Abstract
Objective
To estimate rates of recurrent TB due to reinfection and relapse, by HIV-status, in a general population.
Design
Long-term cohort study in Karonga district, rural Malawi
Methods
All TB patients with culture-proven disease in Karonga district were followed-up after treatment. HIV testing was offered and all M.tuberculosis isolates were fingerprinted using IS6110 RFLP. Fingerprints from initial and recurrent disease episodes were compared to distinguish relapse and reinfection: a second episode was considered a relapse if the fingerprint was identical or differed by only 1-4 bands and was the first occurrence of that pattern in the population. Rates of and risk factors for recurrence, reinfection disease, and relapse were estimated using survival analysis and Poisson regression.
Results
584 culture-positive episodes of TB were diagnosed and completed treatment in 1995-2003 in patients with known HIV status; 53 culture-positive recurrences occurred by May 2005. Paired fingerprints were available for 39 of these. Reinfections accounted for 1/16 recurrences in HIV-negatives and 12/23 in HIV-positives. Rates of relapse were similar in HIV-positive and HIV-negative individuals. Using multiple imputation to allow for missing fingerprint information, the rate of reinfection disease in HIV-positives was 2.2/ 100 person years, and in HIV-negatives 0.4/100 person years.
Conclusions
HIV increases the rate of recurrent TB in this setting by increasing the rate of reinfection disease, not relapse.
Keywords: TB, recurrence, reinfection, relapse, cohort, HIV, Africa
Introduction
In sub-Saharan Africa, rates of tuberculosis (TB) recurrence after treatment completion of up to 20/100 person years are reported1. Recurrent TB occurs due to relapse of persistent infection despite symptomatic and microbiological “cure”, or due to reinfection. Estimates of which predominate are inconsistent2, and vary between populations with different treatment regimens and infection risks. Clarification is important for monitoring the effectiveness of control programmes, and for understanding immune responses to TB. Relapse can be distinguished from reinfection disease by molecular fingerprinting strains from successive episodes.
The impact of HIV on rates of relapse and reinfection disease is not clear. A Cape Town study reported that 77% (24/31) of recurrences in a cohort suspected to have low HIV-prevalence were due to reinfection3, and the incidence of reinfection disease exceeded that of primary TB. If this were a generalised phenomenon, and not limited to individuals with unusual susceptibility or exposure, this has implications for vaccine development: if natural infection does not prevent subsequent infection, then an effective vaccine has to induce some “unnatural” immune response. However, in another study in South Africa, recurrent disease due to reinfection was common in HIV-positive and rare in HIV-negative goldminers, while rates of relapse were similar4. Other studies have examined the proportion of recurrences due to reinfection, but not calculated rates5-8.
We present results from the first study of relapse and reinfection disease to calculate rates by HIV-status in a general population, and show that rates of reinfection, but not relapse, differ by HIV-status.
Methods
The study was conducted in Karonga, a rural district in Malawi, population approximately 250,000. In 1998-2001, adult HIV prevalence was 14%9 and the annual incidence of smear-positive pulmonary TB in adults was around 100/100,00010. Antiretroviral therapy was not available in the district during the study.
TB case ascertainment was through enhanced passive surveillance; major health facilities are staffed by project staff and remote health centres visited bi-monthly. TB suspects with chronic cough have three sputa collected according to Malawi National TB Programme (NTP) guidelines and undergo standard smear (auramine and Ziehl-Neelson) and culture (acidified Lowenstein-Jensen slopes). A UK reference laboratory undertakes species confirmation and drug sensitivities. Suspected extrapulmonary TB is investigated with appropriate specimens.
Treatment follows the NTP schedule: prior to 1997, for smear-positive patients, 2 months of streptomycin, isoniazid, rifampicin and pyrazinamide (2SHRZ) plus 6 months of thiacetazone and isoniazid (6 TH). In 1997 ethambutol (E) was substituted for thiacetazone and in 2001 for streptomycin. For smear-negative patients the course was 1SHT/11TH until 1997, then 1SHE/11EH until 2001, then 2HRZ/6EH. Two review sputum specimens are collected at each of 2, 5 and 8 months of treatment for smear-positive patients.
Patients were followed throughout treatment and outcomes recorded. Patients with culture-positive TB were included if their initial episode of TB was diagnosed between September 1995 and March 2003 and if they completed treatment without change of regimen, thus including some who were smear-positive (“failed”) at two months in whom the intensive treatment phase was prolonged without re-treatment being started. Pulmonary TB patients were “cured” if their end of treatment review specimens were negative on smear and culture.
Outcomes to the end of the study (May 2005) were ascertained from community-study databases, and from home visits for those with uncertain vital status. Further episodes of TB were ascertained through usual procedures. (The Karonga Prevention Study has developed high quality tracing procedures which reliably identify individuals over decades, even after migration)11.
Isolates confirmed as M.tuberculosis were subcultured and DNA extracted for fingerprinting (RFLP typing of IS611012). Strains with fewer than 5 bands were spoligotyped. Strains were compared, using Gelcompar™ and visually. Beijing strains were identified by comparison of RFLP patterns and confirmed using spoligotyping13. Recurrences with identical strains, or with changes of 1-4 bands on RFLP where the new pattern was the first occurrence of that pattern in the population, were defined as relapses14,15. Changes of more than 4 bands or differences of 1-4 bands where the second pattern had been found in previous patients were taken as evidence of reinfection. In addition, comparisons to strains processed on the same day were made, if reinfection disease was suspected, to exclude contamination15. Cross-contamination is more likely in an isolated positive culture (IPC; single positive culture from a patient with no other specimens smear or culture-positive); these were included but are indicated in the table.
Strain comparison was carried out blind to patient HIV-status. The frequency of each strain in the population was noted16. Drug sensitivity patterns (streptomicin, isoniazid, rifampicin, ethambutol and pyrazinamide (S,H,R,E,Z)) were recorded.
TB patients were counselled for HIV testing if consent was given on initiation of treatment. In cases where an HIV test from the time of treatment initiation was not available, HIV status was inferred from a prior positive or a subsequent negative test.
Data are presented on the 584 individuals with known HIV status, with the exception of the univariable analysis of risk factors and the analysis of Beijing strains, for which knowledge of HIV status is not required.
Survival analysis and Poisson regression were used. To estimate recurrence rates, follow-up was censored at time of death, at time of departure from district or loss to follow up, at the time of diagnosis with recurrent TB, or at the end of the study, whichever came first. All those treated for recurrent TB were censored at diagnosis, but if they were culture positive, re-entered the analysis when they completed treatment. Robust standard errors were used to account for non-independence of multiple episodes.
To improve estimates of culture-positive relapse and reinfection disease rates, multiple imputation of relapse/reinfection disease status was done for the 14 culture-positive recurrences for which paired fingerprint data were not available. Five imputations were done, using a logistic regression model including age group, sex, HIV status, drug resistance of the intial episode, and timing of the recurrence to predict if the recurrence was a relapse or reinfection, and combined in a Poisson regression analysis to estimate (i) the rate of relapse and reinfection, by HIV status and time since completion of the previous episode of TB (ii) the rate ratio for relapse and reinfection, comparing HIV-positive to HIV-negative individuals, controlling for age group, sex, and time since completion of the previous episode of TB.
The studies were approved by the National Health Sciences Research Committee of Malawi and the London School of Hygiene and Tropical Medicine Research Ethics Committee.
Results
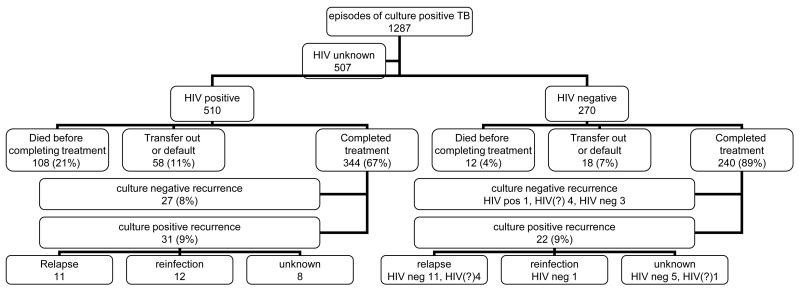
From September 1995 to March 2003 there were 1287 episodes of culture-positive TB diagnosed. HIV-status of patient was available in 780 cases (510 (65%) were HIV-positive) of which 584 (75%) completed treatment: (HIV-positive, 67% and HIV-negative, 89%) (Figure 1). Other episodes resulted in transfer or default from treatment (76, 10%) or death (120, 15%) with higher rates, in the HIV-positive patients.
Figure 1.
Outcome of episodes of culture positive tuberculosis
One patient was known to have seroconverted during the period of the study, between between initiation of treatment of first and second (culture-negative) episode, 3 years later. She was included as HIV-negative when overall rates of recurrence were calculated to avoid numerator/denominator bias as seroconvertors without recurrences might not be identified.
For the 537/584 pulmonary episodes, 360 (67%) had smear-negative 8 month sputa; the other patients could not produce sputum. The 584 episodes occurred in 558 people. 47 episodes (8%) were in people who had previously been treated for TB. 59% of the episodes were in females, and the median age at recruitment was 33 years for females and 37 for males.
For 11 of the 584 episodes, no further information was available after treatment completion. The remaining 573 contributed 1646 person years (py) of follow-up (HIV-negative 871 py, HIV-positive 775 py). At the end of the study period, 208 (87%) HIV-negative patients were alive, 15 (6%) had died and 17 (7%) had left the district. For HIV-positive patients the numbers were 143 (42%) alive, 180 (52%) died and 21 (6%) left.
Mortality
The mortality was much higher in HIV-positive than HIV-negative TB patients post-treatment with less than 50% surviving beyond 3 years compared to more than 95%.
Recurrent TB
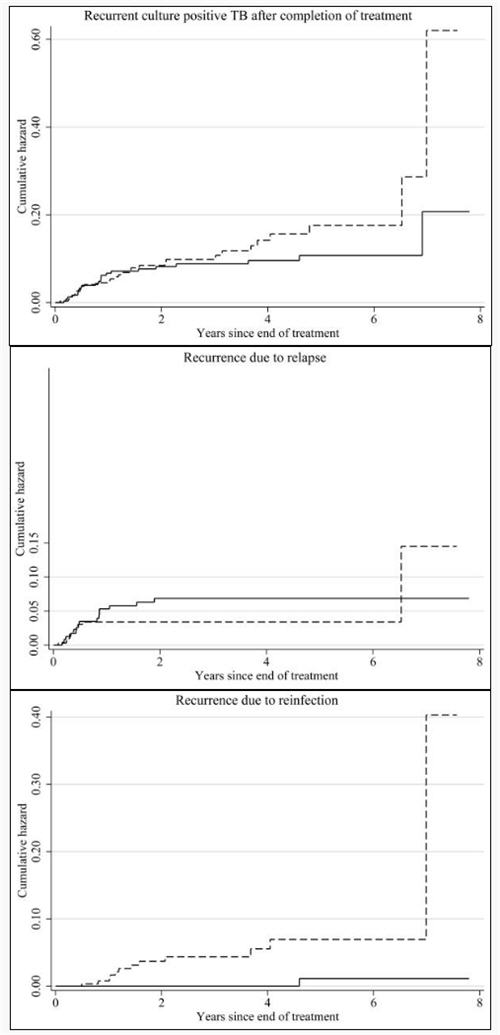
During follow-up from September 1995 to May 2005, the 584 episodes were followed by 88 clinically-diagnosed recurrences, of which 53 were culture-positive; the remainder were culture-negative on two or more specimens. The overall rate of recurrence was higher in HIV-positive than in HIV-negative individuals (RR 2.1, p=0.002 adjusted for age, sex and time since treatment completion) but not when restricted to culture-positive recurrence (adj. RR 1.4, p=0.22 & Figure 2a).
Figure 2.
In 39/53 of the culture-positive recurrences, RFLP data were available from both episodes. This included 16/22 recurrences in HIV-negatives and 23/31 in HIV-positives (Figure 1). Details of paired episodes are presented in Table 1, ordered by HIV-status and increasing time since completion of treatment for index episode: 26 cases were relapses and 13 reinfections. Three of the “relapses” were in people who were culture-positive (but smear-negative) at the end of treatment but are included as relapses rather than treatment failures, as under standard NTP conditions, smear-negativity is the marker for “cure” and culture is not routine. Table 1 also presents data relevant to the accuracy of the classification of relapse and reinfection: number of bands, whether the band pattern had changed, presence of IPC, and population frequency of the second strain. Over this period 494 distinct strains were identified in the district, the most common strain accounting for 4.1% of isolates.
Table 1.
Details of episodes from individuals with paired RFLP fingerprints on initial and recurrent episodes, by HIV status in order of time since completion treatment
| Patient information | Index episode | Recurrent episode | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||
| A or B= same patient H & W=husband/wife |
Sex | Age at diagnosis of index episode |
HIV status at end of treatment |
Outcome in study | Previous TB | TB type a | Drug resistance b (SHREZ) |
IPC c | Failure during Rx | 8 month review culture d |
Rif in 1st episdoe | TB type | Outcome | Drug resistance b (SHREZ) |
Poss contam | Number of bands (1st/ 2 nd episode) |
Beijng either episdoe | IPC c | Recurrence | Frequency of second strain (%) |
Months since 1st isolate |
Months since completion Rx |
| A 1 | F | 35 | Neg | Alive | N | SPP | RSSS - | N | N | Neg | Y | SPP j | Completed | SSSSS e | - | 14 / 15 | N | N | Relapse | 1.7 | 12 | 2 |
| 2 | F | 44 | Neg | Alive | N | SPP | HH - - - | N | N | Neg | Y | SPP | Completed | HHSSS | - | 9 / 9 | N | N | Relapse | 0.1 | 10 | 2 |
| A 3 | F | 36 | Neg | Alive | Y | SPP j | SSSSS | N | N | Neg | Y | SPP | Completed | HSSSS | - | 14 / 14 | N | N | Relapse | 1.7 | 11 | 4 |
| f H 4 | M | 39 | Neg | Alive | Y | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | SSSSS | - | 16 / 16 | N | N | Relapse | 3.1 | 19 | 5 |
| 5 | M | 70 | Neg | Alive | N | SPP | - SS - - | N | F | Pos | Y | SPP | Completed | - SS - - | - | 16 / 16 | N | N | Relapse | 0.3 | 13 | 5 |
| 6 | F | 27 | Neg | Alive | N | SPP | - SS - - | N | N | Neg | Y | SPP | Completed | - SS - - | - | 9 / 9 | N | N | Relapse | 4.1 | 14 | 6 |
| 7 | F | 63 | Neg | Died | N | SPP | SHSSS | N | N | Neg | Y | SPP | Completed | SHSSS | - | 9 / 9 | N | N | Relapse | 4.1 | 13 | 6 |
| 8 | F | 41 | Neg | Died | N | SPP | HHSSS g | N | N | Pos | Y | SPP | Died | SHS –S e | - | 9 / 9 | N | N | Relapse | 4.1 | 17 | 9 |
| 9 | M | 71 | Neg | Died | N | SPP | - SS - - | N | N | - | Y | SPP | Died | - SS - - | - | 10 / 10 | N | N | Relapse | 0.6 | 19 | 10 |
| 10 | F | 68 | Neg | Alive | N | SPP | - SS - - | N | N | - | Y | SPP | Completed | SRSSS | - | 10 / 10 | N | N | Relapse | 0.1 | 18 | 10 |
| 11 | M | 68 | Neg | Alive | N | SPP | - SS - - | N | N | Neg | Y | SPP | Completed | - SS - - | - | 11 / 11 | N | N | Relapse | 0.9 | 18 | 10 |
| B 12 | F | 28 | Neg | Alive | Y | SPP k | -SS--- | N | F | Neg | Y | SPP | Completed | - SS - - | - | 11 / 11 | N | N | Relapse | 0.9 | 19 | 10 |
| B 13 | F | 26 | Neg | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP k | Completed | - SS - - | - | 11 / 11 | N | N | Relapse | 0.9 | 24 | 13 |
| f W 14 | F | 32 | Neg | Alive | Y | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | SSSSS | - | 16 / 16 | N | N | Relapse | 3.1 | 27 | 19 |
| 15 | M | 66 | Neg | Alive | N | SPP | - SS - - | N | N | - | Y | SPP | Completed | - SS - - | - | 10 / 10 | N | N | Relapse | 0.1 | 33 | 23 |
| 16 | F | 31 | Neg | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | - SS - - | N | 12 / 17 | 2nd | N | Reinfection | 1.3 | 63 | 55 |
| 17 | F | 26 | Pos | Died | N | LN | SHSSS | N | N | Pos | N | SPP | Completed | SHSSS | - | 17 / 16 | N | N | Relapse | 0.8 | 9 | 1 |
| 18 | F | 19 | Pos | Died | N | SPP | SSSSS | N | N | Neg | Y | SPP | Died | SSSSS | - | 10 / 10 | N | N | Relapse | 2.6 | 12 | 3 |
| 19 | F | 54 | Pos | Alive | N | SNP | - SS - - | N | N | - | Y | SNP | Completed | - SS - - | - | 10 / 10 | N | N | Relapse | 1.4 | 15 | 3 |
| 20 | M | 36 | Pos | Died | N | SPP | SSSSS | N | N | Neg | Y | SPP | Died | - SS - - | - | 16 / 16 | N | N | Relapse | 3.1 | 11 | 4 |
| 21 | M | 35 | Pos | Left | N | SPP | - SS - - | N | F | - | Y | SPP | Left | - SS - - | - | 15 / 15 | N | N | Relapse | 0.3 | 16 | 4 |
| 22 | M | 48 | Pos | Alive | N | SPP | - SS - - | N | N | - | Y | SPP | Completed | - SS - - | - | 15 / 15 | N | N | Relapse | 0.4 | 12 | 5 |
| 23 | F | 26 | Pos | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | SSSSS | - | 9 / 9 | N | N | Relapse | 0.3 | 12 | 5 |
| 24 | F | 46 | Pos | Died | N | SNP | - SS - - | Y | N | Neg | N | SPP | Completed | - SS - - | - | 14 / 14 | N | N | Relapse | 0.3 | 17 | 6 |
| 25 | M | 31 | Pos | Alive | N | SPP | - SS - - | N | N | Neg | Y | SNP | Completed | - SS - - | - | 14 / 14 | N | N | Relapse | 1.0 | 13 | 6 |
| 26 | F | 31 | Pos | Alive | N | SNP | SSSSS | Y | N | Neg | N | SNP | Completed | - SS - - | N | 8 / 11 | N | N | Reinfection | 0.1 | 18 | 6 |
| 27 | F | 28 | Pos | Died | N | SPP | - SS - - | N | N | Neg | Y | SPP | Died | - SS - - | - | 13 / 13 | N | N | Relapse | 0.4 | 14 | 7 |
| 28 | F | 16 | Pos | Died | N | SPP | - SS - - | Y | N | Neg | Y | SPP | Completed | - SS - - | N | h15 / 14 | N | N | Reinfection | 0.3 | 21 | 10 |
| 29 | M | 29 | Pos | Alive | N | SPP | - SS - - | N | F | Neg | Y | SPP | Completed | - SS - - | N | i 9 / 9 | N | N | Reinfection | 4.1 | 26 | 12 |
| 30 | F | 49 | Pos | Died | N | SNP | SSSSS | N | N | - | N | SNP | Died | - SS - - | N | 13 / 10 | N | Y | Reinfection | 0.1 | 25 | 14 |
| 31 | F | 33 | Pos | Died | N | SPP | SSSS - | N | N | Neg | Y | SNP | Died | SSSSS | N | 10 / 6 | N | N | Reinfection | 0.3 | 21 | 14 |
| 32 | F | 22 | Pos | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | - SS - - | N | 9 / 22 | 2nd | N | Reinfection | 1.1 | 26 | 17 |
| 33 | F | 27 | Pos | Died | N | SNP | SSSSS | N | N | Neg | Y | SPP | Completed | - SS - - | N | 10 / 14 | N | N | Reinfection | 1.7 | 27 | 19 |
| 34 | F | 33 | Pos | Died | N | SPP | SSSSS | N | N | Neg | Y | SPP | Died | - SS - - | N | 9 / 11 | N | N | Reinfection | 0.9 | 27 | 19 |
| 35 | F | 29 | Pos | Died | N | SPP | SSSSS | N | N | Neg | Y | SPP | Died | - SS - - | 13 / 10 | N | N | Reinfection | 0.6 | 34 | 25 | |
| 36 | F | 33 | Pos | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | - SS - - | N | 10 / 11 | N | N | Reinfection | 0.3 | 51 | 44 |
| 37 | M | 32 | Pos | Died | N | SPP | - SS - - | N | N | Neg | Y | SPP | Died | - SS - - | N | 19 / 9 | 1st | N | Reinfection | 0.3 | 56 | 48 |
| 38 | F | 19 | Pos | Alive | N | SPP | SSSSS | N | N | Neg | Y | SPP | Completed | - SS - - | - | 16 / 16 | N | N | Relapse | 3.1 | 87 | 78 |
| 39 | F | 49 | Pos | Alive | N | LN | SSSSS | N | N | - | N | SNP | Died | - SS - - | N | 9/20 | N | N | Reinfection | 0.3 | 84 | 96 |
SPP= smear positive pulmonary, SNP=smear negative pulmonary, LN=lymphnode
S=fully sensitive, R=resistant, H=highly resistant, - =not done
Isolated Positive Culture defined here as a positive culture (which may or not be smear positive) with no other smear or culture positive specimens from the same patient in the same episode
According to the Malawi National TB programme, end of treatment review specimens undergo microscopy only. Therefore patient with smear negative, culture positive specimens will be considered “cured”. Under more stringent criteria, these would be considered treatment failures.
Resistance to streptomycin not identified on relapse – likely technical failure or mixed infection in initial episode
Same strain, so could be relapse or reinfecting each other
A second isolate from the same episode with the same RFLP strain, had drug resistance pattern SHSSS
One band different from the previous strain, but was not the first occurrence of this strain
Two bands different from the previous strain (same number of bands but different positions), but was not the first occurrence of this strain
Same episode
Same episode
Among the 14 recurrences without paired RFLP data, 5 had the fingerprint missing from the initial episode and 9 from the recurrent episode.
Relapses are shown in figure 2b: 15 relapses occurred in HIV-negative patients (11 confirmed HIV-negative at relapse, 4 refused further testing) and 11 in HIV-positive patients. The rate of relapse was highest in the first 6 months, and was similar for HIV-positive and HIV-negative individuals (adj RR 0.8, p=0.48). The people who had two episodes of culture-positive recurrence (Table 1, “A” and “B”) were HIV-negative, and all four episodes were confirmed relapses.
Recurrences due to reinfection (when possibilities of cross-contamination were excluded) occurred in 1 HIV-negative patient (confirmed HIV-negative at recurrence) and 12 HIV-positive patients. There was a steady rate of reinfection disease for HIV-positive individuals from the early months post-treatment and no reinfection disease in the HIV-negative patients until one case more than 4 years after treatment (Figure 2c). The rate of culture-positive reinfection disease was higher in HIV-positive than in HIV-negative patients (adj RR 7.5, p=0.041). Restricting the definition of relapse to those with exactly matching fingerprints, one HIV-negative case with recurrence at two months and one HIV-positive case at one month would be reclassified as reinfection disease (Table 1).
Beijing strains
Thirty one of the 759 initial episodes (4.1%) involved Beijing genotype strains. None were followed by proven relapses, one was followed by reinfection disease and one by culture-negative recurrence. Three of the 14 episodes of reinfection disease (21%, 95% ci 5-51%) were due to Beijing strains, including the single case in an HIV-negative patient (Table 1). Though numbers are small, there are more than expected; only 4.3% of patients had Beijing strains in their first episode of disease over this period13, giving a relative risk of Beijing strain associated with reinfection disease of 4.6 (95% ci 1.6-13.0) when adjusted for year.
Risk factors for recurrence
Table 2 shows rates and (univariable) rate ratios for culture-positive recurrence, relapse and reinfection. Rates of recurrent culture-positive TB, and reinfection disease were higher in HIV-positive patients, but rates of relapse were not. Recurrence rates were also higher in women, those who had had previous TB, those with drug resistance, and those who had a smear-positive specimen during treatment. Time since treatment completion was a strong determinant of relapse vs. reinfection, with high rates of relapse in the first 6 months. There was no evidence of an association between HIV-status and relapse when adjusted for other factors, or when early and late relapses were considered separately. Overall, there remained a strong association (RR=5.4, 95% ci 2.1-14.1) between drug resistance and relapse when adjusted for other factors.
Table 2.
Rates and crude rate ratios of recurrent culture positive TB, relapses and reinfections
| Recurrent culture positive TB | Relapse | Reinfection | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | Rate per 100 py (95% ci) |
Crude RR (95% ci) |
Rate per 100 py (95% ci) |
Crude RR (95% ci) |
Rate per 100 py (95% ci) |
Crude RR (95% ci) |
||
| HIV | Negative | 22 | 2.5 (1.7-3.8) | ref | 1.7 (1.0-2.9) | ref | 0.11 (0.16-0.8) | ref |
| Positive | 31 | 4.0 (2.8-5.7) | 1.6 (0.9-2.7) | 1.4 (0.8-2.6) | 0.8 (0.4-1.8) | 1.5 (0.9-2.7) | 13.5 (1.8-103.7) | |
| Sex | Male | 20 | 2.0 (1.3-3.1) | ref | 1.0 (0.5-1.9) | ref | 0.3 (0.1-0.9) | ref |
| Female | 39 | 3.4 (2.5-4.7) | 1.7 (0.99-2.9) | 1.7 (1.1-2.6) | 1.7 (0.8-3.6) | 1.1 (0.6-1.9) | 3.5 (1.0-12.4) | |
| Age group | <=34 | 30 | 2.6 (1.8-3.8) | ref | 1.0 (0.5-1.7) | ref | 1.1 (0.6-1.9) | ref |
| 35-49 | 17 | 2.9 (1.8-4.6) | 1.1 (0.6-2.0) | 1.9 (1.0-3.4) | 1.9 (0.8-4.4) | 0.3 (0.08-1.4) | 0.3 (0.07-1.4) | |
| 50+ | 12 | 2.9 (1.7-5.2) | 1.1 (0.6-2.2) | 1.7 (0.8-3.6) | 1.8 (0.7-4.6) | 0.2 (0.03-1.7) | 0.23 (0.03-1.8) | |
| TB prior to index episode |
None | 51 | 2.3 (2.0-3.4) | ref | 1.3 (0.9-1.9) | ref | 0.7 (0.4-1.1) | ref |
| >=2 years | 3 | 3.2 (1.0-9.8) | 1.2 (0.4-3.9) | 1.1 (0.1-7.5) | 0.8 (0.1-6.1) | 1.1 (0.1-7.5) | 1.6 (0.2-12.2) | |
| < 2 years | 4 | 6.0 (2.3-16.1) | 2.3 (0.8-6.4) | 4.5 (1.6-14.0) | 3.6 (1.1-11.8) | 0.0 (−) | - | |
| Rifampicin | None | 6 | 2.8 (1.3-6.3) | ref | 0.5 (0.06-3.3) | ref | 1.4 (0.5-4.4) | ref |
| 2+ months | 52 | 2.7 (2.1-3.6) | 0.97 (0.4-2.3) | 1.5 (1.0-2.1) | 3.1 (0.4-23.0) | 0.6 (0.3-1.0) | 0.4 (0.1-1.5) | |
| Drug resistance | None | 50 | 2.4 (1.9-3.2) | ref | 1.1 (0.7-1.6) | ref | 0.7 (0.4-1.2) | ref |
| Any | 9 | 10.2 (5.3-19.6) | 4.2 (2.0-8.5) | 7.9 (3.8-16.6) | 7.4 (3.1-17.2) | 0.0 (−) | - | |
| TB type | Smear pos pulm | 47 | 2.7 (2.0-3.5) | ref | 1.4 (1.0-2.1) | ref | 0.6 (0.3-1.0) | ref |
| Smear neg pulm | 8 | 3.8 (1.9-7.6) | 1.4 (0.7-3.0) | 1.4 (0.5-4.4) | 1.0 (0.3-3.3) | 1.4 (0.5-4.4) | 2.5 (0.7-9.1) | |
| Extra pulm | 4 | 2.6 (2.0-3.5) | 0.98 (0.4-2.7) | 0.7 (0.09-4.6) | 0.5 (0.06-3.4) | 1.3 (0.3-5.2) | 2.3 (0.5-10.5) | |
| Treatment failure a |
None | 53 | 2.3 (2.0-3.4) | ref | 1.2 (0.8-1.8) | ref | 0.7 (0.4-1.2) | ref |
| Any | 6 | 6.8 (3.1-15.2) | 2.6 (1.1-6.1) | 4.6 (1.7-12.1) | 3.7 (1.3-10.7) | 1.1 (0.2-8.1) | 1.7 (0.2-12.7) | |
| Time since treatment completion |
0-6 months | 21 | 6.0 (3.9-9.2) | ref | 5.2 (3.2-8.2) | ref | 0.3 (0.04-2.0) | ref |
| 6 - 36 months | 25 | 2.2 (1.5-3.2) | 0.4 (0.2-0.6) | 0.8 (0.4-1.5) | 0.2 (0.07-0.3) | 0.8 (0.4-1.5) | 2.7 (0.3-21.5) | |
| 36 months + | 13 | 2.0 (1.2-3.5) | 0.3 (0.3-0.7) | 0.3 (0.08-1.3) | 0.06 (0.01-0.3) | 0.8 (0.3-1.9) | 2.7 (0.3-23.4) | |
Univariate analyses of other risk factors include individuals with unknown HIV status.
Culture or smear positive specimen during treatment
Rates of reinfection disease and relapse by HIV
Rates of recurrence, relapse and reinfection disease by HIV-status are shown in Table 3, with and without imputed data. Including imputed data, the overall rates of relapse were 2.1/100 py for HIV-negative and 1.8/100py for HIV-positive patients, although rates were not constant over the period of the study with high initial rates and substantial decline after six months. The rates of reinfection disease were estimated as 0.4/100py for HIV-negative and 2.2/100py for HIV-positive patients, and, although low in the first six months, appeared, with these few data, to be fairly constant thereafter. Adjusted for age, sex and timeband (to account for differing rates over time) the rate ratio for relapse comparing HIV-positive patients to HIV-negative patients, was 0.8 (0.3-1.7, p=0.48) and for reinfection, 6.3 (1.3-31.5, p=0.02).
Table 3.
Rates of recurrence in patients of known HIV status
| RECURRENCES | CULTURE POSITIVE RECURRENCES |
RELAPSES | REINFECTIONS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| RFLP confirmed | Incl. imputed missing values |
RFLP confirmed | Incl. imputed missing values |
|||||||
| n | rate per 100 py (95% ci) |
n | rate per 100 py (95% ci) |
n | rate per 100 py (95% ci) |
rate per 100 py (95% ci) |
n | rate per 100 py (95% ci) |
rate per 100 py (95% ci) |
|
| HIV − | 30/239 | 22/239 | 15/239 | 1/239 | ||||||
| Overall | 30 | 3.4 (2.4-4.9) | 22 | 2.5 (1.7-3.) | 15 | 1.7 (1.0-2.9) | 2.1 (1.3-3.5) | 1 | 0.1 (0.02-0.8) | 0.4 (0.09-1.5) |
| First 6 months | 12 | 10.3 (5.9-18.2) | 9 | 7.7 (4.0-14.9) | 8 | 6.9 (3.4 – 13.8) | 18.7 (4.9-15.4) | 0 | 0.0 (−) | 10.09 (0.008-0.9) |
| 6 – 36 months | 13 | 2.8 (1.6-4.8) | 10 | 2.0 (1.1-4.0) | 7 | 1.5 (0.7-3.2) | 11.5 (0.7-3.2) | 0 | 0.0 (−) | 10.3 (0.06-1.5) |
| 36+ months | 5 | 1.7 (0.72-4.2) | 3 | 2.9 (0.3-3.2) | 0 | 0.0 (−) | 10.5 (0.08-2.9) | 1 | 0.3 (0.05-2.4) | 10.6 (0.1-2.3) |
| HIV+ | 58/344 | 31/344 | 11/344 | 12/344 | ||||||
| Overall | 58 | 7.8 (6.0-10.1) | 31 | 4.0 (2.8-5.7) | 11 | 1.4 (0.8 – 2.6) | 1.8 (1.0-3.1) | 12 | 1.5 (0.9-2.7) | 2.2 (1.3-3.6) |
| First 6 months | 15 | 10.2 (6.1-16.9) | 11 | 7.2 (4.0-12.9) | 9 | 5.9 (3.1-11.0) | 15.8 (3.2-10.6) | 1 | 0.7 (0.1-4.6) | 10.6 (0.08-4.2) |
| 6 – 36 months | 30 | 7.6 (5.0 – 10.3) | 12 | 2.8 (1.6-4.9) | 1 | 0.2 (0.03-1.6) | 11.0 (0.4-2.3) | 8 | 1.8 (0.9-3.7) | 12.1 (1.0-4.3) |
| 36+ months | 13 | 7.1 (4.2-12.4) | 8 | 4.2 (2.1-8.5) | 1 | 0.5 (0.07-3.8) | 10.3 (0.05-2.1) | 3 | 1.6 (0.5-4.9) | 13.7 (1.7-8.1) |
| Crude rate ratio for HIV |
2.2 (1.4-3.4) p<0.001 |
1.6 (0.9-2.7) p=0.10 |
0.8 (0.4-1.8) p=0.62 |
0.8 (0.4-1.8) p=0.63 |
13.5 (1.8-103.7) p=0.001 |
6.1 (1.3-27.4) p=0.02 |
||||
| Adj. rate ratio (age, sex, timeband) |
2.1 (1.2-3.2) p=0.002 |
1.4 (0.8-2.4) p=0.22 |
0.7 (0.3-1.4) p=0.29 |
0.7 (0.3-1.7) p=0.48 |
- | 6.3 (1.3-31.5) p=0.02 |
||||
These rates were estimated from a Poisson regression model including HIV status and time since completion of the previous episode of TB, assuming that the effect of HIV status on the rate of relapse and reinfection is the same for each of the 3 categories of time since completion of previous episode. The 5 imputed datasets were created using the ice (imputation using chained equations) procedure in Stata. The imputed datasets were then combined in a Poisson regression analysis, using the Stata procedure micombine.
Discussion
We demonstrate that rates of relapse, given successful completion of treatment under routine programme conditions in Karonga, are not influenced by HIV-status, in contrast to the rate of reinfection disease. In HIV-positive individuals the rate of reinfection disease was equal to the rate of relapse, but the rate of reinfection disease in HIV-negative individuals was very low. Apart from HIV, the major predictor of relapse or reinfection disease was time since completion of treatment as different causes of recurrence predominated in different time periods.
A considerable proportion of diagnosed culture-positive patients were not HIV tested (39%). These were predominantly (65%) amongst those who failed to complete treatment due to death or default and thus would not contribute to rates of recurrence. Of those who completed treatment and were eligible for this study, the majority of the 175 (23%) who were not tested for HIV, were diagnosed in years when KPS TB studies were quiescent, unrelated to individual patient characteristics. Rates of mortality after treatment completion, recurrence, relapse and reinfection for HIV-unknown patients were intermediate when compared to rates for HIV-negative and HIV-positive, which is consistent with there being no important bias introduced.
People who initially refused testing but were later found HIV-negative (in two cases, at time of recurrence) were included for analysis in the HIV-negative group. Thus rates of recurrence in HIV-negatives might be slightly inflated.
Whereas the missing RFLP results did not affect the estimates of the rate ratios (as shown by the similarity before and after addition of imputed values), they give a more realistic estimate of the absolute rates of relapse and reinfection, which would otherwise be underestimated because of the missing data.
Missing RFLP fingerprints were more frequent at the beginning of the study period due to technical difficulties in recovering stored cultures, again unrelated to patient characteristics, with equal proportions missing for HIV-positive and HIV-negative patients. Given the methods of ascertainment and follow-up of TB cases in Karonga there should be no difference in whether recurrent cases were identified or patients lost to followup, by HIV-status.
Our findings are similar to those in South African goldminers4, despite the different setting and much lower TB incidence in Malawi. The predominance of relapse as a cause of recurrence in HIV-negative individuals is consistent with two studies from high TB prevalence settings: in Hong Kong 37/42 recurrences17, and in Vietnam 39/50 recurrences had identical strains18. However, findings from two other studies where HIV prevalence is unknown but thought to be low, differed considerably: 32/52 recurrences in Shanghai19 and 24/31 in Cape Town3 were considered reinfections. In Cape Town the incidence of TB is extremely high, three times that of Karonga, while the incidence in China is similar to Malawi. In each of these studies, the HIV prevalence may have been considerably higher in those with reinfection disease than in the population, and among TB patients in general, and there may be other important risk factors, such as alcohol use. There may also be methodological reasons for the differences.
Errors of patient identification, sample handling, or cross-contamination will favour “reinfections”; wrongly attributed specimens in either episode are unlikely to match. In addition, apparent reinfections could be due to mixed infections20-23. Wrongly classifying “relapse” is unlikely, unless there is homogeneity in the molecular fingerprints of circulating strains. The potential for misclassification based on IPC is higher17,24,25. In this study none of the HIV-negative patients with recurrences had IPC. Among the HIV-positives with recurrences, 3 individuals had IPC in initial strains and 1 in recurrent strains, 1 classifed as relapse, 3 as reinfections (Table 1); thus, if anything, we overestimated reinfection disease rates, although none of the IPC were processed on the same day as an isolate with the same strain. The longstanding population-based epidemiological studies in Karonga District make incorrect patient identification very unlikely, and sample handling procedures are rigorous.
Forty four percent (46/105) of recurrent episodes of TB were culture negative and diagnosed clinically or radiographically. Radiographic changes may be unreliable in this group as signs of previous TB are often unresolved. This compares with 51% (1352/2639) of episodes from the recruitment period who were excluded from the cohort as there was no positive culture. HIV positive patients were more likely at both initial (54%, 597/1107) and recurrent episodes (47%, 27/58) to have a diagnosis of culture negative TB than were HIV negative patients (50%, 268/538 and 27%, 8/30 respectively).
Although higher rates of relapse might be expected in HIV-positive patients, as effective chemotherapy needs support from a functioning immune system, this may be counter-balanced by the lower rates of cavitatory disease, associated with relapse4. Immunosuppression may also increase the proportion of culture-negative and undiagnosed TB, and will increase mortality rates. There will be true relapses (and reinfections) among the culture-negative recurrences and deaths. Cause of death was unknown for most patients, but 4/11 HIV-negative patients and 53/145 HIV-positive patients who died without having been diagnosed with recurrent TB, had submitted sputa post-treatment suggesting that they had had symptoms consistent with TB: all were negative. Five (all HIV-positive) submitted sputa within three months of death. HIV-positive patients with drug resistant isolates were twice as likely to die during treatment as those with sensitive strains (p=0.02), but there were few deaths in HIV-negative patients during treatment whether or not there was a drug resistant strain, and drug resistance is rare in this setting.
Most of the relapses were early and might be identified as treatment failures prior to treatment completion, if more sensitive clinical and laboratory investigations were available.
HIV may exert its effect on reinfection disease by increasing risk of re-infection with M. tuberculosis after treatment, increasing risk of development of disease given re-infection, or both. HIV-infection may also increase the risk of exposure, through attendance at health facilities.
High rates of HIV-negative reinfection disease would imply that previous disease does not induce protective immunity at least in a proportion of individuals known to be susceptible to TB26,27. Using our previous estimates of year and age specific rates of TB and of HIV prevalence in the district10,28, less than one new primary case of TB would be expected among an HIV-negative group age- sex- and area-matched to the population in this study. One HIV-negative reinfection case was found. Though numbers are small, this is consistent with natural TB providing little or no protection against subsequent infection and disease, and with the hypothesis that individuals who have one episode of disease are at increased risk of another3.
Beijing genotype strains have been associated with higher recurrence rates29 30; although in one study the association was lost after controlling for drug resistance. In our study, we found no association with relapse rates. However, the single confirmed HIV-negative reinfection disease case was due to a Beijing strain, and overall the Beijing genotype was over-represented among reinfection disease cases. This could represent a higher probability of a Beijing strain establishing infection; faster progression to disease, given reinfection (consistent with findings in the Gambia31); or a chance finding. In South African goldminers, 3/14 reinfection disease strains were Beijing32, and areas where Beijing strain prevalence is high report high rates of reinfection disease in HIV-negative patients 33,34.
The mortality during treatment in the HIV-positive patients with reinfection disease episodes was high (5/11) and these findings strengthen the case for secondary prophylaxis for HIV-positive TB patients. CD4 counts were not available to estimate the risk of recurrence at different levels of immunosuppression. While 9/10 recurrences by 6 months post-treatment amongst HIV-positive patients were relapses, 10/12 thereafter were reinfections, perhaps because immunocompromised patients who have residual bacilli after treatment will relapse early on. Secondary prophylaxis could thus be delayed to avoid inducing drug resistance, but still prevent reinfection disease. The rates of reinfection disease were not so high as to suggest that there is a greater risk after TB treatment than at other times in the course of HIV infection, and primary preventive therapy based on CD4 count is an alternative, adding to the protection assumed to be afforded by antiretroviral therapy35.
Identifying underlying reasons for the higher recurrence rate in HIV-positive than in HIV-negative individuals is important operationally. Relapse rates vary according to drug regimens, programme effectiveness and prevalence of drug resistant strains; reinfection disease rates will vary according to background risk of M.tuberculosis infection. Our findings are consistent with another cohort study in a contrasting setting, and emphasise that in an area of medium to high TB incidence, HIV-negative patients are predominantly affected by relapse, while HIV-positive patients are at risk of both relapse and reinfection.
Acknowledgements
ACC, FDM, PEF and JRG designed the study. ACC and FDM implemented the study. ACC and SF undertook statistical analysis and SF imputed the missing variables. JNM undertook data handling and cleaning. DTM and KM recruited and followed up patients. JRG analysed the molecular data. ACC, SF, JRG and PEF wrote the paper. FDM died in a tragic accident prior to analysis.
The Ministry of Population and Health, the National Health Sciences Research Committee and the people of Karonga provided much appreciated support. Malcolm Yates, Hamidou Traore and Kim Mallard undertook the RFLP typing and Annelies van Rie provided helpful comments.
Footnotes
Meeting at which data have been previously presented
International Union of TB and Lung Disease, November 2007, Cape Town, RSA
Abstract No: 0071256
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Korenromp EL, Scano F, Williams BG, Dye C, Nunn P. Effects of human immunodeficiency virus infection on recurrence of tuberculosis after rifampin-based treatment: an analytical review. Clin Infect Dis. 2003;37(1):101–12. doi: 10.1086/375220. [DOI] [PubMed] [Google Scholar]
- 2.Lambert ML, Hasker E, Van Deun A, Roberfroid D, Boelaert M, Van der Stuyft P. Recurrence in tuberculosis: relapse or reinfection? Lancet Infect Dis. 2003;3(5):282–7. doi: 10.1016/s1473-3099(03)00607-8. [DOI] [PubMed] [Google Scholar]
- 3.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171(12):1430–5. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 4.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358(9294):1687–93. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JL, Okwera A, Vjecha MJ, Byekwaso F, Nakibali J, Nyole S, et al. Risk factors for relapse in human immunodeficiency virus type 1 infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1997;1(5):446–53. [PubMed] [Google Scholar]
- 6.van Rie A, Warren R, Richardson M, Victor TC, Gie RP, Enarson DA, et al. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N Engl J Med. 1999;341(16):1174–9. doi: 10.1056/NEJM199910143411602. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey-Faussett P, Githui W, Batchelor B, Brindle R, Paul J, Hawken M, et al. Recurrence of HIV-related tuberculosis in an endemic area may be due to relapse or reinfection. Tuber Lung Dis. 1994;75(3):199–202. doi: 10.1016/0962-8479(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 8.Hawken M, Nunn P, Gathua S, Brindle R, Godfrey-Faussett P, Githui W, et al. Increased recurrence of tuberculosis in HIV-1-infected patients in Kenya. Lancet. 1993;342(8867):332–7. doi: 10.1016/0140-6736(93)91474-z. [DOI] [PubMed] [Google Scholar]
- 9.Crampin AC, Glynn JR, Ngwira BM, Mwaungulu FD, Ponnighaus JM, Warndorff DK, et al. Trends and measurement of HIV prevalence in northern Malawi. Aids. 2003;17(12):1817–25. doi: 10.1097/00002030-200308150-00011. [DOI] [PubMed] [Google Scholar]
- 10.Glynn JR, Crampin AC, Ngwira BM, Mwaungulu FD, Mwafulirwa DT, Floyd S, et al. Trends in tuberculosis and the influence of HIV infection in northern Malawi, 1988-2001. Aids. 2004;18(10):1459–63. doi: 10.1097/01.aids.0000131336.15301.06. [DOI] [PubMed] [Google Scholar]
- 11.Jahn A, Crampin A, Glynn J, Mwinuka V, Mwaiyeghele E, Mwafilaso GJ, et al. Evaluation of a village-informant driven demographic surveillance system in Karonga, Northern Malawi. Demographic Research. 2007;16:2119–248. [Google Scholar]
- 12.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31(2):406–9. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glynn JR, Crampin AC, Traore H, Yates MD, Mwaungulu FD, Ngwira BM, et al. Mycobacterium tuberculosis Beijing genotype, northern Malawi. Emerg Infect Dis. 2005;11(1):150–3. doi: 10.3201/eid1101.040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warren RM, van der Spuy GD, Richardson M, Beyers N, Borgdorff MW, Behr MA, et al. Calculation of the stability of the IS6110 banding pattern in patients with persistent Mycobacterium tuberculosis disease. J Clin Microbiol. 2002;40(5):1705–8. doi: 10.1128/JCM.40.5.1705-1708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glynn JR, Yates MD, Crampin AC, Ngwira BM, Mwaungulu FD, Black GF, et al. DNA fingerprint changes in tuberculosis: reinfection, evolution, or laboratory error? J Infect Dis. 2004;190(6):1158–66. doi: 10.1086/423144. [DOI] [PubMed] [Google Scholar]
- 16.Glynn JR, Crampin AC, Yates MD, Traore H, Mwaungulu FD, Ngwira BM, et al. The importance of recent infection with Mycobacterium tuberculosis in an area with high HIV prevalence: a long-term molecular epidemiological study in Northern Malawi. J Infect Dis. 2005;192(3):480–7. doi: 10.1086/431517. [DOI] [PubMed] [Google Scholar]
- 17.Das S, Chan SL, Allen BW, Mitchison DA, Lowrie DB. Application of DNA fingerprinting with IS986 to sequential mycobacterial isolates obtained from pulmonary tuberculosis patients in Hong Kong before, during and after short-course chemotherapy. Tuber Lung Dis. 1993;74(1):47–51. doi: 10.1016/0962-8479(93)90068-9. [DOI] [PubMed] [Google Scholar]
- 18.Quy HT, Cobelens FG, Lan NT, Buu TN, Lambregts CS, Borgdorff MW. Treatment outcomes by drug resistance and HIV status among tuberculosis patients in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2006;10(1):45–51. [PubMed] [Google Scholar]
- 19.Shen G, X Z, Shen X, Sun B, Gui X, Shen M, Mei J, Gao Q. Recurrent tuberculosis and exogenous reinfection, Shanghai, China. Emerg Infect Dis. 2006;12(11):1776–1778. doi: 10.3201/eid1211.051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, Gey van Pittius NC, et al. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med. 2004;169(5):610–4. doi: 10.1164/rccm.200305-714OC. [DOI] [PubMed] [Google Scholar]
- 21.Yeh RW, Hopewell PC, Daley CL. Simultaneous infection with two strains of Mycobacterium tuberculosis identified by restriction fragment length polymorphism analysis. Int J Tuberc Lung Dis. 1999;3(6):537–9. [PubMed] [Google Scholar]
- 22.Shamputa IC, Jugheli L, Sadradze N, Willery E, Portaels F, Supply P, et al. Mixed infection and clonal representativeness of a single sputum sample in tuberculosis patients from a penitentiary hospital in Georgia. Respir Res. 2006;7:99. doi: 10.1186/1465-9921-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer AS, Borgdorff MW, de Haas PE, Nagelkerke NJ, van Embden JD, van Soolingen D. Analysis of rate of change of IS6110 RFLP patterns of Mycobacterium tuberculosis based on serial patient isolates. J Infect Dis. 1999;180(4):1238–44. doi: 10.1086/314979. [DOI] [PubMed] [Google Scholar]
- 24.de Boer AS, Blommerde B, de Haas PE, Sebek MM, Lambregts-van Weezenbeek KS, Dessens M, et al. False-positive mycobacterium tuberculosis cultures in 44 laboratories in The Netherlands (1993 to 2000): incidence, risk factors, and consequences. J Clin Microbiol. 2002;40(11):4004–9. doi: 10.1128/JCM.40.11.4004-4009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruddy M, McHugh TD, Dale JW, Banerjee D, Maguire H, Wilson P, et al. Estimation of the rate of unrecognized cross-contamination with mycobacterium tuberculosis in London microbiology laboratories. J Clin Microbiol. 2002;40(11):4100–4. doi: 10.1128/JCM.40.11.4100-4104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Repique CJ, Li A, Collins FM, Morris SL. DNA immunization in a mouse model of latent tuberculosis: effect of DNA vaccination on reactivation of disease and on reinfection with a secondary challenge. Infect Immun. 2002;70(7):3318–23. doi: 10.1128/IAI.70.7.3318-3323.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orme IM. The search for new vaccines against tuberculosis. J Leukoc Biol. 2001;70(1):1–10. [PubMed] [Google Scholar]
- 28.White RG, Vynnycky E, Glynn JR, Crampin AC, Jahn A, Mwaungulu F, et al. HIV epidemic trend and antiretroviral treatment need in Karonga District, Malawi. Epidemiol Infect. 2007:1–11. doi: 10.1017/S0950268806007680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan NT, Lien HT, Tung le B, Borgdorff MW, Kremer K, van Soolingen D. Mycobacterium tuberculosis Beijing genotype and risk for treatment failure and relapse, Vietnam. Emerg Infect Dis. 2003;9(12):1633–5. doi: 10.3201/eid0912.030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis. 2006;12(5):736–43. doi: 10.3201/eid1205.050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong BC, Hill PC, Aiken A, Awine T, Antonio M, Adetifa IM, et al. Progression to active tuberculosis, but not transmission, varies by Mycobacterium tuberculosis lineage in The Gambia. J Infect Dis. 2008;198(7):1037–43. doi: 10.1086/591504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonnenberg P. 2007 personal communication. [Google Scholar]
- 33.Victor TC, de Haas PE, Jordaan AM, van der Spuy GD, Richardson M, van Soolingen D, et al. Molecular characteristics and global spread of Mycobacterium tuberculosis with a western cape F11 genotype. J Clin Microbiol. 2004;42(2):769–72. doi: 10.1128/JCM.42.2.769-772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei J, Shen X, Zha J, Sun B, Shen M, Shen GM, et al. Study on the molecular epidemiology of Myobacterium tuberculosis in Shanghai. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(9):707–10. [PubMed] [Google Scholar]
- 35.Golub JE, Saraceni V, Cavalcante SC, Pacheco AG, Moulton LH, King BS, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. Aids. 2007;21(11):1441–8. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]