Abstract
Using hydrofluoric acid, scanning electron microscope-assisted X-ray microanalysis, and energy-filtered transmission electron microscopy, we present the first definite proof of biomineralized silicon [(SiO2)]n in a ciliophoran protist, Maryna umbrellata, a common inhabitant of ephemeral pools. In the trophic specimen, the amorphic silicon (glass) granules are accumulated in the anterior half of the body. When entering the dormant stage, most glass granules are excreted to form the surface cover of the globular resting cyst. Most likely, the silicon granules are synthesized in vesicles of the Golgi apparatus. First, nanospheres with a size of 20–40 nm are formed in a fibrous matrix; they grow to be spongious complexes, eventually becoming amorphous glass granules with an average size of 819 nm × 630 nm. In the transmission electron microscope, the silicon granules show the characteristic fracture pattern of glass known from many other silicon-bearing organisms. A literature survey suggests that silicon is very rare in ciliates. The fine structure and genesis of silicon granules in M. umbrellata are very similar to those of other organisms, including vascular plants and animals, indicating a common mechanism. Light perception and protection against mechanical stress and predators might be functions of the silicon granules in M. umbrellata. The palaeontological significance of glass cysts in ciliates is also discussed.
Keywords: Electron energy loss spectra, fossil protists, palaeontology, resting cysts, silicon genesis
THE element silicon (Si) is one of the most abundant in the earth’s crust (27.7% w/w), ranking second only to oxygen (46.6% w/w). In nature, silicon generally occurs in the form of silicates (H3SiO4−) and silicon (silica) dioxide [(SiO2)]n, i.e. as quartz (e.g. SiO4 tetraeders) or as amorphous, hydrated, polymerized silicic acid often called opal or glass (Ehrlich 1990).
Many organisms, ranging from protists to sponges and vascular plants, take advantage of the common occurrence of silicon, using it to build internal or external skeletons and/or scale structures (for reviews, see Anderson 1994; Bovee 1981; Perry, Belton, and Shafran 2003). Among the protists, the decorative radiolarians, diatoms, and silicoflagellates are well-known examples. Possibly, few protist phyla lack this property, for instance, the ciliates, a phylum with about 10,000 described species, of which Bovee (1981) states: “The Ciliophora do not metabolically accumulate silicon”.
As early as 1873, Haeckel reported siliceous loricae in some marine, tintinnid ciliates, such as Dictyocysta and Codonella. However, he did not test this rigorously, but likely concluded it from the glassy appearance and the overall similarity of the loricae with radiolarian skeletons. This might explain the disappearance of Haeckel’s notion in the literature (Kofoid and Campbell 1939). More recent electron microscopic investigations show that Dictyocysta, codonellids, and some other tintinnids have organic loricae possibly made of chitinous materials; however, environmental siliceous particles (quartz grains) are frequently agglutinated as extrinsic silicon (Bovee 1981; Foissner, Berger, and Schaumburg 1999; Laval-Peuto 1994 for TEM micrographs).
Many protists contain crystalline structures, often called “excretion crystals”. They consist mainly of calcium phosphate and calcium carbonate as well as small amounts of other ions, such as magnesium, sulphur, and chloride (Hausmann 1982; Hausmann and Walz 1979; Kalmus 1931; Pautard 1976); barium sulphate rarely occurs as a gravity device (Williams 1989). The function of most other crystals is still obscure. Possibly, they are an ion storage essential for metabolic processes or they are used to regulate the intracellular concentration of certain ions (Hausmann and Walz 1979).
In ciliates, the senior author often observed minute (≤ 2 μm) granules conspicuously sparkling under interference contrast optics (Foissner 1993; Foissner, Agatha, and Berger 2002). When investigating Maryna umbrellata, a common colpodid ciliate in ephemeral pools, in the transmission electron microscope (TEM), we immediately recognized the glassy nature of the “sparkling granules” due to their conchoidal fracture pattern when sectioned with a diamond knife (Garrone, Simpson, and Pottu-Boumendil 1981).
Further studies showed the glass granules to contribute significantly to the wall of the resting cyst and their origin in vesicles of the Golgi apparatus. Finally, a survey of the literature suggested that our observation is most likely the first definite proof of biomineralized silicon in the phylum Ciliophora.
MATERIALS AND METHODS
Material and cultivation
Maryna umbrellata (Gelei, 1950) Foissner (1993) was isolated from an ephemeral meadow pond in the Donnenberg Park near the so-called House of the Hangman (47°47′N, 13°02′E), Salzburg City, Austria.
Maryna umbrellata is a planktonic, rapidly swimming ciliate, which reproduces in division cysts formed on the bottom of the cultivation dish. Inspection of the food vacuoles and crude experiments with potential food sources (heterotrophic and autotrophic flagellates, small ciliates) showed that M. umbrellata fed mainly on bacteria, while flagellates were taken sparsely; ciliates were rejected. Thus, about 20 specimens were isolated to set up a pure culture with French table water (Eau de Volvic) and some crushed wheat kernels to stimulate growth of the natural bacterial flora. Cultures were grown at room temperature and were renewed each week by transferring about 20 cells and a decaying wheat kernel into fresh medium.
After the logarithmic growth phase, when few dividing cells occurred, most specimens encysted, possibly due to the accumulation of excretory products produced by both the ciliates and the bacteria. Cysts were morphologically fully developed and thus possibly mature after 1–2 d. To be at the safe side, all investigations were performed on cysts older than 10 d.
Identification
The species was determined by live observation (bright-field and interference contrast), silver impregnation, and scanning electron microscopy (SEM), as described by Foissner (1991).
Maryna umbrellata was insufficiently known when Foissner (1993) revised the genus. Thus, Foissner et al. (2002) redescribed M. umbrellata from Costa Rican and Australian populations. However, later investigations (this paper) showed that the resting cysts of the Austrian and Australian populations were quite different, suggesting the latter as an undescribed species. This matter will be treated in a separate publication. For the present study it is sufficient to know that the Austrian population represents the “true” M. umbrellata, as described by Gelei (1950) and reviewed in Foissner (1993).
Classic electron microscopic methods
For SEM, mature resting cysts were collected from a 2-wk-old culture, fixed in cacodylate-buffered osmium tetroxide (2% w/v) at pH 6 for 30 min, rinsed and cleaned with tap water, and then transferred to a special preparation chamber, where they were dehydrated with a graded ethanol series and critical point dried (Foissner 1991). Finally, specimens were mounted on a SEM stub and gold sputtered. Observations were made with a Cambridge Stereoscan 250 (Cambridge Instruments, Cambridge, United Kingdom) operated at 20 kV.
For TEM, resting cysts were fixed for 30 min in a mixture of 10 ml glutaraldehyde (25% v/v), 6 ml aqueous osmium tetroxide (2% w/v) and 10 ml aqueous, saturated mercuric chloride. After three washes in tap water, the specimens were transferred to glycid ether 100 (Serva, Heidelberg, Germany) via a graded ethanol series and propylene oxide. Flat embedding in aluminium weighing pans allowed specimens to be investigated light microscopically (up to 400X) to select well-preserved individuals. See Foissner (2005) for further treatment and details.
Silicon (glass) detection
We used three different methods for analysing the “sparkling granules” in the cytoplasm of trophic cells and in the granule layer of the cyst surface. Appropriate controls were made for all detection methods. To save space, we show the controls only for the hydrofluoric acid (HF) treatments and for one EDAX analysis.
HF treatment
For the light microscopical investigations, trophic cells and cysts were air-dried on albumen-coated plastic slides. Then, the albumen was hardened in 98% ethanol and the slides rewetted in tap water. The rewetted slides were put for 1 min in 4% (w/v) HF, washed in tap water, dehydrated with ethanol, embedded in resin, and analysed under bright-field and interference contrast. A simpler method was finally applied to the resting cysts. Selected cysts were directly put in HF for 2 min, washed in tap water, and investigated in wet condition (Fig. 13, 16).
Fig. 13–21.
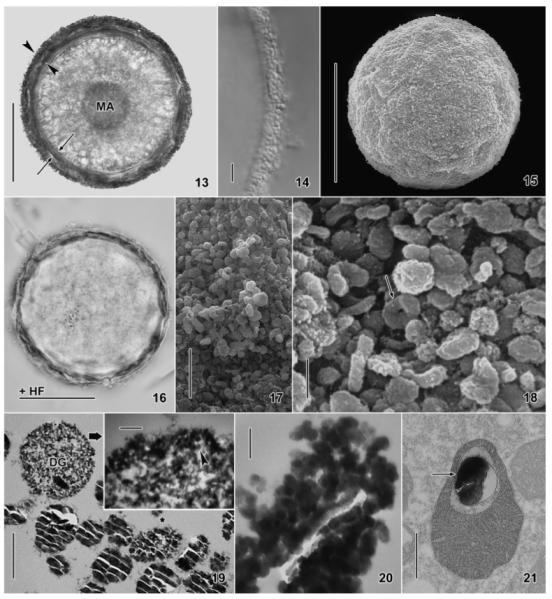
Maryna umbrellata, resting cysts in the bright field (13, 16) and interference contrast microscope (14), in the SEM (15, 17, 18), and in the TEM (19–21). 13, 14, 16. The same cyst before (13, 14) and after (16) hydrofluoric acid (HF) treatment, which removes the external granular silicon layer of the cyst wall (13, arrowheads; 14), while the internal organic layer remains (13, arrows; 16). Further, the granules covering the nuclear apparatus disappear (13, 16). 15, 17, 18. In the SEM, the cysts are finely granulated (15) due to countless silicon granules (17, 18), some of which are torus shaped (Fig. 18, arrow). 19. The silicon granules in the external layer of the cyst wall (cf. Fig. 22, 23) are about 1 μm in size and show the typical conchoidal fracture pattern of glass; one of the granules was partially pulverized by the diamond knife (asterisk). A developing granule shows silicon nanospheres (inset, arrowhead) embedded in fibrous material. 20. Grazing section showing the silicon granules to be composed of nanospheres with a diameter of 20–70 nm.21. Some mitochondria contain a silicon granule (arrow). DG, developing silicon granule; HF, hydrofluoric acid treated; MA, macronucleus. Scale bars = 50 μm (Fig. 13, 15, 16), 5 μm (Fig. 14, 17), 1 μm (Fig. 18, 19), 500 nm (Fig. 21), and 100 nm (Fig. 19, inset; 20).
For the TEM investigations, specimens were fixed as described above. The preparations were first studied in the ordinary way and interesting sections marked. Then, the grids were placed for 2–3 min on drops of HF (0.4%, 1%, 3%, 5%), 3 times rinsed in distilled water, and the marked sections re-analysed directly or after ordinary uranyl acetate/lead citrate staining in a Zeiss TEM 910 (Zeiss, Oberkochen, Germany) (Fig. 9–12, 22–25).
Fig. 9–12.
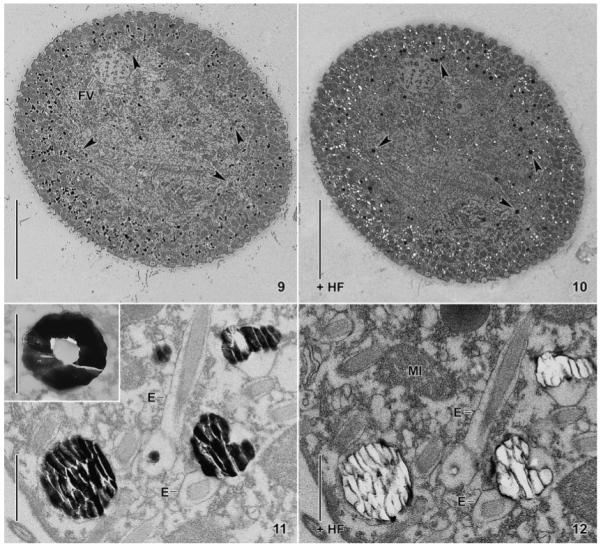
Maryna umbrellata, trophic specimens in the transmission electron microscope (TEM). 9, 10. Transverse section before and after hydrofluoric acid (HF) application. When treated with HF, the black-spotted periphery (9) becomes white-spotted (10) because the dense silicon granules have been dissolved. Hydrofluoric acid appears to darken the section and some granule-like structures, ordinarily stained in the untreated section (9, arrowheads), become dark, like silicon granules (10, arrowheads). 11, 12. Higher magnification of a section through the cytoplasm before (11) and after (12) HF treatment. The glass granules show the typical fracture pattern (11) and become lucent when the silicon is removed by HF with the exception of some (organic?) material at the fracture edges. The inset in Fig. 11 shows a torus-shaped silicon granule from another section. E, extrusomes; FV, food vacuole; HF, hydrofluoric acid treated; MI, mitochondrion. Scale bars = 20 μm (Fig. 9, 10) and 1 μm (11, 12).
Fig. 22–29.
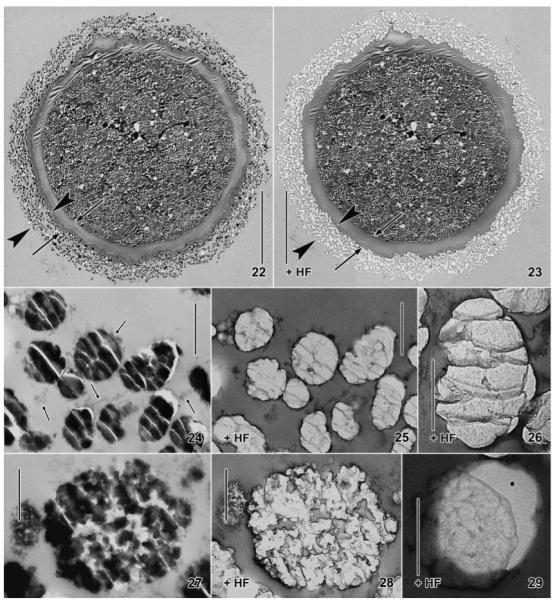
Maryna umbrellata, resting cysts in the transmission electron microscope. 22, 23. Overview before and after application of hydrofluoric acid (HF). The external granule layer of the cyst wall (22, arrowheads) becomes lucent when the silicon has been removed by HF (23), while the organic layer remains (arrows). 24, 25. As Fig. 22 and 23 but at higher magnification. Arrows mark mucous material that holds together the silicon granules. 26. High magnification of a granule where the silicon has been removed by HF. There remain grey, membrane-like structures arranged in the fracture pattern of glass granules. 27, 28. A late developmental stage of a silicon granule before and after treatment with HF. Grey, membrane-like structures remain in those areas that appear “empty” or lucent in the untreated section (27), suggesting that the membranous structures are resin dragged behind the knife. 29. The membrane-like structures recognizable after HF treatment are not caused by the grid film, which appears structureless when the granule is slightly displaced (asterisk). Scale bars = 20 μm (Fig. 22, 23), 1 μm (Fig. 24, 25), and 500 nm (Fig. 26–29).
Scanning electron microscope-assisted X-ray microanalysis
Two variations were applied. First, live cells were concentrated and washed 3 times with distilled water in a centrifuge (2,000 rotations/min for 30 s = 500 g). Then, a minute drop with some cells was put on a plastic SEM stub and the cells squashed with a needle so that the cytoplasm could distribute over the stub. The sparkling granules were then easily identified in the SEM by their globular shape, their typical size (~1 μm), and the element composition (Fig. 6, 7). Second, 95–140-nm-thick sections of trophic and encysted cells were mounted on carbon-coated grids and investigated with an ESEM XL30 (Fei, Hillsboro, OR) instrument (Fig. 8).
Fig. 1–8.
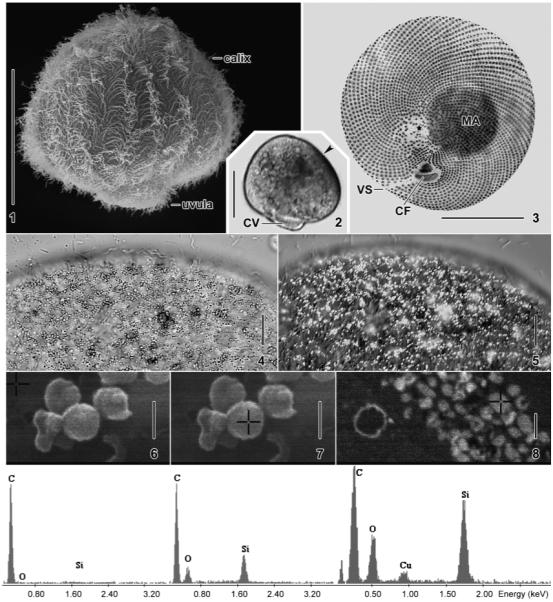
Maryna umbrellata from life (2, 4, 5), after silver carbonate impregnation (3), and in the SEM (1, 6–8). 1. Dorsolateral view showing body shape and holotrichous ciliation. 2. Bright-field micrograph showing the dark spot (arrowhead) produced by the sparkling granules shown in Fig. 4, 5. 3. Silver impregnation shows the spiral ciliary rows, the small oral ciliary fields, and the more sparse ciliation of the distal uvular surface (asterisk). 4, 5. The dark spot (cp. Fig. 2) consists of minute (≤ 2 μm) granules appearing dark in the bright-field microscope, while conspicuously sparkling under interference contrast. 6, 7. X-ray microanalysis (EDAX) of sparkling granules from a squashed, trophic cell. The crosses mark the sites measured: the carbon film as a control (6) and a granule (7) consisting of silicon and oxygen. 8. EDAX analysis of a 140-nm-thick section through the cyst wall, whose external layer consists of countless granules (cross) composed of silicon and oxygen. The carbon and copper peaks are from the sputter coating and the grid, respectively. CF, oral ciliary fields; CV, contractile vacuole; MA, macronucleus; VS, ventral suture. Scale bars = 50 μm (Fig. 1–3), 10 μm (Fig. 4, 5), and 900 nm (Fig. 6–8).
Electron energy loss spectra (EELS in the TEM)
These were acquired from 40 to 50 nm thick, unstained thin sections mounted on copper grids. The sections were studied with a Zeiss LEO 912 AB Omega TEM operated at 120 kV using an LaB6 cathode (Fig. 40). A 100-μm spectrometer entrance aperture was used for defining the measurement area. The EELS were acquired at a TEM magnification of 25,000X using a spectrum magnification of 125X, illumination angles up to 1.6 mrad, and exposure times up to 20 s. Images and EELS were captured by a dual speed CCD Slow Scan Camera TRS Sharpeye (Troendle, Moorenwies, Germany) and were processed by an iTEM Software (Olympus-SIS, Münster, Germany).
Fig. 40.
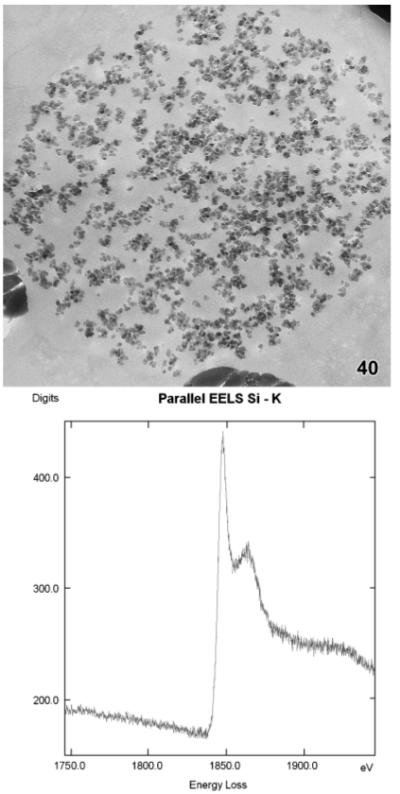
Maryna umbrellata, electron energy loss spectrum (EELS) of the K-edge acquired from a granule in the middle developmental stage (cp. Fig. 34).
Genesis of the silicon granules
Trophic and dividing cells from a logarithmically growing culture were fixed for 30 min in a mixture of 3% (v/v) glutaraldehyde in 0.05 M Na–cacodylate buffer, pH 7.0, and 2% (w/v) aqueous osmium tetroxide, then rinsed in the same buffer, and stored for some days in 3% (v/v) glutaraldehyde. The cells were dehydrated and flat-embedded in resin as described above. Appropriate specimens were selected light microscopically, removed from the Epon (glycid ether 100), and mounted on TEM stubs. Thin sections were stained with uranyl acetate and lead citrate and viewed in a Zeiss TEM 910 (Fig. 30–39).
Fig. 30–39.
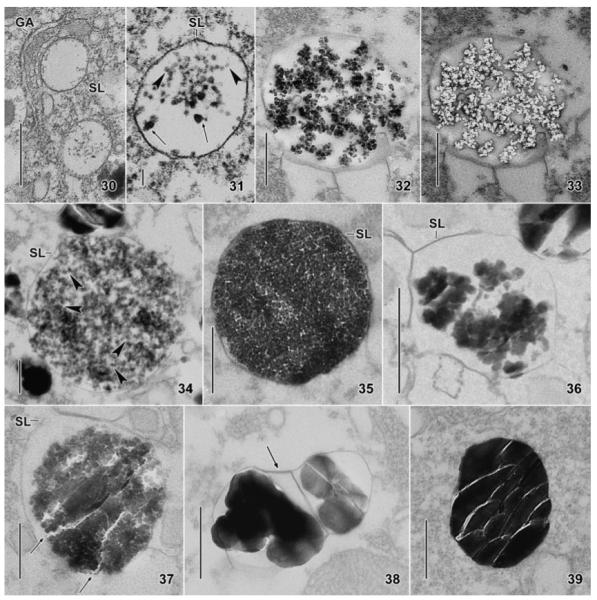
Maryna umbrellata, transmission electron micrographs showing the origin and development of the silicon granules. 30, 31. Two early developmental stages, showing silicon deposition vesicles (SDV) very likely originating from the Golgi apparatus. Note small, pale nanospheres (31, arrowheads) and some larger and darker ones (31, arrows) very likely consisting of two or three spheres. 32, 33. A middle developmental stage before and after treatment with hydrofluoric acid (HF), showing reticulate aggregates of silicon nanospheres (32) disappearing after application of the acid (33). 34. A middle to late developmental stage, similar to that used for the electron energy loss spectrum (Fig. 40), showing the silicon nanospheres (some marked by arrowheads) embedded in a fibrous, possibly organic matrix. 35. A late developmental stage, where the SDV is packed with silicon nanospheres embedded in a fibrous matrix. 36, 37. Very late developmental stages showing the polymerization of the silicon nanospheres to amorphous silica gel. Arrows in Fig. 37 mark the typical fracture pattern of glass recognizable in more advanced areas of the granule. 38, 39. Fully developed silicon granules: that shown in Fig. 38 is partitioned by a silicalemma (arrow), which is hardly recognizable in Fig. 39. GA, Golgi apparatus; SL, silicalemma. Scale bars = 500 nm (Fig. 30, 32, 33, 35–39), 250 nm (Fig. 34), and 100 nm (Fig. 31).
As there was continuous production of silicon granules both in trophic and in dividing cells, it was impossible to correlate the genesis of the silicon granules with the life cycle. Thus, we used three other markers: the size of the silica deposition vesicles (SDV), the abundance of nanospheres, and the structure of the granules.
Terminology
General ciliate terminology follows Lynn (2008). Cyst terminology follows Gutiérrez et al. (2003), Foissner (2005), and Foissner, Müller, and Weisse (2005). Silicon terminology is according to Simpson and Volcani (1981). Silicon (Si) is used to denote the element, and as a generic term when the nature of the specific silicon compound is not known. The term silica [(SiO2)]n refers to amorphous, hydrated, polymerized silicic acid with unknown molecular weight. Silicate (SiO3−2) is the ionic form of silica. Silica deposition vesicle denotes the membrane-bounded sac within which an endogenous siliceous structure is formed. The bounding membrane of a SDV is the silicalemma. The silicon particles recognizable light microscopically are termed granules (~ 800 nm in size), which themselves are composed of nanospheres (≤ 100 nm).
RESULTS
A brief description of trophic and cystic Maryna umbrellata
A detailed knowledge of the trophic and cystic morphology of M. umbrellata is not needed for understanding the present paper. Thus, and because full descriptions are in preparation, the presentation will be very brief. For a revision of species, see Foissner (1993) and Foissner et al. (2002).
Maryna umbrellata belongs to the class Colpodea and is a common inhabitant of ephemeral, limnetic habitats, such as road and meadow puddles and rock pools, where it catches one’s eye by the rapid, dancing movement. It is an umbrella-shaped ciliate with a size of about 100 μm and is usually rather dark in transmitted light due to the high refractivity of the sparkling (silicon) granules (Fig. 2); the umbrella is called the calix, and the short stalk is called the uvula (Fig. 1). The small oral funnel is at the boarder of calix and uvula and contains two ciliary fields or oral polykinetids used to collect bacterial food (Fig. 3). Maryna umbrellata has 70–110 spiral ciliary rows composed of paired, about 10-μm-long cilia (Fig. 1, 3). The nuclear apparatus is near or in the calix centre, while the contractile vacuole is in the posterior end of the uvula (Fig. 2, 3). The cytoplasm usually contains many food vacuoles 5–15 μm across and countless, minute (< 2 μm) granules (“crystals”) hardly recognizable under transmitted light but conspicuously sparkling and dancing under interference contrast illumination (Fig. 4, 5). The granules are concentrated in the anterior body half, forming a rather distinct subapical spot dorsally in the bright-field microscope (Fig. 2).
When encysting, the sparkling granules and mucous material are released to form the external layer of the cyst wall. These processes will be shown in a forthcoming study, describing the formation and structure of the cyst wall. The granules stop sparkling when they become located in the cyst wall, although their fine structure and chemical composition do not change visibly.
The globular resting cysts have a diameter of about 100 μm and appear dark to black under transmitted light. They are packed with globules 0.5–7 μm across, up to 2-μm-sized lipid droplets, and brownish granules, some of which cover the macronucleus, which thus appears as a large, dark globule in the bright-field microscope (Fig. 13). The brownish granules, although sparkling under interference contrast illumination and becoming invisible (dissolve?) after HF treatment, are not made of silicon but resemble lipid droplets in the TEM (Foissner, unpubl. data) The cyst wall is about 10 μm thick and consists of two distinct layers, each about 5 μm thick: the inner, colourless layer is bright and structureless, while the yellow-brown external layer consists of countless granules, which have a size of 0.5–2 μm and are embedded in mucous material (Fig. 13, 14).
Chemical composition of the sparkling granules
The three methods applied (HF, EDAX, EELS) showed that the sparkling cytoplasmic granules and the granules of the cyst cover consist of glass or silica (Fig. 6–8, 9–13, 16, 22–26, 40). No other elements could be detected. Quantitative analyses were not performed but the oxygen peak was lower than the silicon peak in all EDAX analyses. The granules dissolved readily in various concentrations of HF (i.e. 0.4–5% [v/v] for 2 min), but did not dissolve in NaOH (0.5% [v/v] for 5 min) and phosphoric acid (20% [v/v] for 5 min).
Fine structure of the silicon granules
The size and structure of the silicon granules are very similar in trophic and cystic specimens and in SEM and TEM. Thus, they will be described together.
The granules are highly variable in size and shape, producing coefficients of variation between 17% and 43% (Table 1). The size varies from 235 to 1,750 nm, with averages of 788–860 nm (length) and 571–688 nm (width), corresponding to a maximum difference of 12% between sites (trophic, cystic) and methods (SEM, TEM). We interpret these small differences to be caused by measurement problems, such as sections not going through the granule centre and/or the main granule axis. Using all measurements, i.e. 540 granules from the 45 trophic and cystic specimens sectioned, the granules have an average size of 819 nm 630 nm (Table 1), and are thus broadly ellipsoidal (Fig. 11, 18, 19, 24, 39). However, some shape variation occurs (i.e. globular, ellipsoidal, elongate ellipsoidal, reniform), of which torus-like, distinctly flattened granules are the most conspicuous (Fig. 11, 18).
Table 1.
Morphometric data on silicon granules in trophic and cystic cells, on the nanospheres composing the silicon granules, and on the size of the silicon depositing vesicles
| Characteristicsa | Method | Mean | M | SD | SE | CV | Min | Max | n | i |
|---|---|---|---|---|---|---|---|---|---|---|
| Cytoplasmic granules, lengthb |
TEM | 787.9 | 789 | 297 | 23 | 37.7 | 250 | 1,750 | 164 | 8 |
| Cytoplasmic granules, widthb |
TEM | 570.6 | 572 | 243 | 19 | 42.6 | 250 | 1,500 | 164 | 8 |
| Cystic granules, length |
SEM | 813.9 | 812 | 313 | 28 | 38.5 | 333 | 1,667 | 129 | 9 |
| Cystic granules, width |
SEM | 688.2 | 689 | 249 | 23 | 36.2 | 235 | 1,481 | 129 | 9 |
| Cystic granules, length |
TEM | 815.8 | 815 | 240 | 19 | 29.5 | 400 | 1,500 | 156 | 8 |
| Cystic granules, width |
TEM | 653.9 | 655 | 210 | 17 | 32.2 | 250 | 1,325 | 156 | 8 |
| Nanospheres from mature granules or very late developmental stages, diameter |
TEM | 37.3 | 38 | 12 | 1.5 | 32.6 | 21 | 64 | 64 | 18 |
| Nanospheres from developing granules, diameter | TEM | 27.1 | 27 | 10 | 1.2 | 36.3 | 16 | 53 | 64 | 11 |
| SDV, length: stage 1c | TEM | 769.5 | 800.0 | 227.1 | 85.8 | 29.5 | 442 | 1,000 | 26 | 7 |
| SDV, width: stage 1c | TEM | 607.2 | 625.0 | 179.1 | 67.7 | 29.5 | 380 | 835 | 26 | 7 |
| SDV, length: stage 2c | TEM | 1,347.7 | 1,260.8 | 292.1 | 92.4 | 21.7 | 886 | 1,732 | 57 | 10 |
| SDV, width: stage 2c | TEM | 1,126.7 | 1,143.4 | 279.4 | 88.4 | 24.8 | 500 | 1,430 | 57 | 10 |
| SDV, length: stage 3c | TEM | 851.4 | 890.1 | 194.8 | 68.9 | 22.9 | 589 | 1,150 | 44 | 8 |
| SDV, width: stage 3c | TEM | 639.5 | 637.4 | 133.6 | 47.2 | 20.9 | 444 | 826 | 44 | 8 |
| SDV, length: stage 5b,c | TEM | 859.9 | 860.4 | 145.2 | 43.8 | 16.9 | 625 | 1,188 | 91 | 11 |
| SDV, width: stage 5b,c | TEM | 606.9 | 573.0 | 130.2 | 39.3 | 21.5 | 438 | 891 | 91 | 11 |
Measurements in nm.
Measured 2 times by independent persons as a control.
Stages see “Results.”
CV, coefficient of variation in %; i, individuals investigated; M, median; Max, maximum; Min, minimum; n, number of structures measured in i individuals; SD, standard deviation; SDV, silicon deposition vesicles; SE, standard error of mean; SEM, scanning electron microscopy; TEM, transmission electron microscopy.
In the cytoplasm, the silicon granules are surrounded by the silicalemma (Fig. 36) which, however, is sometimes difficult to recognize (Fig. 39). In the cyst cover, the granules are embedded in mucous material, gradually decreasing in structural density from proximal to distal (Fig. 22). Indeed, most of the distal granules are lost in old cysts, where the slime has been partially decomposed; thus, the granular silica layer is usually thinner in older than in younger cysts. The granule surface appears smooth in the TEM (Fig. 11, 39), while often rough or spongious in the SEM, partially due to some adhering slime and middle developmental stages, which have a more or less wrinkled surface (Fig. 18, 19, 24). Developing silica granules are rather frequent in the distal zone of the cyst cover, indicating that they are excreted very early (Fig. 19). Although these granules lost the silicalemma, they do not disperse, possibly because the nanospheres are embedded in fibrous material (see genesis of the silicon granules); however, they tend to be larger than the same developmental stages in the cytoplasm, indicating some loss of structural integrity (Fig. 19, 34, 35). Rarely, silicon granules are enclosed in mitochondria (Fig. 21) or food vacuoles.
Both, the cytoplasmic and cystic silicon granules show the highly characteristic, conchoidal fracture pattern of glass sectioned with a diamond knife (Fig. 11, 19, 24, 39). Depending on the preparation processes and section conditions, few to many granules may be broken into pieces, some of which lie one upon the other; in the latter case, the fracture pattern disappears and the granules become very dense, sometimes showing “large” nanospheres (~ 100–200 nm) along the margin. The fracture pieces appear structureless (Fig. 11, 24, 38, 39). However, when granules are crushed by the physical forces of the diamond knife, they appear to be composed of nanospheres with an average diameter of 37 nm (Table 1; Fig. 19, 20).
Treating the thin sections with HF has no visible effect on the fine structure of the cells. However, HF has some staining effect, i.e. the grid film and the cell structures become more electron dense than in conventional preparations. After HF treatment, the silicon disappears, leaving membrane-like structures tracing the conchoidal fracture pattern both in developing (Fig. 27, 28) and mature granules (Fig. 11, 12, 24–26). These “residue lines” could not be removed by prolonged application and/or high concentrations of HF.
Genesis of the silicon granules
Silicon granules were produced throughout the life cycle, except in the cysts, and developmental stages were found in 12 trophic specimens and in five dividers, where they were most numerous. Although granule genesis is a continuous process, we distinguished four stages.
Generally, the silicon granules originate from dense nanospheres with a size of 16–53 nm, usually 20–40 nm (Table 1). We use nanospheres as a descriptive term for the minute globules composing developing and mature silicon granules. The nanospheres have the same shape (globular) and structure (amorphic with smooth surface) in all developmental stages (Fig. 31–37) and in mature granules (Fig. 20), while the average size is distinctly lower (27 vs. 38 nm) in maturing granules (Table 1). The SDV are broadly ellipsoidal throughout development, just as are the mature granules (Table 1). These features of the nanospheres will not be repeated in the stage descriptions.
Stage 1 (Table 1 and Fig. 30, 31)
Clear vesicles with some dense nanospheres, single and in small groups, occur near the distal side of the dictyosomes. The nanospheres dissolve in hydroflouric acid. The SDVs have an average size of 769 nm × 607 nm and are bounded by the silicalemma, i.e. a unit-type membrane. The nanospheres are sometimes concentrated near the silicalemma and are usually embedded in some fluffy (organic?) material.
Stage 2 (Table 1 and Fig. 32, 33, 40)
The SDV have grown considerably to an average size of about 1,348 nm × 1,127 nm. They contain a coarsely meshed accumulation of nanospheres embedded in fibrous material and showing silicon with HF (Fig. 32, 33) and EELS analysis (Fig. 40).
Stage 3 (Table 1 and Fig. 19, 34, 35)
In this and the following stage, the SDVS decrease in size to an average of 851 nm × 640 nm. The vesicles are now packed with nanospheres in a more or less distinct fibrous (organic?) matrix forming a fine-meshed, spongious pattern. Silicon deposition vesicles of about this stage are possibly the first to become secreted during encystment because rather many of them occur in the distal zone of the cyst envelope (Fig. 19).
Stage 4 (Fig. 19, 27, 28, 36, 37)
The nanospheres polymerize to larger, amorphic masses occasionally already showing the characteristic fracture pattern of glass in some areas (Fig. 36, 37). Concomitantly, the diameter of the silicon mass decreases slightly, exposing the silicalemma.
Stage 5 (Table 1 and Fig. 24, 38, 39)
Finally, the nanospheres polymerize to an amorphic granule, showing the typical size and fracture pattern of mature granules (Fig. 24, 39). The silicalemma now attaches to the amorphous contents and is thus often not clearly recognizable (Fig. 39). Rarely, the SDVs are divided by the silicalemma in two or three chambers (Fig. 38).
DISCUSSION
Silicon (glass) in ciliates
To the best of our knowledge, biomineralized silicon has never been recorded from the phylum Ciliophora. At first glance, this appeared surprising because crystals and sparkling, crystal-like granules are quite frequent in ciliates (Berger 2006; Foissner 1993; Foissner et al. 2002). Thus, we made a survey of the TEM literature of all main ciliate groups (for a review, see Lynn 2008). Although crystals and crystal-like structures were frequently mentioned, none showed the characteristic fracture pattern of glass (Fig. 11, 24). Then, we supposed that glass granules could have been overlooked or not mentioned, especially in colpodids and hypotrichs, where crystals and sparkling, crystal-like granules are frequent (Berger 1999, 2006, 2008; Foissner et al. 2002). Accordingly, we re-evaluated not only the detailed papers by Lynn (1976a–d) but also thousands of unpublished TEM micrographs from species we had investigated over the years: the colpodids Bryometopus (Wirnsberger, Foissner, and Adam 1985), Grossglockneria (Aescht, Foissner, and Mulisch 1991), Bursaridium (Foissner 1993), Cosmocolpoda (Foissner and Foissner 1994), and Pseudocyrtolophosis (Foissner and Foissner, unpubl. data) as well as the hypotrichs Euplotes (Foissner 1977), Kahliella (Foissner and Foissner 1987), Pseudokeronopsis (Wirnsberger and Hausmann 1988), and Engelmanniella (Wirnsberger-Aescht, Foissner, and Foissner 1989, 1990). No indication of silicon was found. Thus, silicon seems to be rare in ciliates and is possibly confined to marynid colpodids (for reviews of species, see Foissner 1993; Foissner et al. 2002). However, even in that group glass is not a general feature because it is lacking in the cyst wall of a species very similar to M. umbrellata (see identification; Foissner, unpubl. data).
Coming back to the uniqueness of our record, the rather recent study by Warren and Carey (1983) must be mentioned. They observed minute spheres on the dorsal surface of the lorica of Platycola truncata, a common sessile peritrich ciliate in limnetic habitats (for a review, see Foissner, Berger, and Kohmann 1992). These spheres had a usual size of 30–60 nm and appeared to have no internal structure although some were completely dense while others appeared to possess a clear centre, with varying degrees of wall thickness. Their EDAX analysis showed the spheres to be composed mainly of silicon and phosphor, followed by calcium and iron.
Unfortunately, Warren and Carey (1983) could not clarify the origin of the spheres. Thus, they could not exclude that environmental silicon has been chemically bound to organic matter, as it is widely assumed for iron and manganese, which cause a browning of organic loricae and stalks. The spheres did not show the typical fracture pattern of glass sectioned with a diamond knife (see “Results”, Fig. 11, 24), but the size and the appearance of these dense spheres highly resembled the nanospheres composing the silicon granules of M. umbrellata (Fig. 20) and other organisms (Lins et al. 2002; Perry et al. 2003). It is also strange that oxygen was not detected in their EDAX analysis, excluding silica (SiO2) as a main component (cp. Fig. 6–8).
Fine structure of the silicon granules
We mentioned several times the highly characteristic, conchoidal fracture pattern of the silicon granules when sectioned with a diamond knife (Fig. 11, 19, 24). This phenomenon is well known from a great variety of silicon-using organisms and has been excellently reviewed by Garrone et al. (1981). These authors also discussed the residue (fracture) lines remaining after HF treatment (Fig. 12, 25, 26) and suggested three models to explain this pattern. (i) The residue lines might be composed of organic or inorganic material that impregnates the silicon. (ii) The residue lines could be caused by a “sweeping along” of the epoxy resin surrounding the embedded silicon structure. They considered it as conceivable that this resin is thus deposited at the edges of the silica fragments and prevents the action of HF. (iii) The regularity in size and shape of the fracture units and their constancy may also be due to an underlying, repeating pattern of a matrix of some type that is involved in polymerization. Such a matrix could contain a repeating pattern of “centres of polymerization,” the edges of which are more prone to fracture and stress (Garrone et al. 1981).
Our observations on M. umbrellata could not exclude model (iii) but favour a modified model (ii) because we could exclude that the residue lines were caused by the grid film pushed together (Fig. 29). We also could not exclude model (i), although the residue lines did not disappear after treatment of the sections with 0.5 N NaOH and 20% (v/v) phosphoric acid for 5 min each, indicating that they are made of inorganic material. However, we now know that biosilica contains organic material, mainly polycationic peptides termed silaffins (Kröger et al. 2000, 2002). Unfortunately, it is still not known where the silaffins are located within the silicon spheres or structures/granules.
Finally, a curious property of the silicon granules must be mentioned: in the cytoplasm, they sparkle conspicuously under interference contrast illumination (Fig. 5), while they are of ordinary brightness when becoming part of the cyst wall (Fig. 14). However, all methods used in the present investigation show that cytoplasmic and cystic granules are identical. First, we supposed that this could be due to the loss of Brownian molecular movement because the cystic silicon granules are firmly anchored in the slime of the wall. However, the cytoplasmic granules maintain sparkling when any movement is excluded by air-drying or chemical fixation of the cell (data not shown). Thus, we cannot provide an explanation for this phenomenon, but it indicates structural changes when the granules have left the cytoplasm.
Silicon genesis
Originally, we supposed that the silicon of M. umbrellata could originate from food bacteria, which themselves contained dense inclusions highly similar to the nanospheres composing the silicon granules; further, many of these inclusions disappeared after HF application (Foissner, Peukert, and Krautgartner 2007). This idea was not unlikely because various bacteria can dissolve polymeric silicon and accumulate silicate ions (Lauwers and Heinen 1974). However, EELS analyses showed the bacterial inclusions to be composed of organic material (data not shown).
Further observations suggested that the silicon granules of M. umbrellata developed in vesicles produced by the Golgi apparatus (Fig. 30, 31). This kind of genesis has been observed in many, but not all, organisms, for instance, in diatoms, testate amoebae, chrysophytes, and sponges (for reviews, see Anderson 1994; Simpson and Volcani 1981, Uriz, Turon, and Becerro 2003). Based on these data, Uriz et al. (2003) suggested a general model of biogenic silification: “Nanosphere formation is the first observable feature of silica polymerization both in organisms and in vitro; they increase in size and fuse with each other forming a network as the silification proceeds.” This model matches our data from M. umbrellata: the nanospheres composing developing granules are smaller than those composing the mature granules (Table 1) and are arranged in a spongious pattern (Fig. 32–34) before polymerizing to glass granules (Fig. 36–39). Indeed, our Fig. 32–35 and 40 are conspicuously similar to the pattern generated by silaffins in vitro (cp. with Fig. 4A–D in Kröger et al. 2002). Silaffins are polycationic peptides very likely guiding polymerization and arrangement of biosilica (Kröger et al. 2000).
Function of silicon in Maryna umbrellata
As in many other organisms (Patterson and Dürrschmidt 1986), concrete data on the function of silicon in M. umbrellata are not available. However, the location of the silicon granules gives some indication. In the interphase cell, many granules are concentrated in a dense, subapical area, suggesting some function in light perception (for a review, see Kuhlmann 1998). Indeed, M. umbrellata is positively phototactic: when half of a culture cup is covered with black paper, the specimens accumulate in the bright half.
A second main function of the silicon granules may be to strengthen the cyst wall because glass is more solid and stable than organic matter. Further, the glass cover may reduce desiccation (Pigon 1955) and cyst predation because the organic contents will not be discernible for other organisms.
Palaeontological significance
Silicon fossilizes excellently, as shown by many protists and sponges (Bovee 1981; Foissner and Schiller 2001; Morris 1998). Thus, the silicon cyst cover of M. umbrellata should also fossilize. There are, indeed, numerous globular microfossils collectively termed “spheres,” many of which highly resemble protist cysts (for a review, see Pokorný 1958). Most of the microfossils are calcareous or chitinous in nature, but few have been tested for silicon. Unfortunately, the cyst of M. umbrellata does not have a specific external morphology, e.g. spines or pustules. Thus, a definite identification in the fossil record will be very difficult.
ACKNOWLEDGMENTS
This study was supported by grants of the Austrian Science Foundation, FWF projects P-19699-B17, P-20360-B17, and P-18869-B16. Thanks to Prof. Dr. Erko Stackebrandt (DSMZ, Braunschweig) for helpful comments on bacteria. The technical assistance of Mag. Gudrun Fuss, Mag. Barbara Harl, Robert Schörghofer, and Andreas Zankl is greatly acknowledged.
LITERATURE CITED
- Aescht E, Foissner W, Mulisch M. Ultrastructure of the mycophagous ciliate Grossglockneria acuta (Ciliophora, Colpodea) and phylogenetic affinities of colpodid ciliates. Eur. J. Protistol. 1991;26:350–364. doi: 10.1016/S0932-4739(11)80156-2. [DOI] [PubMed] [Google Scholar]
- Anderson OR. Cytoplasmic origin and surface deposition of siliceous structures in Sarcodina. Protoplasma. 1994;181:61–77. [Google Scholar]
- Berger H. Monograph of the Oxytrichidae (Ciliophora, Hypotrichia) Kluwer; Dordrecht: 1999. [Google Scholar]
- Berger H. Monograph of the Urostyloidea (Ciliophora, Hypotricha) Springer; Dordrecht: 2006. [Google Scholar]
- Berger H. Monograph of the Amphisiellidae and Trachelostylidae (Ciliophora, Hypotricha) Springer; Dordrecht: 2008. [Google Scholar]
- Bovee EC. Distribution and forms of siliceous structures among Protozoa. In: Simpson TL, Volcani BE, editors. Silicon and Siliceous Structures in Biological Systems. Springer; New York: 1981. pp. 233–279. [Google Scholar]
- Ehrlich HL. Geomicrobiology. 2nd ed. Dekker; New York: 1990. [Google Scholar]
- Foissner W. Euplotes moebiusi f. quadricirratus (Ciliophora, Hypotrichida) II. Die Feinstruktur einiger cytoplasmatischer Organellen. Naturk. Jb. Stadt Linz. 1977;23:17–24. [Google Scholar]
- Foissner W. Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. Eur. J. Protistol. 1991;27:313–330. doi: 10.1016/S0932-4739(11)80248-8. [DOI] [PubMed] [Google Scholar]
- Foissner W. Colpodea (Ciliophora) Protozoenfauna. 1993;4:I–X–798. [Google Scholar]
- Foissner W. The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): transmission electron microscopy and phylogeny. Acta Protozool. 2005;44:217–230. [Google Scholar]
- Foissner I, Foissner W. The fine structure of the resting cysts of Kahliella simplex (Ciliata, Hypotrichida) Zool. Anz. 1987;218:65–74. [Google Scholar]
- Foissner W, Foissner I. Fine structure of Cosmocolpoda naschbergeri (Ciliophora, Colpodida) Arch. Protistenk. 1994;144:129–136. [Google Scholar]
- Foissner W, Schiller W. Stable for 15 million years: scanning electron microscope investigation of miocene euglyphid thecamoebians from Germany, with description of the new genus Scutiglypha. Eur. J. Protistol. 2001;37:167–180. [Google Scholar]
- Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha region and the Namib Desert. Denisia. 2002;5:1–1459. [Google Scholar]
- Foissner W, Berger H, Kohmann F. Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems – Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1992;5/92:1–502. [Google Scholar]
- Foissner W, Berger H, Schaumburg J. Identification and ecology of limnetic plankton ciliates. Informationsberichte des Bayer. Landesamtes für Wasserwirtschaft. 1999;3/99:1–793. [Google Scholar]
- Foissner W, Müller H, Weisse T. The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora, Oligotrichea): light and scanning electron microscopy, cytochemistry. Acta Protozool. 2005;44:201–215. [Google Scholar]
- Foissner W, Peukert B, Krautgartner W-D. A unique association between bacteria and ciliates: silicious residues from food bacteria are the main component of the cyst wall of Maryna umbrellata (Ciliophora, Colpodea). Abstract. Protistology. 2007;5:30A. [Google Scholar]
- Garrone R, Simpson TL, Pottu-Boumendil J. Ultrastructure and deposition of silica in sponges. In: Simpson TL, Volcani BE, editors. Silicon and Siliceous Structures in Biological Systems. Springer; New York: 1981. pp. 495–525. [Google Scholar]
- Gelei J. v. Die Marynidae der Sodagewässer in der Nähe von Szeged. Hidrol. Közl. 1950;30:107–119. [Google Scholar]
- Gutiérrez JC, Diaz S, Ortega R, Martín-González A. Ciliate resting cyst walls: a comparative review. Recent Res. Dev. Micobiol. 2003;7:361–379. [Google Scholar]
- Haeckel E. Ueber einige neue pelagische Infusorien. Jenaische Zeitschrift. 1873;7:561–568. plus plates XXVII, XXVIII. [Google Scholar]
- Hausmann K. Kristalle in Wimpertieren. Mikrokosmos. 1982;2:33–39. [Google Scholar]
- Hausmann K, Walz B. Feinstrukturelle und mikroanalytische Untersuchungen an den Kristallen und Lithosomen des Ciliaten Euplotes vannus. Protoplasma. 1979;99:67–77. [Google Scholar]
- Kalmus H. Eine monographische Zusammenfassung der wichtigsten Kenntnisse. Fischer; Jena: 1931. Paramecium. Das Pantoffeltierchen. [Google Scholar]
- Kofoid CA, Campbell AS. The Ciliata. The Tintinnoinea. Bull. Mus. Comp. Zool. Harv. 1939;84:1–473. [Google Scholar]
- Kröger N, Deutzmann R, Bergsdorf C, Sumper M. Species-specific polyamines from diatoms control silica morphology. Proc. Natl. Acad. Sci. USA. 2000;97:14133–14138. doi: 10.1073/pnas.260496497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger N, Lorenz S, Brunner E, Sumper M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science. 2002;298:584–586. doi: 10.1126/science.1076221. [DOI] [PubMed] [Google Scholar]
- Kuhlmann H-W. Photomovements in ciliated protozoa. Naturwissenschaften. 1998;85:143–154. [Google Scholar]
- Lauwers AM, Heinen W. Bio-degradation and utilization of silica and quartz. Arch. Microbiol. 1974;95:67–78. doi: 10.1007/BF02451749. [DOI] [PubMed] [Google Scholar]
- Laval-Peuto M. Classe des Oligotrichea Bütschli, 1887. Ordre des Tintinnida Kofoid et Campbell, 1929. In: de Puytorac, P., editor. Infusoires Cilies, Systematique. Traité de Zoologie. Vol. 2. Masson, Paris, Milan, Barcelone: 1994. pp. 181–219. [Google Scholar]
- Lins U, Barros CF, Cunha da M, Costa Miguens F. Structure, morphology, and composition of silicon biocomposites in the palm tree Syagrus coronata (Mart.) Becc. Protoplasma. 2002;220:89–96. doi: 10.1007/s00709-002-0036-5. [DOI] [PubMed] [Google Scholar]
- Lynn DH. Comparative ultrastructure and systematics of the Colpodida (Ciliophora): structural differentiation in the cortex of Colpoda simulans. Trans. Am. Microsc. Soc. 1976a;95:581–599. [Google Scholar]
- Lynn DH. Comparative ultrastructure and systematics of the Colpodida. An ultrastructural description of Colpoda maupasi Enriquez, 1908. Can. J. Zool. 1976b;54:405–420. [Google Scholar]
- Lynn DH. Comparative ultrastructure and systematics of the Colpodida. Fine structural specializations associated with large body size in Tillina magna Gruber, 1880. Protistologica. 1976c;4:629–648. [Google Scholar]
- Lynn DH. Comparative ultrastructure and systematics of the Colpodida. Structural conservatism hypothesis and a description of Colpoda steinii Maupas. J. Protozool. 1976d;23:302–314. [Google Scholar]
- Lynn DH. Characterization, Classification, and Guide to the Literature. 3rd ed. Springer; Dordrecht: 2008. The Ciliated Protozoa. [Google Scholar]
- Morris SC. The question of metazoan monophyly and the fossil record. In: Müller WEG, editor. Progress in Molecular and Subcellular Biology. Vol. 21. Springer; Berlin: 1998. pp. 1–19. [DOI] [PubMed] [Google Scholar]
- Patterson DJ, Dürrschmidt M. Siliceous structures formed by Heliozoa and heliozoon-like amoebae. In: Leadbeater BC, Riding R, editors. Biomineralization in Lower Plants and Animals. Clarendon Press; Oxford: 1986. pp. 361–374. [Google Scholar]
- Pautard FGE. Calcification in single cells: with an appraisal of the relationship between Spirostomum ambiguum and the osteocyte. Belle W. Baruch Libr. Mar. Sci. 1976;5:33–53. [Google Scholar]
- Perry CC, Belton D, Shafran K. Studies of biosilicas; structural aspects, chemical principles, model studies and the future. In: Müller WEG, editor. Progress in Molecular and Subcellular Biology. Vol. 33. Springer; Berlin: 2003. pp. 269–299. [DOI] [PubMed] [Google Scholar]
- Pigon A. Permeability to water of active forms and cysts of Actinosphaerium. Bull. Acad. Pol. Sci. 1955;3:235–239. [Google Scholar]
- Pokorný V. Grundzüge der zoologischen Mikropalaöntologie. VEB Deutscher Verlag der Wissenschaften; Berlin: 1958. [Google Scholar]
- Simpson TL, Volcani BE. Introduction. In: Simpson TL, Volcani BE, editors. Silicon and Siliceous Structures in Biological Systems. Springer; New York: 1981. pp. 3–12. [Google Scholar]
- Uriz MJ, Turon X, Becerro MA. Silica deposition in demosponges. In: Müller WEG, editor. Progress in Molecular and Subcellular Biology. Vol. 33. Springer; Berlin: 2003. pp. 163–193. [DOI] [PubMed] [Google Scholar]
- Warren A, Carey PG. Lorica structure of the freshwater ciliate Platycola decumbens Ehrenberg, 1830 (Peritrichida, Vaginicolidae) Protistologica. 1983;19:5–20. [Google Scholar]
- Williams RJP. The functional forms of biominerals. In: Mann S, Webb J, Williams RJP, editors. Biomineralization. Chemical and Biochemical Perspectives. VCH; Weinheim: 1989. pp. 1–34. [Google Scholar]
- Wirnsberger E, Hausmann K. Fine structure of Pseudokeronopsis carnea (Ciliophora, Hypotrichida) J. Protozool. 1988;35:182–189. [Google Scholar]
- Wirnsberger E, Foissner W, Adam H. Morphogenesis, fine structure, and phylogenetic relationships of the “heterotrich” ciliate Bryometopus atypicus (Protozoa, Colpodea) Annls Sci. Nat. (Zool.) 1985;7:113–128. [Google Scholar]
- Wirnsberger-Aescht E, Foissner W, Foissner I. Morphogenesis and ultrastructure of the soil ciliate Engelmanniella mobilis (Ciliophora, Hypotrichida) Eur. J. Protistol. 1989;24:354–368. doi: 10.1016/S0932-4739(89)80006-9. [DOI] [PubMed] [Google Scholar]
- Wirnsberger-Aescht E, Foissner W, Foissner I. Natural and cultured variability of Engelmanniella mobilis (Ciliophora, Hypotrichida); with notes on the ultrastructure of its resting cyst. Arch. Protistenk. 1990;138:29–49. [Google Scholar]


