Abstract
Background
Clinical trials in ischemic patients showed the safety and benefit of autologous bone marrow progenitor cell transplantation. Non–bone marrow progenitor cells with proangiogenic capacities have been described, yet they remain clinically unexploited owing to their scarcity, difficulty of access, and low ex vivo expansibility. We investigated the presence, antigenic profile, expansion capacity, and proangiogenic potential of progenitor cells from the saphenous vein of patients undergoing coronary artery bypass surgery.
Methods and Results
CD34-positive cells, negative for the endothelial marker von Willebrand factor, were localized around adventitial vasa vasorum. After dissection of the vein from surrounding tissues and enzymatic digestion, CD34-positive/CD31-negative cells were isolated by selective culture, immunomagnetic beads, or fluorescence-assisted cell sorting. In the presence of serum, CD34-positive/CD31-negative cells gave rise to a highly proliferative population that expressed pericyte/mesenchymal antigens together with the stem cell marker Sox2 and showed clonogenic and multilineage differentiation capacities. We called this population “saphenous vein–derived progenitor cells” (SVPs). In culture, SVPs integrated into networks formed by endothelial cells and supported angiogenesis through paracrine mechanisms. Reciprocally, endothelial cell–released factors facilitated SVP migration. These interactive responses were inhibited by Tie-2 or platelet-derived growth factor-BB blockade. Intramuscular injection of SVPs in ischemic limbs of immunodeficient mice improved neovascularization and blood flow recovery. At 14 days after transplantation, proliferating SVPs were still detectable in the recipient muscles, where they established N-cadherin–mediated physical contact with the capillary endothelium.
Conclusions
SVPs generated from human vein CD34-positive/CD31-negative progenitor cells might represent a new therapeutic tool for angiogenic therapy in ischemic patients.
Keywords: CD34 antigen, pericytes, angiogenesis factors, ischemia, cell therapy
Recent evidence indicating the presence of progenitor cells in arteries and veins has inspired hope for their application in regenerative vascular medicine.1-3 In fetal and postnatal vessels, putative proangiogenic progenitors reside in the vasculogenic niche, which comprises adventitial stromal cells and mature vascular cells of the vasa vasorum.3 We previously showed that CD34-positive (CD34pos) cells from the human fetal aorta coexpress stem cell markers such as CD133 and c-Kit, are clonogenic, and give rise to vascular cells and skeletal myocytes.2 Local implantation of fetal aorta–derived CD34pos/CD133-positive (CD133pos) cells promoted reparative neovascularization in models of ischemia and diabetic ulcers through incorporation into nascent vessels and paracrine stimulation of resident vascular cells.2,4 Ethical concerns and immunogenic/tumorigenic problems limit the clinical use of embryonic/fetal stem cells. On the other hand, CD34pos progenitor cells from adult vessels show low coexpression of CD133 and show limited expansibility and differentiation potential.1,3 To date, no preclinical data are available on the in vivo regenerative potential of adult vessel–derived progenitor cells. The establishment of standardized protocols for isolation of proangiogenic cells from adult tissues and the demonstration of therapeutic activity are therefore needed.
The saphenous vein is the conduit of choice in coronary and peripheral artery bypass operation. During the surgical procedure, a large portion of the vein is excised, but usually, only part of it is transplanted. Here, we demonstrate the presence of progenitor cells with proangiogenic capacity in saphenous vein leftovers from elderly cardiovascular patients. Furthermore, we show the ability of these progenitor cells to give rise to a clonogenic population able to stimulate in vitro angiogenic activity of endothelial cells (ECs) and to improve in vivo reparative neovascularization.
Methods
An expanded Methods section is provided in the online-only Data Supplement.
Vein Collection
Veins were collected during bypass operations and dissected carefully from the surrounding tissue, washed in PBS with antibiotics, and then processed for immunohistochemistry or cell culture.
Immunohistochemical Analysis of Human Saphenous Veins
Sections were stained with antihuman CD34 in combination with von Willebrand factor (vWF) or with CD31 and either NG2 or platelet-derived growth factor receptor-β (PDGFRβ). Alexa Fluor–conjugated secondary antibodies were used. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Cell Isolation and Culture
Venous samples were minced and digested with Liberase 2 (Roche, Basel, Switzerland). For culture selection, 2×105 cells/mL were plated in the presence of Human Medium (HM; complete NeuroCult medium, StemCell Technologies Inc, Vancouver, British Columbia, Canada) that contained basic fibroblast growth factor (10 ng/mL) and epidermal growth factor (20 ng/mL; both from R&D Systems, Minneapolis, Minn) on uncoated wells (n=5).
CD34pos/CD31-negative (CD31neg) cells were isolated from saphenous vein digests either by magnetic bead–assisted cell sorting (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany; n=6) or by fluorescence-activated cell sorting (n 2). Fluorescence-activated cell sorting was performed with a high-speed cell sorter (MoFlo, Beckman Coulter, Fullerton, Colo). Purity of the preparations was assessed by flow cytometry analysis. Differentiation/expansion was performed by seeding CD34posCD31neg cells obtained with each method on fibronectin-coated plates in endothelial growth medium (EGM2, Lonza, Basel, Switzerland) that contained 2% fetal bovine serum (Lonza).
Flow Cytometry Analysis
Single-cell suspensions of freshly prepared vein digests were stained with a combination of fluorescence-labeled antibodies against CD34, CD31, CD133, CD117/c-Kit, chemokine (C-X-C motif) receptor 4 (CXCR4), kinase insert domain–containing receptor (KDR), CD45, CD11b, PDGFRβ, and NG2. Cultured cells were stained for CD44, CD90, CD105, CD29, CD49a, CD49b, CD13, CD59, CD73, and CD146 in addition to the antibodies reported above. Data were acquired in a flow cytometric sorting Canto II (BD Biosciences, Franklin Lakes, NJ) flow cytometer and analyzed with flow cytometric sorting Diva software (BD Biosciences).
Immunocytochemical Characterization
Cultured cells were analyzed by immunocytochemistry for expression of NG2, PDGFRβ, desmin, vimentin, nestin, sex-determining region Y-box2 (Sox2), CD90, CD44, α-sarcomeric actin, α-smooth muscle actin, CD31, osteocalcin, β3-tubulin, acetylcholine transferase, the dopamine transporter, tyrosine hydroxylase, and N-cadherin. Unconjugated primary antibodies were followed by the appropriate fluorescent secondary antibody. Nuclei were stained with DAPI.
Single-Cell Cloning
Single-cell cloning was accomplished by use of a motorized device connected to the flow cytometric sorter (Cyclone, Beckman Coulter).
Multilineage Differentiation
The ability of saphenous vein–derived progenitor cells (SVPs) to differentiate into osteoblasts, adipocytes, chondrocytes, ECs, hepatocytes, myocytes, and neuronal cells was tested as described previously5 and as detailed in the online-only Data Supplement Methods.
Coculture and Conditioned Media Studies
SVPs were labeled with CM-DiI (Molecular Probes [Invitrogen], Eugene, Ore) and saphenous vein–derived ECs (SVECs) were either stained with FITC-conjugated Ulex europaeus agglutinin I (Sigma-Aldrich Corp, St Louis, Mo) or left unlabeled. Cells were then cocultured (1:4 ratio of SVPs to SVECs) on Matrigel (3D; BD Biosciences) or on gelatin-coated coverslips (2D). Conditioned culture medium (CCM) was obtained from 70% confluent SVPs or SVECs cultured for 48 hours in fresh medium. The unconditioned culture medium served as control. Matrigel angiogenesis assay and cell migration, proliferation, and apoptosis assays were performed on SVECs and SVPs as described previously.4,6
Hindlimb Ischemia Model
Male 8-week-old CD1 Foxn1nu/nu mice (Charles River Laboratories, Wilmington, Mass) underwent unilateral limb ischemia as described previously.7 One day after induction of ischemia, 8×104 DiI-labeled SVPs (passage 6), so-called early-culture endothelial progenitor cells,8 or vehicle (DMEM, 30 μL) was injected into 3 different points of the ischemic adductor muscle (n=7 mice per group). Blood flow recovery was followed up by laser Doppler flowmetry as reported previously.7
Assessment of Neovascularization in Ischemic Muscles
Sections (3 μm) from paraffin-embedded adductor muscles, harvested at day 14 after ischemia (n=4 mice per group), were stained with isolectin B4 and α-smooth muscle actin to recognize ECs and smooth muscle cells, respectively, and to calculate capillary and arteriole density. SVP phenotype in vivo was confirmed by staining with anti-human CD44. Proliferating cell nuclear antigen was used as a marker for proliferation.
Position of SVPs in the muscle was assessed in 100-μm-thick sections after staining with directly fluorophore-labeled isolectin B4 and anti-N-cadherin antibody. Z-stack images were taken by confocal microscope and 3D reconstructed by use of Volocity imaging software (Improvision, Coventry, United Kingdom).
Statistical Analysis
Values are presented as mean±SEM. Analyses were performed with GraphPad Prism 5 software (GraphPad Software, San Diego, Calif). Statistical significance was assessed by use of paired or unpaired t test for comparison between 2 groups. For analysis of blood flow recovery and cell biology assays in which measurements were repeated on the same subject at different time points, repeated-measures ANOVA was used, followed by Bonferroni post hoc test. Finally, 2-way ANOVA for block design was applied when variables that were measured at least in triplicate within an experiment were compared, with the experiment being repeated several times. P<0.05 was interpreted to denote statistical significance.
Results
Human Saphenous Veins Contain CD34pos Progenitor Cells
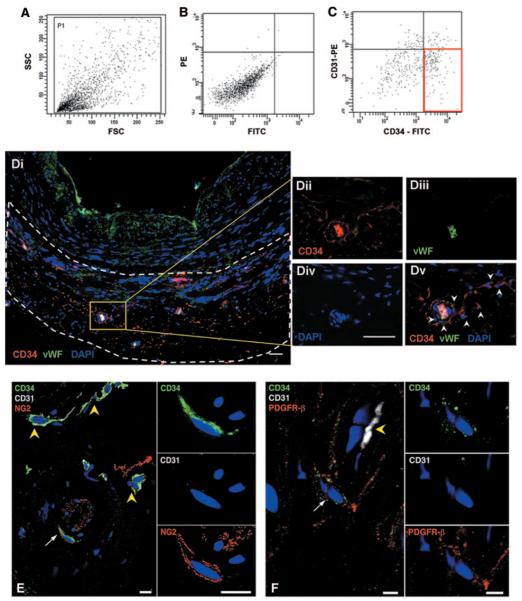
A total of 4×105 to 10×105 cells were obtained from digestion of single saphenous veins (4 to 5 cm length). Flow cytometry analysis of 12 veins demonstrated the presence of a CD34pos cell population that accounted for 28±4% of total cells. The Table shows the relative abundance of the chemokine receptor CXCR4 and markers of hematopoietic cells (CD45, CD11b), progenitor cells (CD133, c-Kit), pericytes (NG2), and ECs (CD31 and KDR) in the CD34pos population. Interestingly, the CD34posCD31neg fraction represented 23.5±4.5% of total cells (Figure 1A through 1C).
Table.
Flow Cytometry Analysis of Cells Obtained From Digested Saphenous Veins (n=12)
| Population | % of Total* | % of CD34 Population*† |
|---|---|---|
| CD34+ | 27.6±4.1 | 100 |
| CD133+ | 4.8±1.6 | 5.6±1.7 |
| c-Kit+ | 2.4±1.3 | 4.5±1.6 |
| KDR+ | 1.1±0.5 | 2.0±0.3 |
| CXCR4+ | 16.9±4.9 | 22.1±9.1 |
| NG2+ | 4.5±0.9 | 2.0±1.4 |
| CD31+ | 12.0±4.6 | 13.8±6.8 |
| CD45+ | 9.7±3.1 | 7.1±1.4 |
| CD11b+ | 8.8±6.5 | 6.7±4.7 |
Values are expressed as mean±SEM.
Values in this column are percentages of each marker in the CD34pos fraction.
Figure 1.
Identification of CD34pos cells in saphenous veins. Representative scattergrams (A and B) showing the relative abundance of CD34posCD31neg cells (C, red box) within saphenous vein digests. Forward-sideward scatterplot (A) and isotype control are shown (B). FSC indicates forward scatter; SSC, side scatter; PE, phycoerythrin; and FITC, fluorescein isothiocyanate. Di, Low-magnification picture shows morphology of saphenous vein and distribution of vasa vasorum in adventitia (delimited by dashed lines). Sections were stained for progenitor/EC marker CD34 (red; Dii), EC marker vWF (green; Diii), and nuclei (DAPI, blue, Div). Representative confocal image merge reveals the presence of CD34posvWFneg progenitor cells located in the perivascular zone of the vasa vasorum (white arrowheads in Dv). Furthermore, in the same region, a few CD34pos cells (green; E and F) coexpressed NG2 (red, E) or PDGFRβ (red, F). Double-positive cells are indicated by white arrows. CD31 (white; E and F) stained ECs of vasa vasorum (yellow arrowheads; E and F). Higher-magnification panels in E and F show adventitial CD34pos cells, negative for CD31 and coexpressing NG2 or PDGFRβ. Scale bar=50 μm (D) and 10 μm (E and F).
The localization of CD34pos cells in saphenous vein sections (n=5) was then examined by fluorescence immunohistochemistry. vWF-positive ECs lined the lumen of the vein and vasa vasorum (Figure 1Di), whereas CD34pos cells negative for the endothelial markers vWF and CD31 were prevalently localized in the adventitia, around or at a short distance from small vessels of the vasa vasorum (Figure 1D through 1F). A few CD34posCD31neg cells stained positively for NG2 and PDGFRβ, thus suggesting their affinity for mesenchymal/perivascular cells (Figure 1E and 1F).
CD34posCD31neg Cells Contain a Population of Mesenchymal Progenitors
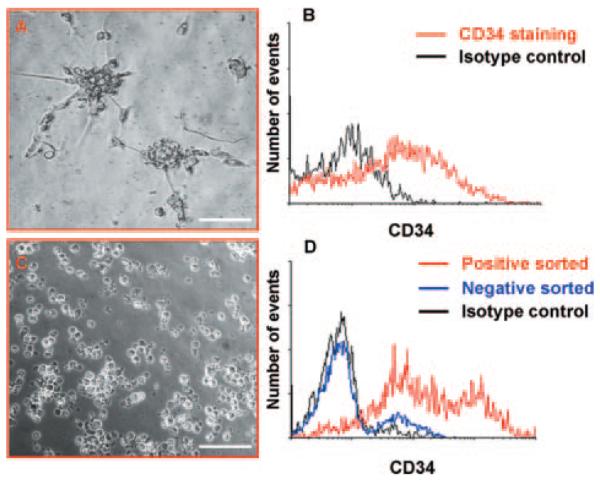
To further characterize the identity and differentiation capacity of saphenous vein–derived CD34pos nonendothelial cells, we used 3 different isolation strategies. First, freshly digested cells were cultured in HM, a medium that we have previously used to select CD34posCD133pos cells from fetal aortas.2 Under these culture conditions, most of the cells died, the few surviving cells appearing as single cells or small clusters. By day 5, cell aggregates grew, forming floating spheroids (Figure 2A). Those spheroids were abundant in CD34pos cells (52.8±5.2%; Figure 2B), whereas only a small fraction expressed CD133 (2.6±0.7%) or CD31 (1.1±1.0%). In addition, CD34posCD31neg cells were isolated by MACSorting, from which we obtained a purity of 70.1±7.1% (Figure 2C and 2D), or by flow cytometric sorting, which yielded 99% purity (online-only Data Supplement Figure IA through IC). In line with immunohistochemistry, flow cytometry analysis showed that the CD34posCD31neg population also contained small fractions that coexpressed NG2 (11.9±5.3%) and PDGFRβ (3.4±1.7%). Expression of the 2 markers was confirmed by reverse-transcription polymerase chain reaction (online-only Data Supplement Figure IID).
Figure 2.
Isolation of CD34pos cells. Morphology of spheroids derived from saphenous vein cells cultured for 5 days in HM (A). Spheroids were stained with CD34 or isotype control. Flow cytometry showed that they comprised ≈50% of CD34pos cells (B). Cells derived from magnetic bead isolation appeared as floating single cells (C). Histogram shows flow cytometry analysis of cells enriched by isolation with magnetic beads (D). Representatives of n=5 (A and B) and n=6 (C and D) experiments. Scale bar=100 μm.
CD34posCD31neg cells selected either by culture, MACS, or flow cytometric sorting (n=2 to 3 veins for each method) were plated on fibronectin in the presence of serum. Five to 10 days after plating, all preparations gave rise to small colonies composed of elongated and fast-growing cells. Of note, the morphology of CD34posCD31neg-derived cells was independent from the isolation procedure (online-only Data Supplement Figure IIA through IIC).
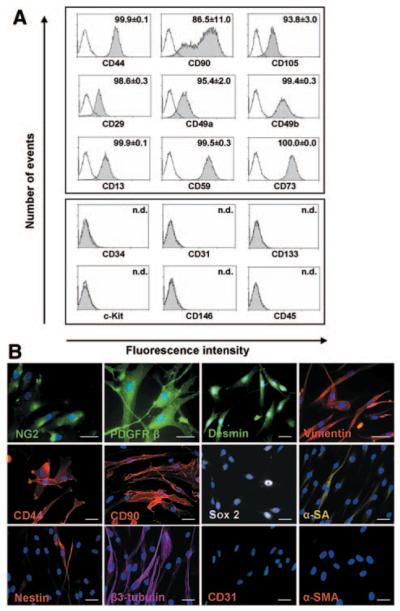
Cells at passage 4 (n=4 preparations) were assayed for their antigen expression by flow cytometry, which showed that serum-induced differentiation was associated with the loss of CD34. In addition, cells expressed the mesenchymal stem cell markers CD44, CD90, CD105, CD29, CD49a, CD49b, CD13, CD59, and CD73, although they were negative for CD31, CD133, c-Kit, CD146, and CD45 (Figure 3A). Immunocytochemical analysis was performed (n=3 preparations) to further define the cell characteristics (Figure 3B). Cells obtained by all 3 procedures consistently expressed desmin (≈99%), vimentin (95±5%), NG2 (62±6%), and PDGFRβ (48±2%). Of note, the expression of NG2 and PDGFRβ was increased remarkably upon differentiation of CD34posCD31neg cells in SVPs (online-only Data Supplement Figure IID).
Figure 3.
Characterization of the CD34posCD31neg-derived population. After culture in serum-containing medium, CD34posCD31neg cells differentiated into cells expressing mesenchymal/pericyte markers as assessed by flow cytometry (A; n.d. indicates not detectable; white, isotype control, gray, specific antibody staining [as indicated]) and immunocytochemistry (B; scale bar=50 μm, nuclei: DAPI [blue]). α-SA indicates α-sarcomeric actin; α-SMA, α-smooth muscle actin. Results are representative of 4 (A) or 3 (B) experiments.
As illustrated in Figure 3B, immunocytochemical analysis further showed that most of the CD34posCD31neg-derived cells were positive for Sox2 (75±17%) but did not express other embryonic-like transcription factors, such as Oct3/4 and NANOG (not shown). The muscle-specific α-sarcomeric actin was expressed in 12±10% of the cells. Interestingly, the early neuronal markers β3-tubulin and nestin were expressed by 58±15% and 20±9% of the population, respectively. In contrast, although isolated from the vessel wall, cells did not express CD31 or α-smooth muscle actin. They were also negative for epithelial cell–associated cytokeratins 8, 18, and 19 and the astrocyte marker glial fibrillary acidic protein (not shown). Thus, the antigenic phenotype of the CD34posCD31neg-derived progeny, henceforth termed SVPs, indicates similarities with undifferentiated cells of the mesenchymal/pericyte lineage.9,10
Importantly, SVPs could be expanded with a doubling time of ≈45 hours (calculated at passage 5), progressively growing in size and decelerating their proliferation rate after passage 10. Expansion of SVPs would potentially allow the generation of 30 to 50×106 cells starting from an average culture (at passage 3) of 2 to 3×105 cells. Furthermore, SVPs were routinely stored in liquid nitrogen and subsequently defrosted during the present study, which illustrates the feasibility of cryopreservation.
Clonogenic and Differentiation Potential
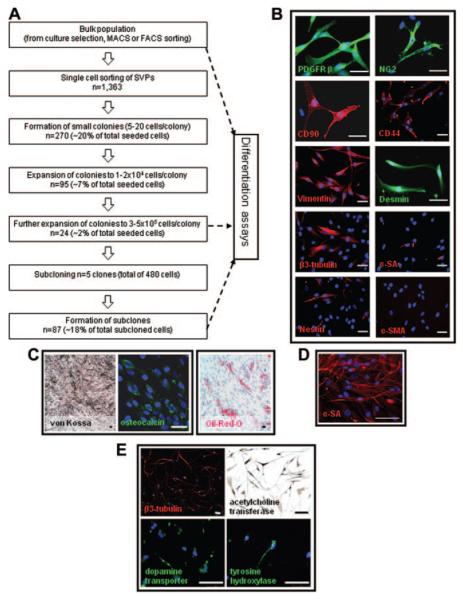
Next, we investigated whether SVPs possess 2 fundamental properties of progenitor cells, clonogenicity and multipotency. For the cloning assay, 1363 CD34posCD31neg cell-derived SVPs from 4 different populations were sorted into 96-well Terasaki plates (1 cell per well; Figure 4A). One week after sorting, 19.8±2.1% of the cells had formed colonies that contained 5 to 20 cells; however, only one third of these early developed clones (≈7% of the initially seeded cells) could be expanded to 1 to 2×104 cells, whereas ≈2% of the initially plated cells did grow further to approximately 3 to 5×105 cells and were subcloned with an efficiency of ≈18%, which generated highly plastic subclones (Figure 4A). Primary clones conserved the mesenchymal/pericyte immunophenotype of the original population (Figure 4B).
Figure 4.
Clonogenic assay and differentiation of SVPs. Single SVPs (1363) were deposited on Terasaki plates. Of the seeded cells, ≈20% formed small colonies, and 2% gave rise to large colonies and could be subcloned (efficiency 18%; A). Clones conserved the mesenchymal/pericyte phenotype of the polyclonal population (B). Under appropriate differentiation conditions, SVPs, their clones, and subclones displayed a wide differentiation capacity, which gave rise to cells of mesodermal lineage such as osteoblasts, adipocytes (C), and myocytes (D; α-sarcomeric actin, [α-SA]). They also generated neuron-like cells (E). Nuclei are identified by DAPI (blue). Scale bar 50 μm (B and C) or 100 μm (D and E). α-SMA indicates α-smooth muscle actin.
Finally, bulk populations of SVPs, clones, and subclones were tested for their ability to differentiate into derivatives of all 3 germ layers. When exposed to differentiation stimuli, SVPs were able to generate mesodermic lineages, such as osteoblasts, adipocytes (Figure 4C), and α-sarcomeric actin–positive myocytes (Figure 4D), whereas no chondrocyte or EC differentiation was obtained. Additionally, SVPs gave rise to neuron-like cells, which expressed early marker β3-tubulin, arrayed in filaments and bundles, and more differentiated markers such as acetylcholine transferase, the dopamine transporter, and tyrosine hydroxylase (Figure 4E). No differentiation toward hepatocytic cell lineage (endodermic differentiation) was detected (not shown). Interestingly, all subclones displayed a wide differentiation capacity, which gave rise to mesodermic lineages (adipocytes, osteoblasts, and myocytes) and neuron-like cells. Taken together, the results demonstrated that SVPs are capable of self-renewal and that their clones possess a broad differentiation capacity.
SVPs Directly Interact With ECs Supporting Their Network Formation Capacity
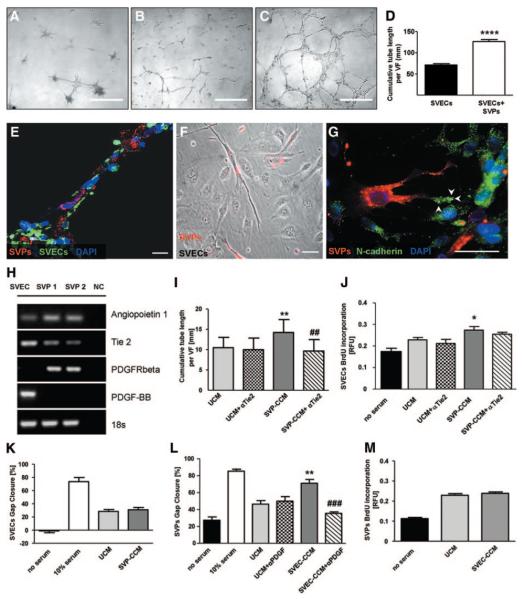
Perivascular mesenchymal cells, namely, pericytes, play a fundamental role in angiogenesis by sustaining the organization of provisional EC structures and strengthening intercellular connections.9 To demonstrate the supportive capacity of SVPs, we cocultured them with SVECs on Matrigel. As shown in Figure 5A, SVPs alone were able to form complex networks, composed of nodes connected by a number of thin branches. Culturing SVPs together with SVECs remarkably improved the network-forming capacity of SVECs (Figure 5B through 5D). We further investigated the connections established between the 2 cell types in coculture. SVECs formed continuous branches that were surrounded by SVPs (Figure 5E; online-only Data Supplement Movie I). Similarly, bidimensional coculture indicated that each SVP connected to multiple SVECs (Figure 5F; online-only Data Supplement Figure III). N-cadherin is expressed by both ECs and pericytes and is implicated in EC/pericyte interaction during vessel formation.11 In the present coculture model, N-cadherin was polarized on the SVP pseudopodia in contact with SVECs. In SVECs, N-cadherin was uniformly distributed on the cellular membrane (Figure 5G). These data indicate the establishment of physical interactions between the 2 populations that favored network enhancement and stabilization.
Figure 5.
Interactions between SVPs and SVECs. SVPs formed peculiar structures in Matrigel (A). Network formation was strongly enhanced in SVP-SVEC coculture (C) compared with culture of SVECs alone (B). D, Bar graph showing average tube length; values are mean±SEM of 4 experiments performed in quadruplicate. VF indicates view field. ****P<0.0001 vs SVECs. Confocal microscopy image shows tubes formed by SVECs (isolectin, green) and covered by SVPs (DiI, red; E). F, Phase-contrast microscopy image showing the interaction of SVPs (DiI, red) with multiple SVECs (unstained) in 2D coculture. G, Representative confocal microscopy image showing different patterns of N-cadherin staining (green fluorescence) in SVPs (localized at filopodia, white arrowheads) and ECs (diffuse to all of the cell membrane). Scale bars: 500 μm (A, B, and C); 50 μm (F and G); 20 μm (E). H, Expression of Ang-1/Tie-2 and PDGF-BB/PDGFRβ in SVECs and SVPs. NC indicates negative control. I through M, Bar graphs show the reciprocal paracrine effect of SVECs and SVP-CCM. SVP-CCM influenced SVEC network-formation capacity (I) and proliferation (J) but not migration (K). Tie-2 blockade inhibited the stimulatory action on the network-formation capacity of SVECs (I). SVP-CCM enhanced SVEC migration (L), this effect being inhibited by PDGF-BB blockade, but did not alter their proliferation (M). Positive control was medium that contained 10% serum, whereas negative control was serum-deprived medium. Values are mean±SEM of 3 experiments performed in quadruplicate. *P<0.05 and **P<0.01 vs unconditioned culture medium; ##P<0.01 and ###P<0.001 vs CCM. UCM indicates unconditioned medium; BrdU, bromodeoxyuridine; and RFU, relative fluorescence unit.
SVPs Support EC Proliferation and Network Formation via Paracrine Mechanisms
By reverse-transcription polymerase chain reaction, we verified that SVECs and SVPs express complementary components of the angiopoietin (Ang)-1/Tie-2 and PDGF-BB/PDGFRβ systems, which are strongly implicated in EC/pericyte cross talk (Figure 5H).9 Furthermore, by ELISA, we confirmed that SVPs release Ang-1 (704±232 pg/mL) and SVECs release PDGF-BB (20.3±1.2 pg/mL) in their respective conditioned medium.
We therefore investigated whether the stimulatory action of SVPs on SVEC angiogenic activity is mediated by paracrine mechanisms. Accordingly, SVP-CCM remarkably supported the persistence of the EC network in the Matrigel assay compared with unconditioned medium (online-only Data Supplement Figure IV). Preincubation of SVECs with Tie-2–blocking antibody abrogated the stimulatory effect of SVP-CCM on network formation (Figure 5I).
Next, we investigated the effect of CCM from SVPs and SVECs on distinct cellular processes, namely, survival, proliferation, and migration. Incubation of SVECs with SVP-CCM significantly increased the rate of bromodeoxyuridine incorporation (Figure 5J) but did not modify the migratory activity of SVECs in the scratch assay (Figure 5K). Furthermore, application of SVP-CCM in a Caspase-Glo assay did not improve the survival of starved SVECs (data not shown).
Conversely, SVEC-CCM improved SVP migration (Figure 5L) but did not show any effect on proliferation compared with unconditioned culture medium (Figure 5M). The addition of PDGF-BB–blocking antibody to the SVEC-CCM abrogated its promotional effect on SVP migration (Figure 5L).
Intramuscular Injection of SVPs Improves Blood Flow Recovery in a Model of Hindlimb Ischemia
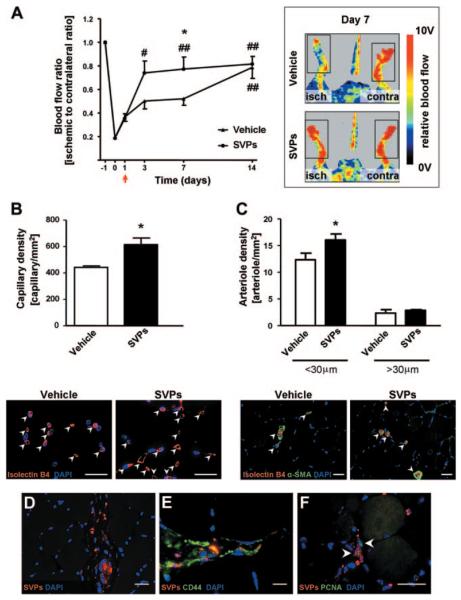
We then determined whether the proangiogenic activity of SVPs translates into improvement of reparative neovascularization in vivo. For this purpose, we injected SVPs or unconditioned medium (vehicle) intramuscularly in immunodeficient mice subjected to unilateral limb ischemia. We found that SVP administration significantly accelerated foot perfusion recovery compared with vehicle. In the SVP-treated group, full recovery was obtained at day 7 after ischemia; the same level was reached 7 days later in the vehicle-injected group (Figure 6A).
Figure 6.
In vivo proangiogenic effect of SVPs. A, Line graphs and representative laser Doppler images taken at day 7 from induction of unilateral limb ischemia (isch) illustrating the improvement of blood flow recovery in SVP-treated compared with vehicle-injected mice (arrow indicates time of cell injection; n=7 animals per group). Contra indicates contralateral. B and C, Bar graphs and representative pictures show increased capillary (B) and arteriole (C) density in muscles injected with SVPs compared with vehicle at 14 days after ischemia. Values are mean±SEM; n=4 animals per group.*P<0.05 vs vehicle; #P<0.05 and ##P<0.01 vs day 1. SVPs (DiI, red) persisted in ischemic muscle 14 days after injection (D), expressed human CD44 (E), and were positive for proliferating cell nuclear antigen (F; PCNA, arrowheads). Scale bar=20 μm. α-SMA indicates α-smooth muscle actin.
Immunohistochemistry demonstrated that the density of capillaries and small arterioles was significantly higher in SVP-injected than in vehicle-injected muscles (Figure 6B and 6C). To verify whether the effect of SVPs on arteriole formation was due to a paracrine action on vascular smooth muscle cells, murine vascular smooth muscle cells were stimulated with SVP-CCM in in vitro assays. We found that SVP-CCM improved vascular smooth muscle cell viability and survival but did not affect bromodeoxyuridine incorporation or migration (online-only Data Supplement Figure V). Transplantation of early-culture endothelial progenitor cells derived from peripheral blood monocytes of healthy subjects8 improved postischemic neovascularization without accelerating limb reperfusion (online-only Data Supplement Figure VI).
Engraftment of Transplanted SVPs Into Ischemic Tissues
At 14 days after injection, we still detected a large number of SVPs deeply engrafted in the injected adductor muscles (31.3±8.8 cells/section; Figure 6D). Furthermore, we confirmed that SVPs retained their phenotype in vivo, as shown by positivity for human CD44 (Figure 6E). Importantly, we detected diffuse proliferating cell nuclear antigen staining in engrafted SVPs (32.7±2.5%), which indicates that the cells were not just surviving in the tissue but were also actively proliferating (Figure 6F). At this late stage, it was still possible to detect proliferating murine capillary ECs and myofibers, but although a trend was evident, no difference in proliferation was detected between the SVP-injected and vehicle-injected groups (online-only Data Supplement Figure VII).
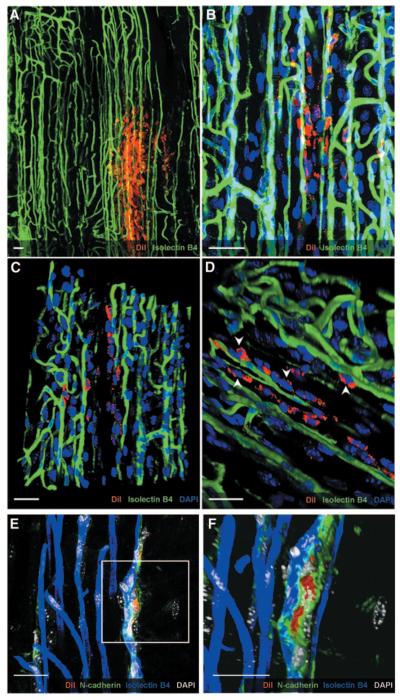
To study the localization of injected cells and the establishment of 3D contacts between host and recipient cells, we analyzed whole-mount preparations of adductor muscles. 3D reconstruction of confocal microscopy images confirmed the presence and abundance of SVPs in the injected tissue (Figure 7A) and indicated that cells were localized mainly around host capillaries (Figure 7B through 7D). Staining for N-cadherin showed polarization of the protein in the junctions formed between SVPs and capillary ECs, thus confirming the physical contact between them (Figure 7E and 7F). Taken together, these data suggest that SVPs have the capacity to incorporate in the host tissue and to localize around the capillaries as natural mural components.
Figure 7.
Interaction of SVPs with the host vasculature. Z-stack of confocal images showing numerous SVPs (CM-DiI; red) surrounding the host vasculature (isolectin B4; green; A and B). 3D reconstruction of the field shows physical contact between SVPs and ECs (C and D; white arrowheads). Direct interaction was also confirmed by N-cadherin staining (green), which accumulated in the junction between SVPs (red) and capillary ECs (blue; E and F). Nuclei were stained with DAPI (blue in A through D; white in E and F). Scale bar=25 μm.
Discussion
Seminal reports described the presence of mesenchymal precursors in vessels from young organ donors12 or donors of unspecified age3,13; however, to the best of our knowledge, the present study is the first to demonstrate the isolation of progenitor cells from saphenous veins of aged patients with coronary artery disease. Furthermore, whereas previous methods relied on plastic adhesion for isolation/selection,12,13 we adopted specific surface markers, namely, CD34 and CD31, to achieve enhanced purity and reproducibility. The differentiation of CD34posCD31neg cells gave rise to a clonogenic and multipotent cell population that could be expanded for at least 10 passages and could be cryopreserved easily. As a result, a relatively small number of CD34posCD31neg cells from a patient's vein could potentially generate 30 to 50 million viable cells, which could be stored to create a bank of ready-to-use cells for autologous therapy. Application to preclinical models of peripheral ischemia demonstrated that the isolated cells possessed a potent reparative action, behaving like perivascular supportive cells and promoting neovascularization by physical and paracrine interaction with ECs.
We previously reported the presence of multipotent CD34posCD133pos cells in the outer layer of the aortic stroma of human fetuses.2,4 After stimulation with growth factors, fetal progenitors generated a population of mature ECs and mural cells. Furthermore, transplantation of fetal aorta CD34posCD133pos cells improved healing of ischemic muscles and diabetic ulcers.2,4 Our new findings suggest that CD34pos progenitors persist in the vasculogenic zone of adult veins, although they appear to be more differentiated, expressing low levels of CD133 and showing limited plasticity compared with their fetal counterparts. Sorted or culture-selected CD34posCD31neg cells also showed restricted expansibility, but on stimulation with serum, they produced highly proliferating cells, which abundantly expressed typical markers of mesenchymal/pericyte lineage. Because those markers were natively expressed at low abundance in vein-resident and freshly isolated CD34posCD31neg cells, the newly emerging phenotype might originate from serum-dependent transcriptional induction or expansion of mesenchymal/pericyte clones within the bulk population of CD34posCD31neg cells. Perpetuation of the mesenchymal/pericyte phenotype in clones and subclones of SVPs supports the second hypothesis. The dual nature of the SVP phenotype recalls recent reports showing strong similarities between pericytes and tissue-specific mesenchymal stem cells.14,15 For instance, mesenchymal stem cells and pericytes are commonly defined as CD34neg, 15,16 and in fact, SVPs quickly stop expressing CD34 on differentiation. Furthermore, SVPs retain a certain level of “stemness,” as indicated by their expression of Sox2; they possess clonogenic capacity and show osteogenic, myogenic, adipogenic, and neuroectodermal potential as previously described for resident mesenchymal progenitor cells17,18 and pericytes.19,20
We showed that SVPs establish interactions with multiple ECs, both in coculture systems and in vivo, recapitulating the natural function of perivascular support cells.21 We also documented a reciprocal paracrine cross talk between the 2 cell types that resulted in stimulation of EC network-formation capacity and proliferation and SVP migration. During angiogenesis, ECs sprout and proliferate to form the capillary lumen, stimulated by factors released by the surrounding cells. This process is followed by the migration of mural cells, which improves the stability and functionality of the vessel.22 Importantly, the present results highlight the involvement of the Ang-1/Tie-2 and PDGF-BB/PDGFRβ systems in the interactive responses of SVPs and ECs.
We found that transplantation of SVPs promotes neovascularization and accelerates blood flow recovery of ischemic limbs, which is relevant to clinical applications. Because no SVP-derived vascular structures were detected, but numerous proliferating SVPs were present in the muscles, we hypothesize that the reparative effect is mainly due to paracrine mechanisms, in line with previous reports on mesenchymal stem cell therapy.23-26 Vascular smooth muscle cells may represent an additional target for the paracrine factors released by SVPs, as documented by in vitro studies that showed increased viability of murine vascular smooth muscle cells exposed to SVP-CCM. However, with respect to the effect of SVP transplantation on arterioles, we also hypothesize an indirect action, consisting of stimulation of capillary sprouting, which then undergoes muscularization.
We were unable to compare SVPs with other tissue-resident or circulating progenitor cells from the same patients because access to other sources for exclusive research finalities was ethically unjustified in those critically ill subjects. Interestingly enough, however, patients' SVPs were therapeutically equivalent in terms of neovascularization promotion and were superior with regard to blood flow recovery compared with early-culture endothelial progenitor cells from young healthy donors, a cell population originally shown to improve the healing of ischemic limbs.27
Further studies are warranted to establish the relative potency and potential synergism of SVPs with other cell types in models of peripheral and myocardial ischemia. From a clinical perspective, we speculate that SVPs might have potential therapeutic application in pathologies such as diabetic retinopathy that are characterized by dysfunction of perivascular support cells.28 Furthermore, resident CD34posCD31neg cells and their progeny might take part in vein graft remodeling and adaptation by supporting the establishment of vascular connections between vasa vasorum of the graft and the recipient.29
In conclusion, we have provided a method to isolate and expand proangiogenic cells from the saphenous vein of aged cardiovascular patients. This is of high clinical relevance, because the starting material is usually available during bypass surgery without additional risk or burden to the patient. The method allows easy preparation and expansion of the cells to provide progenitor cells that accomplish important requirements for autologous cell therapy, being present in an easily accessible adult tissue, expandable in vitro, and able to engraft into host neovascularization in vivo with high efficiency.
CLINICAL PERSPECTIVE.
This study is the first to demonstrate that saphenous leftovers, designed to be discarded after bypass surgery, are indeed an invaluable source of perivascular progenitor cells. These cells can be expanded and stored for subsequent autologous transplantation with the aim of promoting therapeutic angiogenesis. Other potential therapeutic exploitations include use in diseases characterized by impairment of perivascular support cells, such as diabetic microangiopathy, and the application of saphenous vein–derived progenitor cells for the promotion of vein graft adaptation, by seeding them on periadventitial stents. These potential applications will require proof of concept and further preclinical validation.
Supplementary Material
Acknowledgments
We thank Dr Nguyen Tran, Paul Savage, Dr Andrew Herman, and Alan Leard for their technical assistance and helpful support and Dr Andrea Caporali, Brunella Cristofaro, and Mauro Siragusa for valuable advice.
Sources of Funding
This work was supported by a grant (PG/06/096/21325) from the British Heart Foundation and by the National Institute for Health Research, Bristol Biomedical Research Unit in Cardiovascular Medicine. Dr Campagnolo was supported by a British Heart Foundation PhD studentship.
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.899252/DC1.
References
- 1.Howson KM, Aplin AC, Gelati M, Alessandri G, Parati EA, Nicosia RF. The postnatal rat aorta contains pericyte progenitor cells that form spheroidal colonies in suspension culture. Am J Physiol Cell Physiol. 2005;289:C1396–C1407. doi: 10.1152/ajpcell.00168.2005. [DOI] [PubMed] [Google Scholar]
- 2.Invernici G, Emanueli C, Madeddu P, Cristini S, Gadau S, Benetti A, Ciusani E, Stassi G, Siragusa M, Nicosia R, Peschle C, Fascio U, Colombo A, Rizzuti T, Parati E, Alessandri G. Human fetal aorta contains vascular progenitor cells capable of inducing vasculogenesis, angiogenesis, and myogenesis in vitro and in a murine model of peripheral ischemia. Am J Pathol. 2007;170:1879–1892. doi: 10.2353/ajpath.2007.060646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 4.Barcelos LS, Duplaa C, Krankel N, Graiani G, Invernici G, Katare R, Siragusa M, Meloni M, Campesi I, Monica M, Simm A, Campagnolo P, Mangialardi G, Stevanato L, Alessandri G, Emanueli C, Madeddu P. Human CD133+ progenitor cells promote the healing of diabetic ischemic ulcers by paracrine stimulation of angiogenesis and activation of Wnt signaling. Circ Res. 2009;104:1095–1102. doi: 10.1161/CIRCRESAHA.108.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E, D'Aurizio F, Verardo R, Piazza S, Pignatelli A, Poz A, Baccarani U, Damiani D, Fanin R, Mariuzzi L, Finato N, Masolini P, Burelli S, Belluzzi O, Schneider C, Beltrami CA. Multipotent cells can be generated in vitro from several adult human organs (heart, liver, and bone marrow) Blood. 2007;110:3438–3446. doi: 10.1182/blood-2006-11-055566. [DOI] [PubMed] [Google Scholar]
- 6.Caporali A, Pani E, Horrevoets AJG, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 Receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res. 2008;103:e15–e26. doi: 10.1161/CIRCRESAHA.108.177386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emanueli C, Minasi A, Zacheo A, Chao J, Chao L, Salis MB, Straino S, Tozzi MG, Smith R, Gaspa L, Bianchini G, Stillo F, Capogrossi MC, Madeddu P. Local delivery of human tissue kallikrein gene accelerates spontaneous angiogenesis in mouse model of hindlimb ischemia. Circulation. 2001;103:125–132. doi: 10.1161/01.cir.103.1.125. [DOI] [PubMed] [Google Scholar]
- 8.Krankel N, Katare RG, Siragusa M, Barcelos LS, Campagnolo P, Mangialardi G, Fortunato O, Spinetti G, Tran N, Zacharowski K, Wojakowski W, Mroz I, Herman A, Manning Fox JE, MacDonald PE, Schanstra JP, Bascands JL, Ascione R, Angelini G, Emanueli C, Madeddu P. Role of kinin B2 receptor signaling in the recruitment of circulating progenitor cells with neovascularization potential. Circ Res. 2008;103:1335–1343. doi: 10.1161/CIRCRESAHA.108.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:9. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 10.Crisan M, Yap S, Casteilla L, Chen C, Corselli M, Park T, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng P, Traas J, Schugar R, Deasy B, Badylak S, Buhring H, Giacobino J, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, Alviano F, Ricci F, Bonafe M, Orrico C, Bagnara GP, Stella A, Conte R. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 13.Covas DT, Piccinato CE, Orellana MD, Siufi JLC, Silva JWA, Proto-Siqueira R, Rizzatti EG, Neder L, Silva ARL, Rocha V, Zago MA. Mesenchymal stem cells can be obtained from the human saphena vein. Exp Cell Res. 2005;309:340–344. doi: 10.1016/j.yexcr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 15.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MCC, Neder L, Santos ARD, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 18.Tondreau T, Lagneaux L, Dejeneffe M, Massy M, Mortier C, Delforge A, Bron D. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 19.Collett GDM, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 20.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110:2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 21.Shepro D, Morel NM. Pericyte physiology. FASEB J. 1993;7:1031–1038. doi: 10.1096/fasebj.7.11.8370472. [DOI] [PubMed] [Google Scholar]
- 22.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 26.Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006;17:279–290. doi: 10.1159/000094140. [DOI] [PubMed] [Google Scholar]
- 27.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 29.Jeremy JY, Gadsdon P, Shukla N, Vijayan V, Wyatt M, Newby AC, Angelini GD. On the biology of saphenous vein grafts fitted with external synthetic sheaths and stents. Biomaterials. 2007;28:895–908. doi: 10.1016/j.biomaterials.2006.10.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.