Abstract
Adult stem cells have the ability to self-renew and to generate specialized cells. Self-renewal is dependent on extrinsic niche factors but few of those signals have been identified. In addition, stem cells tend to differentiate in the absence of the proper signals, and are therefore difficult to maintain in cell culture. The mammary gland provides an excellent system to study self-renewing signals, as the organ develops post-natally, arises from stem cells and is readily generated from transplanted cells. We show here that adult mammary glands contain a Wnt-responsive cell population that is enriched for stem cells. In addition, stem cells mutant for the negative feedback regulator Axin2 and therefore sensitized to Wnt signals have a competitive advantage in mammary gland reconstitution assays. In cell culture experiments, exposure to purified Wnt protein clonally expands mammary stem cells for many generations and maintains their ability to generate functional glands in transplantation assays. We conclude that Wnt proteins serve as rate-limiting self-renewing signals acting directly on mammary stem cells.
Introduction
Stem cells are characterized by their ability to self-renew as well as to differentiate into specialized cells, properties critical for tissue maintenance and regeneration. The decision to self-renew or differentiate is regulated at multiple levels, including cell-intrinsic transcriptional programs and extra-cellular signals originating from a specialized niche (Morrison and Spradling, 2008). Cell-extrinsic control is important to prevent the unrestrained self-renewal of stem cells and their possible conversion into cancerous cells (Clarke and Fuller, 2006). A critical aspect of the niche model of stem cell regulation is that the availability of self-renewing factors is limited due to their local release. Understanding the nature of the signals and their effect on adult stem cells has been complicated due to the low abundance of these stem cells and the paucity of functional assays. Moreover, because adult stem cells tend to differentiate in the absence of the proper self-renewing signals, they are difficult to maintain in cell culture under defined conditions.
The mammary gland is an excellent model for studying adult stem cell behavior because of its accessibility and unique postnatal development (Deome et al., 1959; Kordon and Smith, 1998; Shackleton et al., 2006; Stingl and Caldas, 2007; Stingl et al., 2006; Visvader and Lindeman, 2006; Welm et al., 2003; Woodward et al., 2005). It is an epithelial organ with a highly branched ductal network, consisting of a basal layer of myoepithelial cells and an inner layer of luminal cells. During pregnancy, under the influences of hormones, luminal cells are induced to differentiate and develop into alveoli that produce milk at parturition. Alveoli regress at the end of lactation, and the mammary gland returns to a morphological state similar to that found in virgin mice (Richert et al., 2000). Transplantation assays have demonstrated the existence of a rare population of mammary stem cells (MaSCs) that are able to reconstitute a functional mammary gland (Deome et al., 1959; Young et al., 1971). These MaSCs are likely to be present in any of the branches, as each portion of the ductal system was shown to be capable of regenerating an entire epithelial tree upon transplantation (Kordon and Smith, 1998). More recently, a MaSC enriched population of cells has been isolated based on specific cell surface markers (Lin−, CD24+, CD29hi) (Shackleton et al., 2006; Stingl et al., 2006). In spite of these advances, it has been difficult to study MaSCs in vitro due to loss of long-term self-renewal. Although short-term mammosphere cultures allow multi-lineage colonies to form in culture, over time these colonies lose their ability to self-renew and start to differentiate in vivo (Dontu et al., 2003). This is likely due to the absence of self-renewing factors in defined tissue culture media.
Wnt signaling has been implicated in different stages of mammary development as well as in mammary oncogenesis (Boras-Granic et al., 2006; Brisken et al., 2000; Chu et al., 2004; Lindvall et al., 2006; Lindvall et al., 2009; Nusse and Varmus, 1982; Roelink et al., 1990). Multiple Wnt genes are expressed in the mammary gland epithelium or stroma (Buhler et al., 1993; Gavin and McMahon, 1992; Huguet et al., 1994; Lane and Leder, 1997; Olson and Papkoff, 1994; Weber-Hall et al., 1994). Moreover, it has been suggested that Wnt signals expand mammary stem cells, as early tumorigenic lesion from transgenic mice overexpressing Wnt1 have increased numbers of stem cells (Nusse and Varmus, 1982; Shackleton et al., 2006; Vaillant et al., 2008). Whether Wnt proteins directly control normal mammary stem cells is however not known. In this study, we use a combination of cell culture and in vivo transplantation experiments to show that Wnt proteins serve as important self-renewing factors for mammary gland stem cells.
Results
Wnt reporter positive cells are enriched for MaSCs
To identify cells that are activated by Wnt/β-catenin signaling in the mammary gland, we used the Axin2-lacZ reporter mouse, which carries a lacZ gene inserted into the Axin2 locus (Lustig et al., 2002), a well-known Wnt/β-catenin target gene (Jho et al., 2002; Leung et al., 2002; Lustig et al., 2002). The expression of lacZ through the endogenous Axin2 promoter faithfully reflects sites of Wnt/β-catenin signaling in multiple tissues. In 8–12 week old virgin animals, lacZ expression was detected in nearly all of the branches in the mammary gland (Figure 1A). Subsequent histological analysis indicated that the Wnt-responsive cells are located in the basal layer of the mammary ducts (Figure 1B) (Baker et al., 2010; Teissedre et al., 2009) where mammary stem cells have been suggested to reside (Shackleton et al., 2006; Stingl et al., 2006). To characterize the reporter-positive cells with respect to previously characterized stem cell populations, we used Fluorescence-Activated Cell Sorting (FACS) and the Lin−, CD24+, CD29hi markers known to enrich for mammary stem cells (Shackleton et al., 2006; Stingl et al., 2006). We found that five percent (n=41/1015) of the Lin−, CD24+, CD29hi cells was positive for the Axin2-lacZ reporter (Figure 1C, D), as measured by X-gal staining. In contrast, no lacZ expressing cells were present in the other epithelial cell population (Lin−, CD24+, CD29low) (n=0/1002; Figure 1E), which has not been found to contain mammary stem cells (Shackleton et al., 2006).
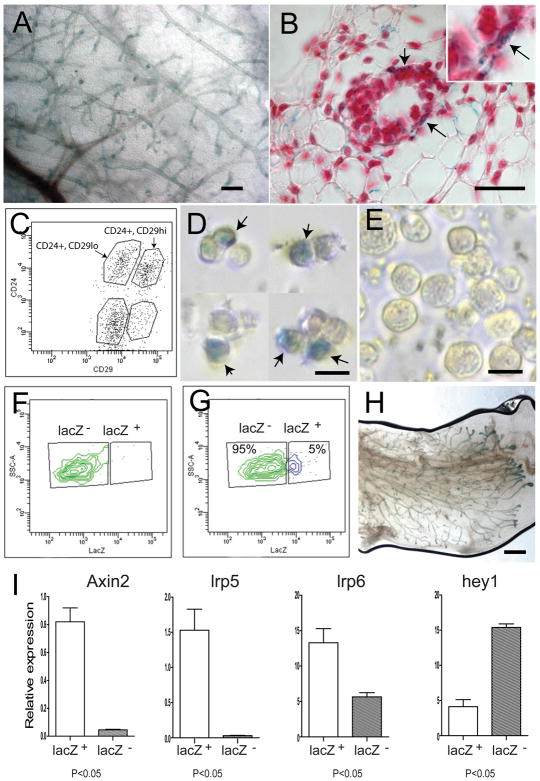
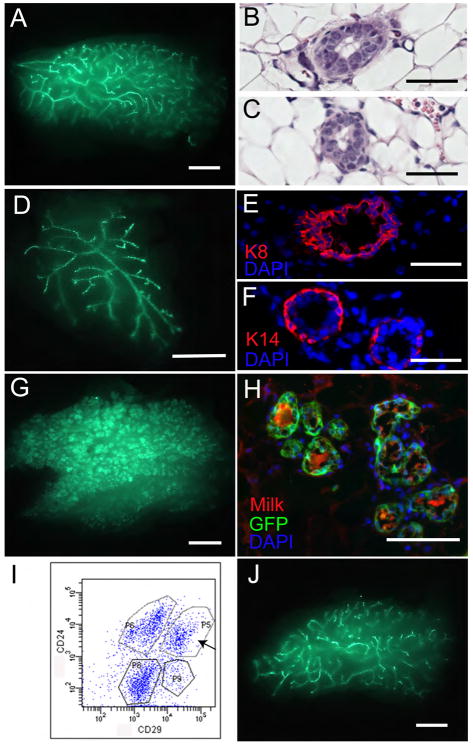
Figure 1. The Wnt-responsive cell population is enriched for mammary stem cells.
A, The expression of Axin2-lacZ was examined by whole-mount X-gal staining in Axin2+/lacZ virgin mammary gland (scale bars, 500um). Wnt-responsive cells are located in all branches. B, Paraffin sections revealed that Wnt-responsive cells (blue cells) reside in the basal layer of the mature ducts (arrows) (scale bar, 50um). Slides were counter-stained with nuclear fast red. C, Primary mammary epithelial cells (MECs) were isolated and analyzed by the expression of the cell surface markers lin−, CD24 and CD29. D and E, lin−, CD24+, CD29hi (stem cell enriched) and lin−, CD24+, CD29low populations were FACS-isolated respectively, followed by X-gal staining. D, 5% of the lin−, CD24+, CD29hi group is Wnt responsive (arrows; scale bar, 10um); E, no Wnt-responsive cells were seen in the lin−, CD24+, CD29low group (scale bar, 10um). F, No lacZ+ cells were detected in the wild type cell control, using a LacZ fluorogenic substrate. G, 5% of lin−, CD24+, CD29hicells are lacZ-positive in Axin2+/lacZ cells, using the lacZ fluorogenic substrate. H, Mammary outgrowths following transplantation were examined by X-gal staining to verify that the epithelium was donor cell derived (scale bar, 1mm). Using lacZ-expressing epithelial cells grown in wild type stroma, it is apparent that most if not all branches, contain Axin2-lacZ cells (compare 1H to 1A). I, q-RT-PCR relatively to HPRT control in the lacZ+, lin−, CD24+, CD29hi and lacZ −, lin−, CD24+, CD29hi cells sorted from 8–12 week old virgin mouse mammary glands. A statistically significant difference of p<0.05 (t test) was observed between the two groups for each of the four genes.
Using a fluorogenic lacZ substrate to isolate live Axin2-lacZ reporter-positive cells in combination with the Lin−, CD24+, CD29hi markers, we confirmed that 5% of the Lin−, CD24+, CD29hi cells were positive for lacZ (Figure 1F, G). The lacZ+ and lacZ− cell fractions were characterized by measuring expression of several genes, using quantitative reverse transcriptase-polymerase chain reaction (q-RT-PCR; Figure 1I). As expected, the Axin2 gene was expressed at higher levels in the lacZ+ cells compared to the lacZ− cells. The Wnt receptors lrp5 and lrp6 expression patterns were similar to that of Axin2 (Figure 1I), in line with the finding that stem cell populations can be enriched by selection for high lrp5 expression (Badders et al., 2009). Conversely, expression of hey1, a Notch target, was significantly lower in the lacZ+ cells (Figure 1I). Notch signaling has been implicated in luminal cell-specific differentiation, while down-regulation of Notch signaling in MaSCs can lead to increased reconstitution (Bouras et al., 2008).
To assess mammary gland reconstitution competence, we transplanted Lin−, CD24+, CD29hi cells that were either lacZ-positive or negative into cleared fat pads. Notably, Wnt-responsive lacZ positive cells generated mammary glands more efficiently than lacZ-negative cells (Table 1). The resulting outgrowths contained lacZ-expressing cells (Figure 1H), indicating that they were derived from the donor mice. Taken together, the CD24+, CD29hi population can be further enriched for mammary stem cells by virtue of their Wnt/β-catenin responsiveness.
Table 1.
Mammary outgrowths derived from Lin−, CD24+, CD29hi , lacZ− (non-Wnt-responsive) and Lin−, CD24+, CD29hi , lacZ+ (Wnt-responsive) sorted mammary cells
| Number of cells injected | Lin−, CD24+, CD29hi, lacZ−* | Lin−, CD24+, CD29hi, lacZ+* |
|---|---|---|
| 500† | 1/5 | 3/5 |
| 100† | 0/5 | 1/5 |
| 100†† | 3/8 | 6/8 |
| 50†† | 5/16 | 11/16 |
| P value <0.01 | ||
Wnt-responsive (lacZ+) and non-Wnt-responsive cells were FACS isolated using a LacZ fluorogenic substrate, in combination with the Lin−, CD24+ and CD29hi markers (Figure 1G), and injected into cleared mammary fat pads. The outgrowths were analyzed at 10 weeks post-transplantation. Implanted lacZ+ cells had higher numbers of outgrows compared with the lacZ− cells. However for those that were scored positive, there were no differences in the extent of the fat pad occupation. Data are pooled from four independent experiments.
shown as number of outgrowths per number of injected mammary fat pads.
transplantation with cells resuspended in 50% serum+PBS.
transplantation performed with cells resuspended in 50% Matrigel, which improves engraftment.
MaSCs that are more responsive to external Wnt signals outcompete wild type stem cells in repopulation assays
The Axin2 gene encodes a ligand-dependent negative feedback regulator of the Wnt pathway that dampens signaling cell-autonomously. Insertion of lacZ into Axin2 inactivates the gene (Lustig et al., 2002) and elevates Wnt signal strength in a ligand-dependent manner. To measure the impact of loss of the Axin2 gene on Wnt signaling activity in mammary cells, we transduced a TCF-dependent luciferase reporter construct (Fuerer and Nusse, 2010) into primary mammary epithelial cells isolated from wild type and Axin2lacZ/lacZ mice. Luciferase activity was monitored following incubation with different concentrations of Wnt3A protein. In the unstimulated state, wild type and Axin2lacZ/lacZ cells had the same luciferase activity, while increasing Wnt3A concentrations activated the reporter to higher levels in Axin2lacZ/lacZ cells (Figure 2A). The difference between WT and Axin2LacZ/LacZ cells was prominent at lower concentrations of Wnt proteins (50–100 ng/ml), while higher concentrations saturated the response in either cell type.
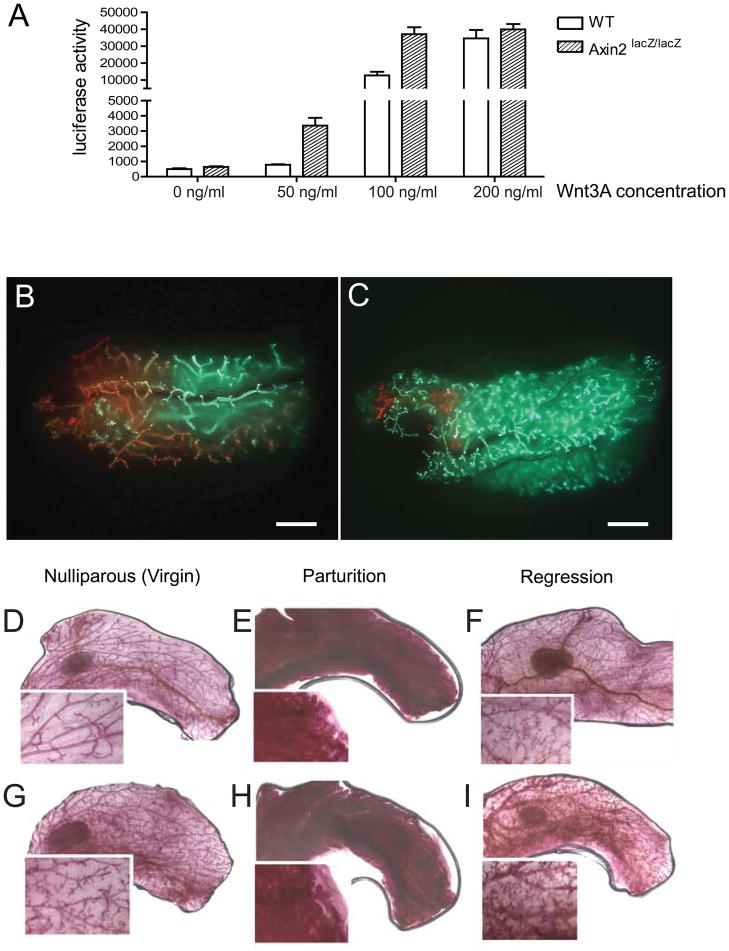
Figure 2. Axin2lacZ/lacZ mutant cells have an advantage over wild type cells in competitive reconstitution assays.
A, WT and Axin2lacZ/lacZ mammary epithelial cells transduced with a Wnt-reporter (7TCF-luciferase) construct were incubated with increasing concentrations of purified Wnt3A protein. The reporter activity was measured after 24 hours. B–C, Lin−, CD24+, CD29hi cells were isolated independently from Actin-GFP mice and Actin-DsRed mice. GFP-positive and DsRed-positive cells were mixed in different ratios and injected into the cleared fat pads of recipients. As a control, B, transplantation of 1:1 ratio mixture of WT; Actin-DsRed and WT; Actin-GFP stem cells resulted in an even distribution of red and green outgrowths (scale bar, 2mm). C, More GFP-positive outgrowths were observed after transplantation of 2:1 mixture of Axin2+/+; DsRed and Axin2lacZ/lacZ ; Actin-GFP cells (scale bar, 2mm). D–F, Whole-mount carmine staining of WT mammary gland in nulliparous stage (D), parturition (E) and regression (F). Whole-mount carmine staining of Axin2lacZ/lacZ mammary gland. While Axin2lacZ/lacZ mammary glands in nulliparous stage display a mildly hypermorphic branching phenotype (G), they have normal differentiation in parturition (H) and regression in post-weaning (I).
The increased response of Axin2lacZ/lacZ mutant MaSCs to Wnt signals suggest that these cells would have a competitive advantage over wild type cells under conditions in which Wnt proteins are limiting self-renewal factors. We tested this in repopulation assays in vivo (and, separately, in cell culture assays; see below) by isolating Lin−, CD24+, CD29hi cells from Axin2LacZ/LacZ mice that also carried an Actin-GFP marker, as well as wild type Lin−, CD24+, CD29hi cells from Actin-DsRed mice. GFP- and DsRed-positive cells were mixed in different ratios and injected into cleared fat pads of recipient animals. As a control, MaSC enriched populations from wild type, Actin-GFP and wild type, Actin-DsRed mice were mixed in a 1:1 ratio, resulting in an even distribution of GFP-positive and DsRed-positive mammary gland branches (Figure 2B, Figure S1A). In contrast, transplanting Axin2LacZ/LacZ; Actin-GFP cells in competition with twice as many Axin2+/+; Actin-DsRed cells resulted in outgrowths that were predominantly GFP-positive and therefore derived from Axin2LacZ/LacZ cells (Figure 2C; Figure S1B). Thus, the potentiated response of Axin2LacZ/LacZ mutant cells to a limiting Wnt source influences the ability of these stem cells to repopulate the mammary gland.
Of note, the Axin2LacZ/LacZ cells generated mammary glands with mild hypermorphic branching phenotypes (GFP-positive branches in Figure 2C), similar to glands in Axin2LacZ/LacZ mutant mice themselves (Figure 2G). However, these glands differentiate normally during parturition and regress after weaning (Figure 2H, I compared to WT glands E, F). Despite their elevated Wnt responsiveness, Axin2LacZ/LacZ mammary glands have normal ordered layers of cells. As demonstrated by histology and FACS analysis (Figure S2), the basal and luminal cell composition was similar to wild type. There was no evidence of hyperplasia or tumors, suggesting that the cellular response to Wnt was not excessive. This is in contrast to the MMTV-Wnt1 mouse model where over-expression of Wnt itself causes cancer (Nusse and Varmus, 1982; Shackleton et al., 2006).
In the presence of Wnt3A protein, MaSCs can be clonally expanded in vitro for many generations
Based on our observations in vivo, we next asked whether Wnt proteins could directly influence MaSCs in vitro by promoting their colony-initiating ability. To ensure that colonies originated from single cells rather than from aggregates, we isolated cells from wild type; Actin-GFP mice and wild type; Actin-DsRed mice, mixed the cells and seeded them in Matrigel. As shown in Figure 3A, individual Lin−, CD24+, CD29hi cells formed monochromatic colonies. In Matrigel, the Wnt3A protein is active as it induces the lacZ reporter in cells from Axin2lacZ/+ mice (Figure 3D), while no lacZ activity was seen in non-treated cells (Figure 3C) or in cells exposed to a Wnt inhibitor, Dkk1 (Figure 3B).
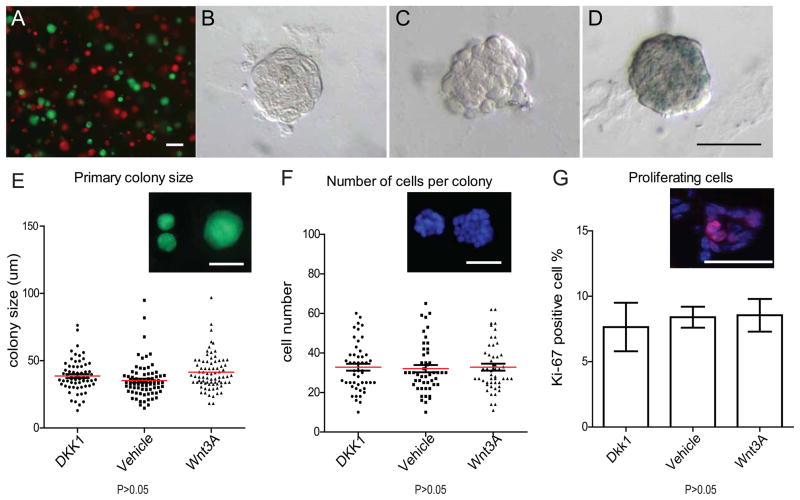
Figure 3. Primary colony formation is not changed by Wnt signaling.
A, FACS-isolated Lin−, CD24+,CD29hi single cells were independently prepared from Actin-GFP mice or Actin-DsRed mice, mixed in a 1:1 ratio and seeded in Matrigel (2x104 cells total in 50 ul pellet). Individual colonies that emerged were exclusively monochromatic, and the number of green and red colonies matched the ratio of the seeded cells (scale bars, 100um). B–D, Lin−, CD24+, CD29hi cells isolated from Axin2lacZ/+ reporter mice were cultured in Matrigel, plus either Dkk1(B), Wnt vehicle control (C) or Wnt3A protein (D). X-gal staining was performed on colonies in 7 days of culture. No Wnt signaling was detectable in Dkk1 or vehicle controls, while the lacZ reporter was activated by Wnt3A protein (F) (scale bar 50um). E, Lin−, CD24+,CD29hi cells isolated from Actin-GFP mice were cultured in Matrigel, plus either Dkk1, Wnt vehicle control or Wnt3A protein. Sizes of the colonies were measured after one week of culture in vitro. In all conditions, there are no differences in the average colony sizes, (which ranged from 20~60um, scale bar, 50um). F, DAPI staining of the colonies in E and cells numbers in each colony were quantified. G, Frozen sections of colonies in E were stained for the proliferation marker Ki-67. There was no difference in the number of Ki-67 positive cells among each condition (P>0.05).
We next plated single sorted Lin−, CD24+, CD29hi cells in the presence of either vehicle, Wnt3A protein or the Wnt-inhibitor Dkk1. The presence or absence of Wnt proteins had no apparent effect on cell proliferation, as the size of the colonies under each of the three conditions was relatively constant, varying between 20 to 60um (Figure 3E) with colonies composed of approximated 15~60 cells (Figure 3F). Moreover, there were no discernible differences in staining for the cell cycle marker Ki67 (Figure 3G). When we examined the clonogenicity of the cells under these conditions, there were no significant differences in primary colony formation: the colonies occurred at a ratio of 1 colony per 15 cells plated (Figure 4A).
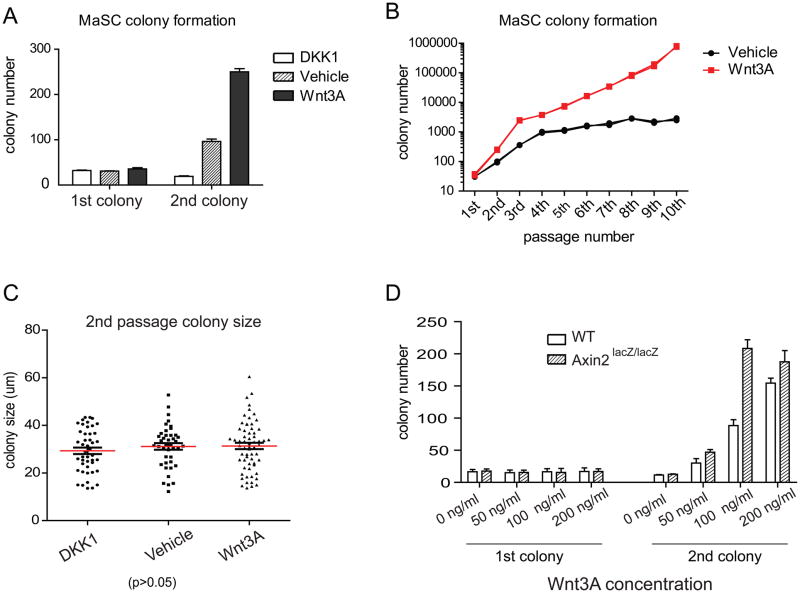
Figure 4. In serial passage, Wnt3A protein leads to continued expansion of colony numbers.
A. Lin−, CD24+,CD29hi cells isolated from Actin-GFP mice were cultured in Matrigel, plus either Dkk1, Wnt vehicle control or Wnt3A protein. Numbers of primary colonies are similar (1 colony out of 15 plated cells in primary culture). However, the number of secondary colonies arising from re-plated single cells is strongly influenced by Wnt. In the presence of Dkk1, the secondary colony number dropped by 50%. In the presence of Wnt3A, the colony number was more than 7 fold higher. B, Colony numbers in vehicle and Wnt3A were followed over 10 passages. In vehicle, colony number reached a plateau in the 4th passage (black line). However, in the presence of Wnt3A, colony numbers expanded continually in each passage (red line). Starting from 35 primary colonies, 106 colonies were calculated to be present in the 10th passage. C, The sizes of the secondary colonies were measured after one week in culture post passage. Under each condition, there was no discernable difference in the average colony size. D. Primary and secondary colony numbers of wild type and Axin2lacZ/lacZ mutant cells grown over a range of Wnt3A protein concentration (X-axis). While primary colony formation efficiency remained unaffected, Axin2lacZ/lacZ mutant cells gave rise to higher numbers of secondary colonies compared to wild type cells (Y-axis), especially at limiting Wnt concentrations. At higher Wnt concentrations, the number of colonies plateaued irrespective of the genotype of the cells.
In contrast to the lack of a Wnt effect on primary colony formation, subsequent serial colony formation, by dissociation of primary colonies and re-plating, was dramatically influenced by the presence of Wnt protein (Figure 4A). Addition of Wnt3A during the first plating increased the number of secondary colonies sevenfold. In contrast, incubation with Dkk1 caused an approximate 50% reduction in secondary colony formation (Figure 4A). In subsequent serial passages, cells cultured in Wnt3A continued to expand and produced increasing numbers of colonies (Figure 4B). This increased clonogenicity was not due to higher proliferation rates, as the size of the colonies was not significantly altered under any condition (Figure 4C and Figure S3A). There was also no discernible change in apoptosis in any of the conditions tested as detected by Annexin V staining (Figure S4). Wnt3A protein not only enhanced the clonogenicity of MaSCs but also lowered the expression of a differentiation marker, Gata-3 (Asselin-Labat et al., 2007; Kouros-Mehr et al., 2006). Levels of Gata-3, a gene expressed and required during early luminal differentiation, were reduced 2–3 fold following exposure to Wnt3A, suggesting a possible role of Wnt-mediated suppression of MaSC differentiation (Figure S3C). However, the colonies contained similar numbers of terminally differentiated basal cells and luminal cells whether cultured with of without Wnt3A (Figure S5 A–D) and expression levels of the markers K8 and K14 in early or late passage colonies were not significantly influenced either (Figure S5 E).
Using colony formation assays, we compared the clonogenic ability of Axin2LacZ/LacZ mutant cells to wild type cells over a range of Wnt protein concentration. In keeping with the enhanced repopulating ability of the Axin2LacZ/LacZ mutant cells when transplanted in vivo (Figure 2B, C), we found that the Axin2LacZ/LacZ cells initiate secondary colonies at a higher rate than wild type cells (Figure 4D). In accordance with the luciferase Wnt reporter experiments (Figure 2A), the differences between Axin2LacZ/LacZ and WT cells in colony initiating assays were more pronounced at lower concentrations of the Wnt protein (50–100 ng/ml) than at saturating concentrations (200 ng/ml). Taken together, these experiments suggest that the clonogenicity of MaSCs is greatly increased by exposure to Wnt protein.
Long-term cultured MaSCs retain full developmental potential in vivo
To test whether the clonally propagated cells had maintained stem cell characteristics --self-renewal and multipotency in vivo -- we transplanted colonies from later passages into cleared fat pads to assess outgrowth. In contrast to vehicle-treated colonies, which failed to repopulate, Wnt-treated colonies displayed robust mammary gland reconstitution ability (Figure 5A–C). Importantly, the repopulating frequencies remained relatively stable (1/34.5–1/48.8) with increasing passage (Table 2). Since the total colony number expanded with each successive passage, this suggests that Wnt increases the absolute number of MaSCs.
Figure 5. Cultured stem cell colonies retain full developmental potential.
A, 100 GFP labeled Lin-,CD24+, CD29hi colonies cultured in the presence of Wnt3A for 2–6 passages were transplanted into cleared fat pads. Virgin recipients fat pads were harvested at 8 weeks post transplantation. Robust GFP outgrowths were detected. (scale bar, 2mm). B, C, Comparing H/E sections of WT (B) and reconstituted mammary glands (C) revealed a normal layered structure in the outgrowths (scale bars, 50um). D, An outgrowth from a transplanted single tertiary colony (scale bar, 2mm). E, F, Immuno-fluorescence staining with anti-K8 (E) and K14 antibody (F) of the sections indicated that single colony derived outgrowths have normal luminal (E) and myoepithelial (F) layers (scale bar, 50um). G, GFP outgrowths harvested from nursing recipients (day 2; scale bars, 2mm). H, Immunofluorescence staining with anti-milk antibody of alveoli arising from nursing recipients (Green, GFP epithelium; red, Milk; blue, DAPI. scale bar, 100um). I, GFP primary outgrowths have the same CD24 and CD29 profile as their donors. J, Normal secondary outgrowths arising from transplantation of cells isolated from primary outgrowths (scale bar, 2mm).
Table 2.
Mammary outgrowths derived from in vitro cultured colonies
| Passage number | Number colonies | EGF+Wnt3A* | Repopulating frequency | EGF+ vehicle* | EGF+Wnt3A, withdrawal of Wnt3A for 7 days* |
|---|---|---|---|---|---|
| 2nd | 100 | 16/18 | 1/10** | 2/14** | |
| 50 | 7/10 | 1/41.5 | - | - | |
| 25 | 8/18 | (27.2–62.2)- | - | - | |
| 3rd | 100 | 23/26 | 1/12** | - | |
| 50 | 12/16 | 1/34.5 | - | - | |
| 25 | 12/20 | (24.7–38.3) | - | - | |
| 1 | 4/40 | - | - | ||
| 4th | 100 | 17/20 | 0/10 | - | |
| 50 | 8/12 | 1/48.8 | - | - | |
| 25 | 6/14 | (33.0–72.3) | - | - | |
| 5th | 100 | 10/18 | 0/10 | - | |
| 6th | 100 | 12/20 | 0/10 | - | |
Lin−, CD24+, CD29hi cells isolated from Actin-GFP mice were cultured in Matrigel in the presence of Wnt3A or vehicle for 2–6 passages. Colonies (ranging from 100-1) were injected into cleared fat pads to test reconstitution. GFP outgrowths were observed for cells cultured with Wnt3A in each passage (Figure 5A). Implantation of single colonies yielded 4/40 GFP-positive outgrowths when examined in nulliparous recipients and yielded 17/40 GFP-positive outgrowths when examined in recipients in parturition. In vehicle or withdrawal of Wnt3A conditions, a small percentage of partial reconstituted glands were detected (Figure S6), as indicated by **.
shown as number of outgrowths per number of injected mammary fat pads.
Notably, single Wnt-treated colonies (derived from single cells in tertiary passage), were frequently able to reconstitute (17 out of 40 when analyzed in parturition recipients, Figure 5G; 4 out of 40 when analyzed in virgin recipients, Figure 5D, Table 2). Immuno-staining for the markers Keratin 8 and Keratin 14 confirmed that the outgrowths had a regular morphology, composed of normally differentiated ductal structures with luminal and myoepithelial cells (Figure 5E, F). When recipient mice were in parturition, the mammary glands resulting from the transplanted colonies consisted of a dense ductal system ending in clusters of alveoli (Figure 5G) that contained abundant milk proteins (Figure 5H).
Are Wnt signals are continuously required to maintain stem cell identity? When we withdrew Wnt protein during secondary plating, we found a marked reduction in the efficiency of mammary gland reconstitution (2 small outgrowths, and 12 implants not generating any detectable tissue; Figure 6; Table 2). This result demonstrates that cells expanded in the presence of Wnt proteins are not transformed into an autonomously self-renewing state.
To confirm unambiguously that the cultured cells maintained full stem cell properties, we performed serial transplantations starting from glands derived from late passage cultured cells. When analyzed by FACS, these primary outgrowths from Wnt-treated colonies displayed a normal CD24 and CD29 profile (Figure 5I, compared to Figure 1C). Moreover, the Lin−, CD24+, CD29hi cells (arrow in Figure 5I) isolated from these primary outgrowths were able to reconstitute and form a normal organ upon subsequent transplantation (Figure 5J; Table S1). Similar results were obtained for the tertiary outgrowths (Table S1). These experiments demonstrated that MaSCs expanded in long-term cell culture in the presence of Wnt3A retained their ability to self-renew and to differentiate normally in vivo.
Discussion
The ability to self-renew while simultaneously generating specialized cells is the critical property of adult stem cells. As currently understood, the choice of a dividing stem cell to self-renew or to differentiate is regulated by factors coming from the niche, or stem cell micro-environment. Implicit in all models of stem cell control is that differentiation is the default choice and that self-renewal depends on the presence of extracellular signals released by the niche. Among the signals implicated in the control of various types of stem cells, the Wnt pathway is prominent (Barker et al., 2007; Jaks et al., 2008; Kalani et al., 2008; Reya et al., 2003; Willert et al., 2003). For example, Wnt target genes, such as lgr5, are expressed in stem cells of the intestine and the hair follicle, and there are stem cell phenotypes associated with loss of Wnt signaling in these tissues (Barker et al., 2007; Jaks et al., 2008). Likewise, in the case of the mammary gland, there is genetic support for a role for Wnt signaling in the maintenance of MaSCs, such as defects in mammary gland development in mice mutant for Wnt pathway components (Boras-Granic et al., 2006; Brisken et al., 2000; Chu et al., 2004; Lindvall et al., 2006). The experiments described in this paper suggest that Wnt signaling, as monitored by the expression of the Wnt target genes Axin2, is a marker for enriching and identifying MaSCs in vivo. Compared to the Axin2-lacZ− counterparts, Axin2-lacZ+ cells reconstitute at higher frequencies. These data are compatible with the recent report that high expression of the Wnt co-receptor Lrp5 marks MaSCs (Badders et al., 2009). It is likely that Axin2-lacZ+ cells overlap with Lrp5hi cells and that Lrp5 expression would allow these cells to be capable of Wnt reception in the first place. Indeed, we detected higher levels of Lrp5 expression in freshly isolated Axin2-lacZ+ cells (Figure 1I). Reduced expression of hey1 in Axin2-lacZ+ cells in vivo is consistent with a previous report of Bouras et al., and supports the notion that inhibition of the Notch pathway in MaSCs enhances their stem cell potential.
In spite of the evidence for a role of Wnt pathway in MaSC regulation, it remained unknown whether Wnt proteins acted directly on mammary stem cells and controlled their self-renewal properties. In this study, we provide multiple lines of evidence that this is indeed the case. In cell culture, we find that MaSCs are dependent on Wnt proteins for long-term expansion and maintenance of self-renewal under defined conditions. In these experiments, Wnt does not act as a mitogenic growth factor, as the proliferation of the cells was not influenced by its presence. Rather, our data support a model of other known growth factors, such as EGF, as proliferative factors, while the Wnt protein prevents differentiation of the dividing cells and thereby leads to self-renewal. In line with this proposal, we find that the expression of Gata-3, a transcription factor that promotes differentiation of mammary stem cells into luminal cells, is suppressed by the Wnt signal (Figure S3C). The combined use of Wnt, EGF and Matrigel has allowed us, for the first time, to expand MaSCs in cell culture, with full retention of their competence to regenerate an organ. This finding may have important implications for attempts to expand breast cancer stem cells (Al-Hajj et al., 2003) in a clonal fashion, as well as stem cells from other tissues. Expanding adult stem cells in culture holds great promise for regenerative medicine but has in general been difficult due to the lack of stem cell markers and knowledge about the required growth factors.
The second line of evidence for a role of Wnt signals as stem cell factors comes from the use of Axin2LacZ/LacZ mutant cells. Axin2 encodes a negative regulator of the Wnt pathway. Unlike other repressors of Wnt signaling, such as Axin1, APC or GSK3, Axin2 is a target gene of Wnt signaling, implying that its expression and signal-dampening function are restricted to Wnt-exposed cells. Any consequence of Axin2 loss is therefore dependent on the presence of active Wnt proteins and indicates that cells showing a phenotype are actively receiving Wnt signals. In competitive repopulation experiments with wild type cells, MaSCs isolated from Axin2LacZ/LacZ mice show a marked increase in repopulating the mammary fat pad and regenerating a functional mammary gland, indicating that the propagation of MaSC in vivo is stimulated by external Wnt signals. While this result leaves open the possibility that Wnt acts as a proliferative signal, we suggest that it reflects the self-renewing ability of the Wnt protein, in part based on the increased ability of the Axin2lacZ/lacZ cells to generate colonies in cell culture (Figure 4D). The Axin2lacZ/lacZ hypermorphic phenotype is unique compared to mutations in other Wnt signaling components, which mostly lead to hyperplasia or even tumors. Moreover, mammary glands originating from Axin2lacZ/lacZ MaSCs have normal ratios of basal and luminal cells. In addition, MaSCs colonies cultured in the presence of Wnt appeared to have normal expression levels of the basal lineage marker K14 and the luminal marker K8, suggesting that Wnt signals promote the self-renewal of MaSCs without altering cell fate specification.
The role of Wnt in the mammary gland is in agreement with current models of stem cell regulation, where the ability to sustain a limited pool of stem cells is a hallmark of the niche (Morrison and Spradling, 2008). This constraint is important in preventing unrestrained expansion of stem cells, which may otherwise lead to cancer (Clarke and Fuller, 2006). Our study suggests that local Wnt signals act as the self-renewal factors in the adult mammary gland. Indeed, previous studies reported that several Wnts, including Wnt2, Wnt4, Wnt5a, Wnt5b, Wnt6 and Wnt7b, are expressed in the epithelium and/or the mesenchyme in the matured virgin mice (Buhler et al., 1993; Gavin and McMahon, 1992; Huguet et al., 1994; Lane and Leder, 1997; Olson and Papkoff, 1994; Weber-Hall et al., 1994). At present, the precise location of the physiological niche for MaSCs remains unknown. It could be that the niche itself is dynamic as exemplified by the hematopoietic system (reviewed in (Kaplan et al., 2007). MaSCs may have several niches as they progress through various developmental stages.
Methods
Mouse Strains
CD1, Axin2lacZ/+ (Lustig et al., 2002), Axin2lacZ/lacZ, Actin-GFP/B6, and Actin-DsRed/B6 and Nude strains were used in this study. Experimental procedures were approved by Animal Care and Use Committee of Stanford University School of Medicine.
Primary cell preparation
Mammary glands from 8–12-week-old-virgin female mice were isolated. The minced tissue was place in culture medium (RPMI 1640 with 25mM HEPES, 5% fetal bovine serum, 1% PSQ, 300U ml−1 Collagenase III (Worthington), and digested for 5hrs at 37C. After lysis of the red blood cells in NH4Cl, a single cell suspension was obtained by sequential incubation with 0.25% trypsin-EDTA at 37C for 5 minutes and 0.1mg/ml DNase I (Sigma) for 5 min with gentle pipetting, followed by filtration through 40um cell strainers.
Cell labeling, flow cytometry
The following antibodies were used: biotinylated and APC conjugated CD31, CD45, TER119 (BD Pharmingen), CD24-PE/cy5, CD24-PE/cy7, CD29-FITC, CD29-APC (Biolegend), Streptavidin-PE/TexasRed and Streptavidin-FITC (BD Pharmingen). Antibody incubation was performed on ice for 15 min in HBSS with 10% fetal bovine serum. LacZ flourogenic substrate staining was performed at 37C for 45min in 2mM CUG concentration (Marker Gene). PE-Annexin V and 7-Amino-Actinomycin (7-AAD) staining were performed follow the manufacturer's instructions (BD Pharmingen). All sorts were performed using a FACSAria (Becton Dickinson). The purity of sorted population was routinely over 95%.
In vitro colony formation assay
FACS-sorted cells were resuspended at a density of 4x105 cells/ml in chilled 100% growth factor reduced Matrigel (BD Bioscience) and the gels allowed to polymerized before covering with culture medium (DMEM/F12, 50ng ml−1EGF, plus either vehicle (1% CHAPS in PBS) or 200ng ml−1Wnt3A (Willert et al., 2003) or 200ng ml−1Dkk1). Culture medium was changed every 24 hrs. Primary colony numbers were scored after 7–10 days in culture. For passaging colonies, media was aspirated and Matrigel was digested by incubation in 500 ul of Matrigel recovery solution (BD Bioscience) for 1 hr on ice. Colonies released from Matrigel were pelleted. Single cells were obtained through incubation with 0.25% trypsin-EDTA at 37C for 5 min, followed by gentle pipetting. Single cells were then re-plated in Matrigel as described above. In assays examining the requirement for Wnt protein in maintaining stem cell properties, Wnt3A protein was withdrawn for 7 days in secondary culture prior to transplantation.
Lentiviral vector and infection
The 7xTcf-FFluc//SV40 viral construct contains seven Tcf/Lef-binding sites, the 5’UTR of the pSuperTopflash reporter plasmid, firefly luciferase gene and a SV40-Puro cassette for Puromycin selection (Fuerer and Nusse, 2010). Lentivirus was produced by transient transfection in 293T cells. Mammary cells were isolated from 8–12 weeks old virgin female glands as described above, followed by pre-plating for 30 minutes to deplete stromal cells. The suspending epithelial cells were collected and plated in DMEM-F12/10% FBS with virus. At 48 hours post infection, cells were put under Puromycin selection. Wnt responsiveness was tested by treating cells with a limiting dilution of Wnt3A and luciferase activates were measured.
Immunostaining
Frozen sections were prepared by air-drying and fixation for 5 min in cold 4% paraformaldehyde. Tissue sections were incubated with primary antibodies at 4C overnight, followed by washes, incubation with secondary antibodies for 1 hr at 25C and counterstained with DAPI (Vector Laboratories). The following antibodies were used: rat anti K8 (1: 250, Developmental hybridoma bank), rabbit anti K14 (1:1000, Covance), rabbit anti milk (1:100, Nordic Immunological Laboratories).
Mammary fat pad transplantation and analysis
Sorted cells were resuspended in 50% Matrigel, PBS with 20% fetal bovine serum and 0.04% trypan Blue (Sigma), and injected in 5~10 ul volumes into 3-week-old female cleared fat pads. Reconstituted mammary glands were harvested after 6–10 weeks. All transplanted cells were labeled with either GPF or LacZ. GFP outgrowths were detected under a fluorescence dissection microscope. LacZ+ outgrowths were detected by whole mount X-gal staining.
Competitive repopulation assays in vivo
In controls, Lin−, CD24+, CD29hi cells from WT ; Actin-GFP mice and from WT; Actin-DsRed mice were isolated respectively and mixed in 1:1 ratio, 3000 WT ; Actin-GFP cells + 3000 WT; Actin-DsRed cells per injection. In Axin2LacZ/LacZ experiments, Lin−, CD24+, CD29hi cells from Axin2LacZ/LacZ ; Actin-GFP mice and from Axin2+/+; Actin-DsRed mice were isolated respectively and mixed in 1:2 ratio, 2000 Axin2LacZ/LacZ ; Actin-GFP cells + 4000 Axin2+/+; Actin-DsRed cells per injection. Mixed cells were injected into cleared fat pads of Nude mice as described above.
Statistics
Limiting dilution analyses used the ‘statmod’ software package for the R computing environment (http://www.R-project.org). MaSC frequencies were estimated using a complementary log-log generalized linear model. Two-sided 95% Wald confidence intervals were computed, except in the case of zero outgrowths, when one-sided 95% Clopper-Pearson intervals were used instead. Student’s t test was used. Significance is indicated on the figures using the following convention: P<0.01. Error bars in all panes represent the standard deviation (SD).
Supplementary Material
Acknowledgments
We thank various colleagues, in particular Renee van Amerongen, Angela Bowman, Margaret Fuller, Esther Verheyen, Jill Helms and Mike Clarke for comments and discussions. Matt van de Rijn kindly provided advice on histology. Priya Rajaraman and Steve Artandi helped in cleared fat pad assays. We thank Christophe Fuerer for providing the 7XTcf-FFluc//SV40 construct and Walter Birchmeier for the Axin2lacZ mouse strain. This work was supported by the Howard Hughes Medical Institute, a fellowship from the California Institute of Regenerative Medicine and the Morton Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Badders NM, Goel S, Clark RJ, Klos KS, Kim S, Bafico A, Lindvall C, Williams BO, Alexander CM. The Wnt receptor, Lrp5, is expressed by mouse mammary stem cells and is required to maintain the basal lineage. PLoS One. 2009;4:e6594. doi: 10.1371/journal.pone.0006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R, Kent CV, Silbermann RA, Hassell JA, Young LJ, Howe LR. Pea3 transcription factors and wnt1-induced mouse mammary neoplasia. PLoS One. 2010;5:e8854. doi: 10.1371/journal.pone.0008854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Dev Biol. 2006;295:219–231. doi: 10.1016/j.ydbio.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell. 2008;3:429–441. doi: 10.1016/j.stem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 2000;14:650–654. [PMC free article] [PubMed] [Google Scholar]
- Buhler TA, Dale TC, Kieback C, Humphreys RC, Rosen JM. Localization and quantification of Wnt-2 gene expression in mouse mammary development. Dev Biol. 1993;155:87–96. doi: 10.1006/dbio.1993.1009. [DOI] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, Glick A, Wysolmerski JJ, Millar SE. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–4829. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerer C, Nusse R. Lentiviral vectors to probe and manipulate the wnt signaling pathway. PLoS One. 2010;5:e9370. doi: 10.1371/journal.pone.0009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin BJ, McMahon AP. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol Cell Biol. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet EL, McMahon JA, McMahon AP, Bicknell R, Harris AL. Differential expression of human Wnt genes 2, 3, 4, and 7B in human breast cell lines and normal and disease states of human breast tissue. Cancer Res. 1994;54:2615–2621. [PubMed] [Google Scholar]
- Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgard R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81. doi: 10.1016/j.molmed.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH. An entire functional mammary gland may comprise the progeny from a single cell. Development. 1998;125:1921–1930. doi: 10.1242/dev.125.10.1921. [DOI] [PubMed] [Google Scholar]
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TF, Leder P. Wnt-10b directs hypermorphic development and transformation in mammary glands of male and female mice. Oncogene. 1997;15:2133–2144. doi: 10.1038/sj.onc.1201593. [DOI] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. The Wnt co-receptor Lrp6 is required for normal mouse mammary gland development. PLoS One. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Olson DJ, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994;5:197–206. [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–241. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- Roelink H, Wagenaar E, Lopes da Silva S, Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990;87:4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–799. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Teissedre B, Pinderhughes A, Incassati A, Hatsell SJ, Hiremath M, Cowin P. MMTV-Wnt1 and -DeltaN89beta-catenin induce canonical signaling in distinct progenitors and differentially activate Hedgehog signaling within mammary tumors. PLoS One. 2009;4:e4537. doi: 10.1371/journal.pone.0004537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7717. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Mammary stem cells and mammopoiesis. Cancer Res. 2006;66:9798–9801. doi: 10.1158/0008-5472.CAN-06-2254. [DOI] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- Welm B, Behbod F, Goodell MA, Rosen JM. Isolation and characterization of functional mammary gland stem cells. Cell Prolif. 2003;36(Suppl 1):17–32. doi: 10.1046/j.1365-2184.36.s.1.3.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- Young LJ, Medina D, DeOme KB, Daniel CW. The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp Gerontol. 1971;6:49–56. doi: 10.1016/0531-5565(71)90048-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.