Abstract
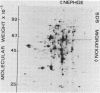
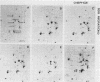
Using a modification of the basic two-dimensional polyacrylamide gel electrophoresis technique, the polypeptides of the protein map of Saccharomyces cerevisiae involved in glycolysis were investigated. This study resulted in a reassignment of two of the seven glycolytic enzyme polypeptides previously identified (Ludwig et al., Mol. Cell. Biol. 2:117-126, 1982), those corresponding to phosphoglycerate kinase and to alcohol dehydrogenase. It also resulted in the identification of two additional glycolytic polypeptides, the enolase B monomer and the glyceraldehyde phosphate dehydrogenase B monomer. The glycolytic enzymes polypeptides so identified were investigated in 5 laboratory strains (all S. cerevisiae) and in 11 commerical strains used for wine making (S. cerevisiae and Saccharomyces bayanus). It appeared highly significant that a particular electrophoretic variant of the glyceraldehyde phosphate dehydrogenase B monomer was found only in the wine yeasts. Furthermore, it was strongly suggested that S. cerevisiae and S. bayanus strains are distinguishible on the basis of a different electrophoretic migration of the enolase B monomer.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucherie H. Protein synthesis during transition and stationary phases under glucose limitation in Saccharomyces cerevisiae. J Bacteriol. 1985 Jan;161(1):385–392. doi: 10.1128/jb.161.1.385-392.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. L., Tekamp-Olson P., Najarian R. The isolation, characterization, and sequence of the pyruvate kinase gene of Saccharomyces cerevisiae. J Biol Chem. 1983 Feb 25;258(4):2193–2201. [PubMed] [Google Scholar]
- Denis C. L., Ferguson J., Young E. T. mRNA levels for the fermentative alcohol dehydrogenase of Saccharomyces cerevisiae decrease upon growth on a nonfermentable carbon source. J Biol Chem. 1983 Jan 25;258(2):1165–1171. [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Synthesis and modification of proteins during the cell cycle of the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Mar;137(3):1185–1190. doi: 10.1128/jb.137.3.1185-1190.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. P., Holland M. J. Structural comparison of two nontandemly repeated yeast glyceraldehyde-3-phosphate dehydrogenase genes. J Biol Chem. 1980 Mar 25;255(6):2596–2605. [PubMed] [Google Scholar]
- Kraig E., Haber J. E. Messenger ribonucleic acid and protein metabolism during sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Dec;144(3):1098–1112. doi: 10.1128/jb.144.3.1098-1112.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J. R., 2nd, Foy J. J., Elliott S. G., McLaughlin C. S. Synthesis of specific identified, phosphorylated, heat shock, and heat stroke proteins through the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Feb;2(2):117–126. doi: 10.1128/mcb.2.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Miller M. J., Xuong N. H., Geiduschek E. P. Identification of proteins whose synthesis is modulated during the cell cycle of Saccharomyces cerevisiae. Mol Cell Biol. 1982 Dec;2(12):1532–1549. doi: 10.1128/mcb.2.12.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Alterations in translatable ribonucleic acid after heat shock of Saccharomyces cerevisiae. J Bacteriol. 1980 Aug;143(2):603–612. doi: 10.1128/jb.143.2.603-612.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister L., Holland M. J. Targeted deletion of a yeast enolase structural gene. Identification and isolation of yeast enolase isozymes. J Biol Chem. 1982 Jun 25;257(12):7181–7188. [PubMed] [Google Scholar]
- Miller M. J., Xuong N. H., Geiduschek E. P. Quantitative analysis of the heat shock response of Saccharomyces cerevisiae. J Bacteriol. 1982 Jul;151(1):311–327. doi: 10.1128/jb.151.1.311-327.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oliver S. G., McCREADY S. J., Holm C., Sutherland P. A., McLaughlin C. S., Cox B. S. Biochemical and physiological studies of the yeast virus-like particle. J Bacteriol. 1977 Jun;130(3):1303–1309. doi: 10.1128/jb.130.3.1303-1309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew B. J., Friesen J. D., Moens P. B. Two-dimensional protein patterns during growth and sporulation in Saccharomyces cerevisiae. J Bacteriol. 1979 Apr;138(1):60–69. doi: 10.1128/jb.138.1.60-69.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]