodes. Mém. Inst. natn. génev. 1859;6:261–482. year 1858. [Google Scholar]
- Cocquyt C. Biogeography and species diversity of diatoms in the northern basin of Lake Tanganyika. In: Rossiter A, Kawanabe H, editors. Ancient Lakes: Biodiversity, Ecology and Evolution. Academic Press; San Diego & Tokyo: 2000. pp. 125–150. [Google Scholar]
- Corliss JO. Silver impregnation of ciliated protozoa by the Chatton-Lwoff technic. Stain Technol. 1953;28:97–100. doi: 10.3109/10520295309105108. [DOI] [PubMed] [Google Scholar]
- Corliss JO. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. 2nd ed Pergamon Press; Oxford: 1979. [Google Scholar]
- Diesing KM. Revision der Prothelminthen. Abtheilung: Amastigen. I. Amastigen ohne Peristom. Sber. Akad. Wiss. Wien. 1865;52:505–579. [Google Scholar]
- Dragesco J, Dragesco-Kernéis A. Free-living ciliates from the coastal area of Lake Tanganyika (Africa) Eur. J. Protistol. 1991;26:216–235. doi: 10.1016/S0932-4739(11)80144-6. [DOI] [PubMed] [Google Scholar]
- Ehrenberg CG. Über die Entwickelung und Lebensdauer der Infusionsthiere; nebst ferneren Beiträgen zu einer Vergleichung ihrer organischen Systeme. Abh. dt. Akad. Wiss. Berl. 1831:1–154. year 1831. [Google Scholar]
- Ehrenberg CG. Dritter Beitrag zur Erkenntniss großer Organisation in der Richtung des kleinsten Raumes. Abh. dt. Akad. Wiss. Berl. 1833:145–336. year 1833. [Google Scholar]
- Ehrenberg CG. Die Infusionsthierchen als vollkommene Organismen. Voss; Leipzig: 1838. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;38:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Foissner W. Taxonomische Studien über die Ciliaten des Großglocknergebietes (Hohe Tauern,Österreich) I. Familien Holophryidae, Prorodontidae, Plagiocampidae, Colepidae, Enchelyidae und Lacrymariidae nov. fam. Annln naturh. Mus. Wien. 1983;84B:49–85. [Google Scholar]
- Foissner W. Infraciliatur, Silberliniensystem und Biometrie einiger neuer und wenig bekannter terrestrischer, limnischer und mariner Ciliaten (Protozoa: Ciliophora) aus den Klassen Kinetofragminophora, Colpodea und Polyhymenophora. Stapfia. 1984;12:1–165. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Biogeography and dispersal of micro-organisms: a review emphasizing protists. Acta Protozool. 2006;45:111–136. [Google Scholar]
- Foissner W, O’Donoghue PJ. Morphology and infraciliature of some freshwater ciliates (Protozoa: Ciliophora) from Western and South Australia. Invertebr. Taxon. 1990;3:661–696. [Google Scholar]
- Foissner W, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems—Band III: Hymenostomata, Prostomatida, Nassulida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1994;1/94:1–548. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsber. Bayer. Landesamtes Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Gorthner A. What is an ancient lake? Arch. Hydrobiol. Beih. Ergeb. Limnol. 1994;44:97–100. [Google Scholar]
- Guhl BE, Finlay BJ, Schink B. Seasonal development of hypolimnetic ciliate communities in a eutrophic pond. FEMS Microb. Ecol. 1994;14:293–305. [Google Scholar]
- Hasegawa M, Kishino H, Yano T. Dating the human-ape split by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Huttenlauch I. SEM study of the skeletal plates of Coleps nolandi Kahl, 1930. Protistologica. 1985;21:449–503. [Google Scholar]
- Huttenlauch I. Morphologie und Morphogenese des Cortex von Coleps amphacanthus. Dissertation. University of Tübingen; Tübingen: 1986. p. 136. [Google Scholar]
- Huttenlauch I. Ultrastructural aspects of the somatic and buccal infraciliature of Coleps amphacanthus Ehrenberg 1833. Protoplasma. 1987;136:191–198. [Google Scholar]
- Huttenlauch I, Bardele CF. Light and electron microscopical observations on the stomatogenesis of the ciliate Coleps amphacanthus Ehrenberg, 1833. J. Protozool. 1987;34:183–192. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 1. Allgemeiner Teil und Prostomata. Tierwelt Dtl. 1930;18:1–180. [Google Scholar]
- Kahl A. Ciliata Libera et Ectocommensalia. Tierwelt Nord- und Ostsee. 1933;23:29–146. Teil II, c3. [Google Scholar]
- Kahl A. Urtiere oder Protozoa I: Wimpertiere oder Ciliata (Infusoria) 4. Peritricha und Chonotricha. Tierwelt Dtl. 1935;30:651–886. [Google Scholar]
- Kent WS. A Manual of the Infusoria: Including a Description of all Known Flagellate, Ciliate, and Tentaculiferous Protozoa, British and Foreign, and an Account of the Organization and Affinities of the Sponges. Vol. I–III. David Bogue; London: p. 913. 1880-1882. [Google Scholar]
- Kreutz M, Foissner W. The Sphagnum ponds of Simmelried in Germany: a biodiversity hot-spot for microscopic organisms. Protozool. Monogr. 2006;3:1–267. [Google Scholar]
- Lepsi I. Über einige insbesondere psammobionte Ciliaten vom rumänischen Schwarzmeer-Ufer. Zool. Anz. 1962;168:460–465. [Google Scholar]
- Martens K. Speciation in ancient lakes. Trends Ecol. Evol. 1997;12:177–182. doi: 10.1016/s0169-5347(97)01039-2. [DOI] [PubMed] [Google Scholar]
- Meunier A. Microplankton des Mers de Barents et de Kara. Campagne Arctique de 1907. Bruxelles; C. Bulens: 1910. [Google Scholar]
- Mori S, Miura T. List of plant and animal species living in Lake Biwa. Mem. Fac. Sci. Kyoto Univ. 1980;8:1–33. [Google Scholar]
- Müller OF. Animalcula Infusoria Fluviatilia et Marina, quae Detexit, Systematice Descripsit et ad Vivum Delineari Curavit. Hauniae; N. Mölleri: 1786. [Google Scholar]
- Nakayama T, Watanabe S, Mitsui K, Uchida H, Inouye I. The phylogenetic relationship between the Chlamydomonadales and Chlorococcales inferred from 18SrDNA sequence data. Phycol. Res. 1996;44:47–55. [Google Scholar]
- Nishino M, Watanabe NC. Evolution and endemism in Lake Biwa, with special reference to its gastropod mollusc fauna. In: Rossiter A, Kawanabe H, editors. Ancient Lakes: Biodiversity, Ecology and Evolution. Academic Press; San Diego & Tokyo: 2000. pp. 151–180. [Google Scholar]
- Nitzsch CL. Cercaria. In: Ersch JC, Gruber JG, editors. Allgemeine Encyclopädie der Wissenschaften und Künste, Theil 16. F. Gleditsch; Leipzig: 1827. Coleps on p. 69. [Google Scholar]
- Noland LE. A review of the genus Coleps with descriptions of two new species. Trans. Am. Micros. Soc. 1925;44:3–13. [Google Scholar]
- Noland LE. Observations on marine ciliates of the Gulf Coast of Florida. Trans. Am. Micros. Soc. 1937;56:160–171. [Google Scholar]
- Obolkina LA. New species of the family Colepidae (Prostomatida, Ciliophora) from Lake Baikal. Zool. Zh. 1995a;74:3–19. [Google Scholar]
- Obolkina LA. Ciliophora. In: Timoshkin OA, editor. Guide and Key to Pelagic Animals of Baikal (with Ecological Notes). Siberian Publishing Firm RAS. Novosibirsk; Nauka: 1995b. pp. 182–250. [Google Scholar]
- Perty M. Zur Kenntniss kleinster Lebensformen nach Bau, Funktionen, Systematik, mit Specialverzeichniss der in der Schweiz beobachteten. Jent & Reinert; Bern: 1852. [Google Scholar]
- Puitika T, Kasahara Y, Miyoshi N, Sato Y, Shimano S. A taxon-specific oligonucleotide primer set for PCR-based detection of soil ciliates. Microbes Environ. 2007;22:78–81. [Google Scholar]
- Rossiter A. Lake Biwa as a tropical ancient lake. In: Rossiter A, Kawanabe H, editors. Ancient Lakes: Biodiversity, Ecology and Evolution. Academic Press; San Diego: 2000. pp. 571–598. [Google Scholar]
- Savi L. Nuovi ciliofori appartenenti alla microfauna del lagostagno craterico di Astroni. Monitore Zool. Ital. 1913;24:95–100. [Google Scholar]
- Small EB, Lynn DH. Phylum Ciliophora Doflein, 1901. In: Lee JJ, Hutner SH, Bovee EC, editors. An Illustrated Guide to the Protozoa. Society of Protozoologists; Lawrence, KS: 1985. pp. 393–575. [Google Scholar]
- Smith JC. Notes on some new, or presumably new, infusoria. Am. Mon. Microsc. J. 1897;28:141–148. [Google Scholar]
- Spiegel A. Einige neue marine Ciliaten. Arch. Protistenkd. 1926;55:184–190. [Google Scholar]
- Stein F. Characteristik neuer Infusorien-Gattungen. Lotos. 1859;9:2–5. 57–60. [Google Scholar]
- Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 1996;13:964–969. [Google Scholar]
- Swofford DL. PAUP* : Phylogenetic Analysis Using Parsimony (* and Other Methods) Version 4.0b10 Sinauer Associates; Sunderland, MA: 2002. [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided y quality analysis tools. Nucl. Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacelet E. La faune infusorienne des “sables á amphioxus” des environs de Marseille. Bull. Inst. océanogr. Monaco. 1961;1202:1–12. [Google Scholar]
- Wilbert N, Schmall G. Morphologie und Infraciliatur von Coleps nolandi Kahl, 1930. Protistologica. 1976;12:193–197. [Google Scholar]
- Yang J, Shen Y. Morphology, biometry and distribution of Difflugia biwae Kawamura, 1918 (Protozoa, Rhizopoda) Acta Protozool. 2005;44:103–111. [Google Scholar]