Abstract
Deficits in cholinergic function have been postulated to cause delirium and cognitive decline. This review examines current understanding of the cholinergic deficiency hypothesis in delirium by synthesizing evidence on potential pathophysiological pathways. Acetylcholine synthesis involves various precursors, enzymes, and receptors, and dysfunction in these components can lead to delirium. Insults to the brain, like ischemia and immunological stressors, can precipitously alter acetylcholine levels. Imbalances between cholinergic and other neurotransmitter pathways may result in delirium. Furthermore, genetic, enzymatic, and immunological overlaps exist between delirium and dementia related to the cholinergic pathway. Important areas for future research include identifying biomarkers, determining genetic contributions, and evaluating response to cholinergic drugs in delirium. Understanding how the cholinergic pathway relates to delirium may yield innovative approaches in the diagnosis, prevention, and treatment of this common, costly, and morbid condition.
Keywords: Acetylcholine, Delirium, Delirium in older persons, Dementia, Cholinergic deficiency
Described by Hippocrates more than 2000 years ago, delirium is an acute confusional state marked by global impairments in attention and cognition (1). Common yet underdiagnosed, delirium occurs in 1%–2% of the general population, and prevalence increases with age to 14% of persons 85 years old or older (2). Delirium is particularly prevalent among hospitalized elderly persons, occurring in 20%–60% and contributing to $6.9 billion (2004 U.S. dollars) in Medicare hospital costs annually (3). Delirium significantly increases the risk for medical complications, institutionalization, functional decline, and dementia (4,5). Mortality rates among hospitalized patients who develop delirium are as high as those among patients with myocardial infarction or sepsis (2).
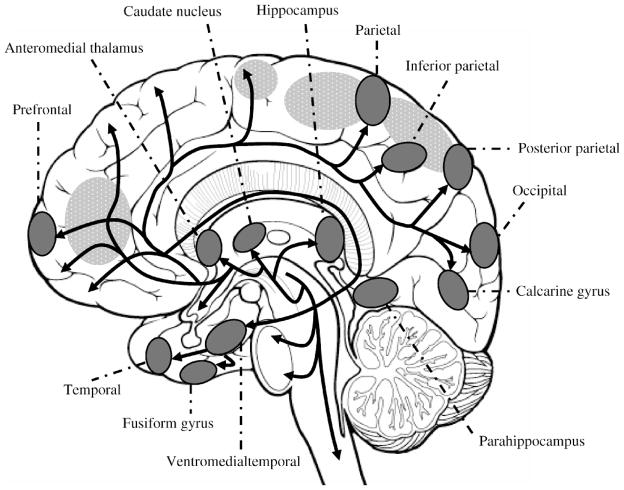
Despite its clinical impact, the pathophysiology of delirium remains poorly understood. To date, central cholinergic deficiency is the leading hypothesized mechanism for delirium (2). Competing hypotheses include dopamine excess, inflammation, and chronic stress; an important subset of delirium also involves toxic-metabolic encephalopathy (6). Acetylcholine plays an extensive role in attention and consciousness. It focuses awareness by acting as a modulator of signal-to-noise ratio in sensory and cognitive input; irregularities in these brain functions cause core symptoms of both hypoactive and hyperactive delirium, including inattention, disorganized thinking, and perceptual disturbances (7). Anatomically, cholinergic pathways have widespread interconnections, projecting from the basal forebrain and pontomesencephalon to interneurons in the striatum and finally to targets throughout the cortex. Although correlating delirium pathophysiology with brain imaging has limitations, particularly specificity and reproducibility, some structural and functional neuroimaging of delirium patients have suggested abnormalities that coincide with areas involved in cholinergic pathways (Figure 1) (8,9).
Figure 1.
Central cholinergic pathways overlap with locations of neuroimaging abnormalities in delirium brain studies. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies on patients with delirium from hepatic encephalopathy, cardiotomy, and traumatic brain injury demonstrate abnormal perfusion in the same cortical, subcortical regions as cholinergic pathways. Cholinergic pathways are indicated by black arrows, locations of abnormal neuroimaging are shaded, brain regions involved in attention are textured.
As in Alzheimer’s disease (AD), deficits in cholinergic function may contribute to the cognitive decline associated with delirium (10). Recent studies suggest that the pathophysiological mechanisms for delirium and AD may have substantial overlap. Thus, delirium and dementia may represent different points along a continuum of cognitive disorders. Based on a comprehensive synthesis of the literature, this review examines current understanding of the cholinergic deficiency hypothesis in delirium by integrating hypotheses and evidence. Potential pathophysiological overlaps between delirium and dementia related to the cholinergic pathway will be examined. Finally, areas of controversy and important avenues for future research will be highlighted. Advancing our understanding of delirium pathophysiology will ultimately yield innovative approaches to diagnosis, prevention, and treatment.
Cholinergic Deficiency Hypothesis
The cholinergic deficiency hypothesis originated in observations that delirium occurred with consumption of toxins and drugs that impair cholinergic function (11). Recent evidence comes from epidemiological studies and anticholinergic assays. Patients with “higher anticholinergic burden,” based on studies rating patients’ drug-related exposure, had more severe cases of delirium (12). Serum anticholinergic activity (SAA) estimates patients’ muscarinic anticholinergic burden from drugs and endogenous sources. SAA is determined by a receptor binding assay in which patients’ serum containing cholinergic drugs or metabolites competes with radiolabeled ligands for muscarinic acetylcholine receptors in homogenized rat forebrain. Increased SAA levels were strongly associated with delirium whereas a decline in SAA was seen with delirium resolution. Elevated anticholinergic activity has also been positively correlated with delirium symptom severity, indicating a dose-response relationship (13).
Despite evidence supporting the cholinergic deficiency hypothesis, there are weaknesses to this theory. To date, human trials of cholinesterase inhibitors have not demonstrated benefit in preventing or treating delirium (14). Cholinergic deficiency also incompletely explains why delirium and AD appear pathophysiologically related yet present differently, with attention and memory deficits, respectively. There is a dearth of literature refuting the cholinergic deficiency hypothesis or candidly discussing its shortcomings. Rather, the focus has been on examining other hypotheses and understanding better the multifactorial complexity of this medical condition. Other divergent factors likely contribute to the pathophysiology of both delirium and dementia, including hypoxia, inflammation, chronic stress, and decreased cerebral metabolism (2).
Several mechanisms, detailed below, can result in cholinergic deficiency and contribute to delirium, including impaired acetylcholine synthesis, cholinergic synaptic mechanisms, ischemia and global stressors, and neurotransmitter imbalance. While acknowledging that each example may have broader pathophysiologic impacts, we have targeted our descriptions primarily to their effects on the cholinergic system.
Impaired Acetylcholine Synthesis
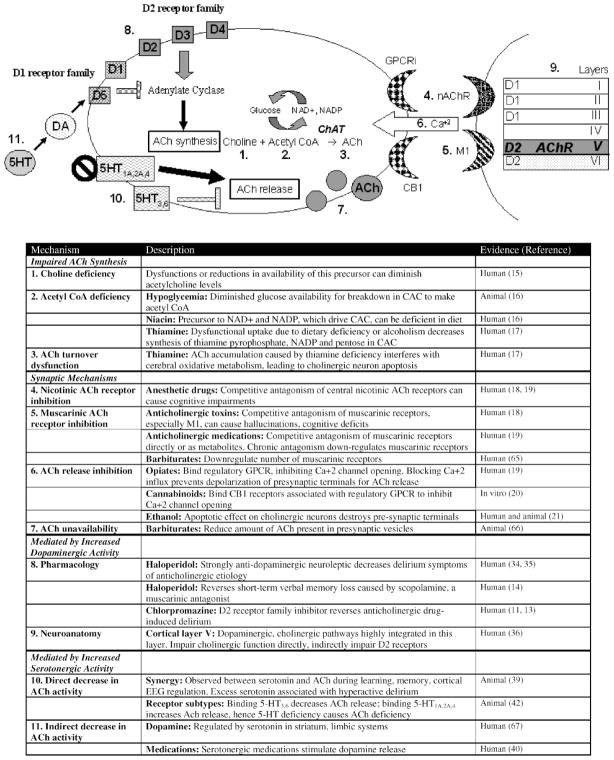
Acetylcholine is produced from the interaction of choline with acetyl coenzyme A (CoA). Thus, dysfunctions in this pathway or reductions in precursor availability can diminish acetylcholine levels (11,15). Acetyl CoA is produced by glucose breakdown in the citric acid cycle. Consequently, hypoglycemia or severe malnutrition may also lead to cholinergic deficit. In experimental animals, vulnerable steps include glucose breakdown, NAD+ and NADP generation from niacin, and enzyme synthesis from thiamine (Figure 2, Item 2) (16). Moreover, thiamine deficiency prevents turnover, leading to selective apoptosis of cholinergic neurons in animal models (Figure 2, Item 3) (17).
Figure 2.
Potential pathophysiological mechanisms for delirium. AD = Alzheimer’s disease; ApoE = apolipoprotein E; βA = β-amyloid; APP = amyloid precursor protein; Ach = acetylcholine; ChAT = choline acetyltransferase; AChE = acetylcholinesterase; IL-1 = interleukin-1; TNF-α = tumor necrosis factor-α; IGF-1 = insulin-like growth factor-1; CoA = coenzyme A; NAD+= nicotinamide adenine dinucleotide; NADP = nicotinamide adenine dinucleotide phosphate; CAC = citric acid cycle; GPCR = G-protein coupled receptor; Ca+2 = calcium; 5-HT = serotonin.
Cholinergic Synaptic Mechanisms
Potential synaptic mechanisms for cholinergic deficiency include impairment of presynaptic, synaptic, or postsynaptic functions of acetylcholine. Nicotinic receptors within the brain bind acetylcholine to modulate cognitive functioning, arousal, learning, and memory. Anesthetic drugs that inhibit postsynaptic nicotinic receptors (e.g., isoflurane, nitrous oxide) can cause cognitive impairments after surgery (18). Muscarinic acetylcholine receptors, more widely distributed throughout the brain, may play a larger role in delirium. Anticholinergic compounds and their metabolites predominantly induce delirium through competitive antagonism of postsynaptic muscarinic receptors (Figure 2, Item 4) (15). The M1 receptor subtype may be especially significant because it is most highly expressed in the central nervous system and is involved in perception, attention, and cognitive functioning. Anticholinergic toxins and medications inhibit strial cholinergic interneurons by blocking postsynaptic M1 muscarinic receptors, leading to hallucinations and cognitive deficits (Figure 2, Item 5) (18). Other muscarinic subtypes (M2–M5) have not been linked to delirium.
Some medications exert their anticholinergic effect presynaptically, preventing acetylcholine release into the synapse (Figure 2, Item 6) (19). Opiates and cannabinoids bind regulatory G-protein-coupled receptors to inhibit calcium channel opening, thereby blocking presynaptic terminal depolarization and affecting cholinergic and other pathways (20). Ethanol causes cholinergic neuron apoptosis, destroying presynaptic terminals and thus lowering the threshold for developing delirium via cholinergic deficiency (21). In addition, some drugs work through multifaceted mechanisms. Chronic barbiturate intake in animal models, for example, reduces the amount of acetylcholine present in the presynaptic vesicles and downregulates the muscarinic receptors on postsynaptic terminals (Figure 2, Item 7).
Ischemia and Global Stressors
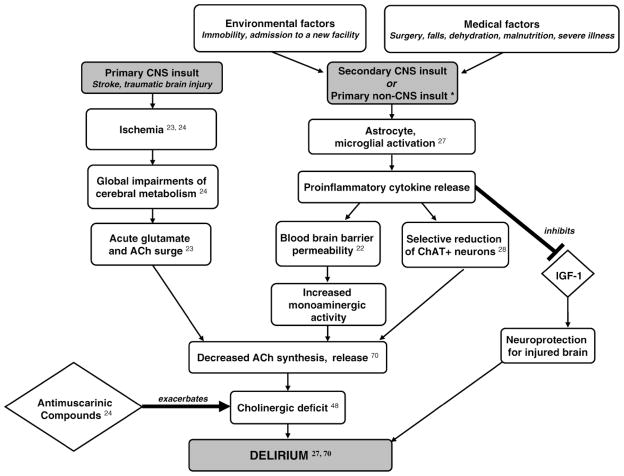
Acetylcholine synthesis, with its dependence on the aerobic citric acid cycle for acetyl CoA, is particularly susceptible to disturbances in cerebral metabolism (22). Global metabolic impairments are hallmarks of two important delirium precipitators: stroke and traumatic brain injury. In both conditions, cerebral ischemia leads to an acute surge of glutamate and acetylcholine, possibly due to decreased removal by impaired circulation (22). The cholinergic deficits that follow can last weeks and have been hypothesized to be a result of ineffective acetylcholine synthesis, release, or uptake caused by the cerebral damage. These deficits coincide with the onset of delirium symptoms following the acute neurological damage (23). Stroke and traumatic brain injury patients are particularly vulnerable to delirium, such as after receipt of antimuscarinic drugs (Figure 3) (24).
Figure 3.
Relationship of stroke, traumatic brain injury, and diverse stressors to delirium. Schematic demonstration of potential pathophysiologic pathways linking stroke, traumatic brain injury and environmental, medical stressors with delirium. Ach = acetylcholine; ChAT = choline acetyltransferase; IGF-1 = insulin-like growth factor 1; CNS = central nervous system. *Represents one potential pathophysiologic pathway to delirium; other mechanisms exist.
Various environmental and medical stressors activate the immune system and trigger cytokine release (25). Cytokines, which mediate inflammatory and immune responses to stress, may increase the risk of delirium through multiple mechanisms, including increasing blood–brain barrier permeability and direct neurotoxic effects (26). They act as conduits between immune cells and the brain, modulating sleep, cognition, and appetite (27). Cytokines may also lead to cholinergic deficits. For instance, bacterial lipopolysaccharides and severe infection trigger a cascade of cytokine release in the brain that selectively reduces choline acetyltransferase (ChAT) immunoreactive neurons; ChAT is the key enzyme in biosynthesis of acetylcholine (28). One major cytokine triggered by stress, tumor necrosis factor-α (TNF-α), can play a role in neurodegeneration by inhibiting insulin-like growth factor-1 (IGF-1), a neurotrophic and neuroprotective peptide in injured brain (26). Cytokines may further contribute to cholinergic deficit by increasing blood–brain barrier permeability and modifying cerebral neurotransmission (29). Cholinergic neurotransmission is exquisitely balanced with other neurotransmitter systems, discussed below. Cytokines can alter this equilibrium, increasing monoaminergic activity and ultimately reducing acetylcholine release (Figure 3) (30). Cytokine production in healthy brains may cause minimal damage; but when coupled with neuronal damage in a vulnerable brain (e.g., stroke, infection), they can precipitate neurotoxic degeneration, especially in the sensitive cells of the cholinergic pathway (31).
Neurotransmitter Imbalance
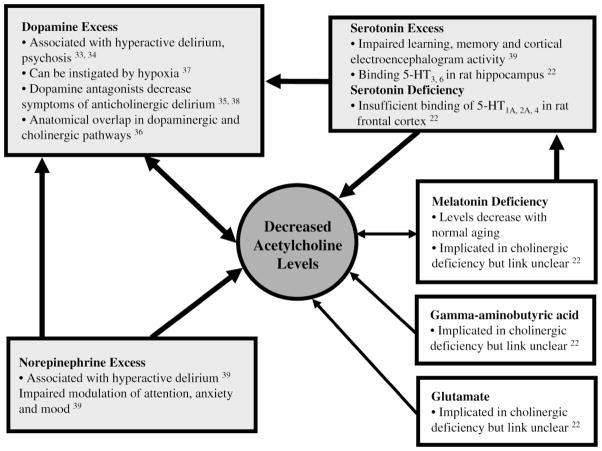
The cholinergic system is balanced by monoamine activity where dysfunction has also been associated with delirium. Dopamine, norepinephrine, and serotonin have roles in arousal and the sleep–wake cycle, mediating physiological responses to stimuli. These responses are modulated by the cholinergic pathway (32). Thus, the development of delirium likely involves interaction between the cholinergic pathway and these monoamines (Figure 4).
Figure 4.
Interactions between acetylcholine and other neurotransmitters in delirium. Schematic demonstration of how decreased acetylcholine levels in delirium alter levels of other neurotransmitters.
Dopamine excesses may contribute to hyperactive delirium, which has been linked to simultaneous acetylcholine decreases. Thus, acetylcholine and dopamine may be inversely related in delirium pathogenesis; pharmacological and neuroanatomical evidence support this model (33). For example, dopamine agonists like D-amphetamine result in frontostriatal abnormalities that correlate with delirium (34). Alternatively, dopamine antagonists, including neuroleptics, have been used to treat cases of delirium related to anticholinergic mechanisms (Figure 2, Item 8) (35).
Anatomically, dopaminergic and cholinergic pathways overlap significantly in the brain, suggesting intimate balance between these neurotransmitters. The prefrontal cortex has six layers of distinct neurotransmitter receptors. The D2 receptor family (dopamine), which inhibits acetylcholine synthesis, coincides with cholinergic fibers in layer V of the prefrontal cortex (36). Dysfunctions in the D2 receptor subtype have been associated with hallucinations, stereotypic behavior, and thought disturbances (Figure 2, Item 9) (36). Various dopamine receptors impact acetylcholine levels differently, which may explain the diverse clinical manifestations of delirium, including its hyperactive and hypoactive forms. Other contributors, like hypoxia, also result in dopamine surges that decrease acetylcholine release, which can lead to delirium (37). This forms the basis for the as-yet controversial proposal for treating all delirium with neuroleptics, which block dopamine receptors in the brain (38).
Serotonin is both directly and indirectly related to the cholinergic deficiency hypothesis. Excess serotonin has been observed in conjunction with diminished acetylcholine when learning, memory, and cortical electroencephalogram activity are impaired in experimental rats. Impairment of these cognitive measures is indicative of delirium (Figure 2, Item 10) (39). Binding of 5-HT3,6 receptors in rat hippo- campus decreases acetylcholine release whereas binding of frontal cortex 5-HT1A,2A and 5-HT4 increases release. Hence, depending on the serotonin receptor bound, both serotonin deficiency and excess may be linked to cholinergic deficiency (22). Serotonin also stimulates dopaminergic activity, thus inhibiting acetylcholine release in the pre- frontal cortex, striatum, and limbic systems (Figure 2, Item 11) (40). Administration of selective serotonin reuptake inhibitors, including bupropion and fluoxetine, has been associated with delirium (41).
Norepinephrine plays an important role in modulating attention, anxiety, and mood; like dopamine, excess noradrenergic activity has been associated with hyperactive delirium (39). Norepinephrine controls dopaminergic neurons in the mesocortex, affecting the prefrontal cortex where cholinergic pathways interface with both monoamines (42). Hence, imbalances of the cholinergic–noradrenergic axis may underlie delirium pathophysiology. Dysfunctional interactions between acetylcholine and other neurotransmitters like glutamate, melatonin, and γ-aminobutyric acid (GABA) are less understood but may play a role in the cholinergic deficit of delirium (43).
Delirium and Dementia
Dementia with Lewy Bodies, the second most common form of dementia, has a clinical presentation overlapping with delirium, including attentional impairment, fluctuations, and visual hallucinations. Loss of cholinergic neurons, resulting in severe acetylcholine deficits, is believed to account for the confusional symptoms present in this disease (44). AD and delirium have also been highly interrelated in clinical and epidemiologic studies, but the mechanisms behind this relationship remain unexplored. Up to two-thirds of patients with delirium have underlying dementia and, conversely, dementia is a significant risk factor for delirium (2). Because late-onset and end-stage AD patients exhibit significant cholinergic deficits, acetylcholine deficiency is implicated as a potential common pathway in both syndromes. Recent studies have further blurred delineation between delirium and dementia. Delirium symptoms have been found to persist for much longer than previously believed, up to months or years after onset (45). Delirium, in addition to its underlying etiologies, may itself also lead to long-term cognitive impairment and dementia (46,47). Moreover, neuroimaging studies have documented regions of hypoperfusion in patients with delirium that overlap with hypoperfused areas in AD patients (48).
The cholinergic deficiency hypothesis has been extensively studied in AD and may shed light on our understanding of delirium. The major neurochemical abnormality seen in AD is central cholinergic deficiency (49), as demonstrated in postmortem, in vivo, and pharmacological studies (50). Pathophysiological mechanisms behind this deficit in AD include decreased ChAT, increased acetylcholinesterase activity, lower M2 and nicotinic receptor densities, and decreased M2 receptor coupling to G proteins (51). Cholinergic neuron death is a central part of AD pathophysiology (50). As discussed above, dysfunction of cholinergic neurons also plays an important role in delirium pathophysiology. Thus, delirium and AD may represent points along a continuum of cognitive disorders, rather than two entirely separate conditions (25).
Apolipoprotein E: Potential Common Mechanism Between Delirium and AD
Apolipoprotein E (ApoE) has been extensively examined in the AD model, but this area is underexplored in delirium. A recent study demonstrated for the first time that the ApoE-ε4 allele results in longer duration of delirium; however, the mechanism remains uncertain (52). ApoE may be linked to cholinergic deficits and clinical characteristics in AD (53). AD patients possessing the ApoE-ε4 allele, for instance, demonstrate electroencephalogram slowing suggestive of more significant cholinergic deficits than what is seen in AD patients with other ApoE genotypes (54). The ApoE4 protein destroys cholinergic neurons via a combination of increased synthesis and impaired clearance of β-amyloid (55).
The ApoE-ε4 allele has been linked to ChAT down-regulation in a dose-dependent fashion. Studies of ChAT polymorphism suggest a genetic basis for this observation, perhaps involving inefficient ChAT gene translation into a functional enzyme (56).
ApoE typically modulates acetylcholinesterase in the synaptic cleft of neurons (57); any variability in ApoE isoforms may result in acetylcholinesterase disinhibition and cholinergic deficit. Initial treatment for AD currently involves administration of acetylcholinesterase inhibitors. Due to its cholinergic deficiencies, delirium is theoretically also treatable with acetylcholinesterase inhibitors (58). Two double-blind, randomized trials of cholinesterase inhibitors recently failed to demonstrate statistically significant prevention of postoperative delirium (Table 1) (58,59).
Table 1.
Potential Overlapping Mechanisms of Cholinergic Deficiency in AD and Delirium
| Mechanism | Finding in AD | Potential Parallels in Delirium |
|---|---|---|
| ApoE4 protein | Cholinergic neuron death: βA tightly binds ApoE4, making the latter unavailable for mobilizing phosphatidylcholine in ACh synthesis. ApoE4 exacerbates this by accelerating APP cleaving into βA and impairing βA clearance (55) | Cholinergic neuron apoptosis: Thiamine deficiency can cause neuronal death by mechanisms similar to ApoE, depleting precursors for ACh synthesis (Figure 2, Item 3) (17) Symptomatic link: APOE-ε4 allele associated with longer duration of delirium, poorer recovery (52,60) |
| ChAT downregulation | ChAT synthesis: Proportionally downregulated with each APOE-ε4 allele; ChAT catalyzes key step of ACh synthesis (56) | Selective ChAT+ neuron reduction: By stressor-induced cytokine release in rat forebrains (Figure 2) (28) |
| AChE dysregulation | AChE inhibitors: Dysregulated AChE levels cause excess of AChE that can lead to cholinergic deficits. AChE inhibitor drugs are currently first-line treatment, prolonging the effects of ACh by preventing its hydrolysis, inactivation in synaptic clefts | AChE inhibitors: Theoretically therapeutic, donepezil undergoing clinical trials has not demonstrated statistically significant delirium prevention, treatment (58,59) |
| Cytokine polymorphisms | IL-1α, 6: Polymorphisms in these cytokines linked with decreased ACh synthesis (68,69) | Decreased ACh synthesis: Due to proinflammatory cytokines released during stress (Figure 2) (70) |
| Cytokine excess | Elevated IL-1, TNF-αlevels: Inhibit IGF-1, which protects against cholinergic neuron apoptosis and deleterious βA binding (64) | IGF-1 inhibition: By proinflammatory cytokines released during stress prevents neuroprotection of injured brain (Figure 2) (27) |
Note: AD = Alzheimer’s disease; ApoE = apolipoprotein E; βA = β-amyloid; APP = amyloid precursor protein; Ach = acetylcholine; ChAT = choline acetyltransferase; AChE = acetylcholinesterase; IL-1 = interleukin-1; TNF-α= tumor necrosis factor-α; IGF-1 = insulin-like growth factor-1.
Neuroimmunology, AD, and Delirium
Cytokines and other immune mediators implicated in the cholinergic deficit of delirium have been more extensively studied in AD. Understanding the role of these immune mediators in AD may elucidate their role in delirium. As in delirium, some neuroprotective factors are inhibited in AD whereas other proinflammatory ones are activated to decrease acetylcholine synthesis (Table 1) (59). ApoE isoforms have also been associated with altered immune responses to exogenous neurotoxins. A predictive model of recovery from delirium was recently formulated based on gender, presence of the ApoE-ε4 allele, and levels of interferon-γ and IGF-1 (60).
Discussion
Although various pathophysiologic mechanisms have been hypothesized, cholinergic deficiency appears to play a central role in the development of delirium. We have reviewed in depth the evidence exploring cholinergic mechanisms in delirium. Acetylcholine synthesis has been demonstrated to be vulnerable to a number of stressors and deficiencies. Delirium-inducing medications often function by antagonizing presynaptic and postsynaptic acetylcholine receptors. Impairments in global metabolism, cytokine interactions, and neurotransmitters have often been considered separate mechanisms contributing to delirium. However, we suggest that all of these mediators play important, interacting roles in delirium. Many of these mediators operate through inducing central cholinergic deficits. With better elucidation of its mechanisms, we can strengthen our ability to elucidate the prognosis, prevention, and treatment of delirium.
Understanding the role of cholinergic deficiency in AD offers new insights into delirium pathophysiology. Delirium may result from insults to the vulnerable brain of AD patients already suffering cholinergic deficit. Delirium and AD may also represent two points along a continuum of cognitive disorders. Our review shows a clear link between these syndromes. Cholinergic deficits present in both conditions can be attributed to neuronal death, decreased ChAT synthesis, and neuroimmunological responses. Studies in both conditions have shown cognitive improvements with administration of acetylcholinesterase inhibitors. Additional pathophysiologic mechanisms, however, also contribute to both conditions. Further investigation of the interface between delirium and dementia, such as confirming the potential mechanisms in Table 1, will help advance our understanding of both conditions.
Important directions for future research include identifying delirium biomarkers, determining genetic contributions, and evaluating response to cholinergic drugs (61). Identification of more effective delirium biomarkers would allow critical advances in both basic and clinical investigation of delirium. One promising area of investigation is development of an SAA assay specific for the cholinergic M1 receptor associated with cognition (62). Other components of the cholinergic pathway, like ChAT levels and acetyl-cholinesterase activity, are measurable in both human cerebrospinal fluid and blood. Hence, they may potentially be useful as biomarkers that allow us to diagnose and follow delirium in a more standardized manner. Further exploration of the contribution of ApoE alleles, as well as other genetic factors, to delirium is another important area for future research. Finally, clinical trials to evaluate effectiveness of cholinergic drugs for the prevention and treatment of delirium are critically needed. Delirium is one of the most preventable conditions in elderly hospitalized patients; previous studies indicate that at least 30%–40% of cases may be preventable (3). Better understanding of the cholinergic system in delirium may help refine our approach to the diagnosis, prevention, and treatment of this devastating condition of older persons.
Acknowledgments
This report was funded in part by the American Federation for Aging Research, by grants from the National Institute on Aging (R21AG027549, R21AG025193, K24AG00949, and P60AG008812), and by the Aging Brain Center, Hebrew SeniorLife. Dr. Marcantonio is a Paul Beeson Physician Faculty Scholar in Aging Research. Dr. Inouye is supported in part by the Milton and Shirley F. Levy Family Chair.
References
- 1.Burns AG, Byrne J. Delirium. J Neurol Neurosurg Psychiatry. 2005;75:362–367. doi: 10.1136/jnnp.2003.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 4.Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Multicomponent geriatric intervention for elderly inpatients with delirium: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61:176–181. doi: 10.1093/gerona/61.2.176. [DOI] [PubMed] [Google Scholar]
- 5.Bellelli G, Speciale S, Barisione E, Trabucchi M. Delirium subtypes and 1-year mortality among elderly patients discharged from a post-acute rehabilitation facility. J Gerontol A Biol Sci Med Sci. 2007;62:1182–1183. doi: 10.1093/gerona/62.10.1182. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Ferrucci L. Elucidating the pathophysiology of delirium and the interrelationship of delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61:1277–1280. doi: 10.1093/gerona/61.12.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb GL, Johnson J, Wanich C, Sullivan E. Delirium in the medically ill elderly: operationalizing the DSM-III criteria. Int Psychogeriatr. 1991;3:181–196. doi: 10.1017/s1041610291000650. [DOI] [PubMed] [Google Scholar]
- 8.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61:1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 9.Fong TG, Bogardus ST, Jr, Daftary A, et al. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006;61:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 10.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 11.Tune LE, Damlouji NF, Holland A, Gardner TJ, Folstein MF, Coyle JT. Association of postoperative delirium with raised serum levels of anticholinergic drugs. Lancet. 1981;2:651–653. doi: 10.1016/s0140-6736(81)90994-6. [DOI] [PubMed] [Google Scholar]
- 12.Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161:1099–1105. doi: 10.1001/archinte.161.8.1099. [DOI] [PubMed] [Google Scholar]
- 13.Flacker JM, Cummings V, Mach JR, Jr, Bettin K, Kiely DK, Wei J. The association of serum anticholinergic activity with delirium in elderly medical patients. Am J Geriatr Psychiatry. 1998;6:31–41. [PubMed] [Google Scholar]
- 14.Cummings JL, Gorman DG, Shapira J. Physostigmine ameliorates the delusions of Alzheimer’s disease. Biol Psychiatry. 1993;33:536–541. doi: 10.1016/0006-3223(93)90009-3. [DOI] [PubMed] [Google Scholar]
- 15.Blass JP, Gibson GE. Cerebrometabolic aspects of delirium in relationship to dementia. Dement Geriatr Cogn Disord. 1999;10:335–338. doi: 10.1159/000017165. [DOI] [PubMed] [Google Scholar]
- 16.Ghajar JB, Gibson GE, Duffy TE. Regional acetylcholine metabolism in brain during acute hypoglycemia and recovery. J Neurochem. 1985;44:94–98. doi: 10.1111/j.1471-4159.1985.tb07117.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawasai O. Behavioral and neurochemical alterations following thiamine deficiency in rodents: relationship to functions of cholinergic neurons. Yakugaku Zasshi. 2005;125:549–554. doi: 10.1248/yakushi.125.549. [DOI] [PubMed] [Google Scholar]
- 18.Pratico C, Quattrone D, Lucanto T, et al. Drugs of anesthesia acting on central cholinergic system may cause post-operative cognitive dysfunction and delirium. Med Hypotheses. 2005;65:972–982. doi: 10.1016/j.mehy.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Coffman JA, Dilsaver SC. Cholinergic mechanisms in delirium. Am J Psychiatry. 1988;145:382–383. doi: 10.1176/ajp.145.3.382b. [DOI] [PubMed] [Google Scholar]
- 20.Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of cAMP response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz P, editor. The Neuropathophysiology of Delirium. Oxford: Oxford University Press; 2002. [Google Scholar]
- 23.Dixon CE, Bao J, Bergmann JS, Johnson KM. Traumatic brain injury reduces hippocampal high-affinity [3H]choline uptake but not extra-cellular choline levels in rats. Neurosci Lett. 1994;180:127–130. doi: 10.1016/0304-3940(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 24.Dixon CE, Hamm RJ, Taft WC, Hayes RL. Increased anticholinergic sensitivity following closed skull impact and controlled cortical impact traumatic brain injury in the rat. J Neurotrauma. 1994;11:275–287. doi: 10.1089/neu.1994.11.275. [DOI] [PubMed] [Google Scholar]
- 25.Eikelenboom P, Hoogendijk WJ. Do delirium and Alzheimer’s dementia share specific pathogenetic mechanisms? Dement Geriatr Cogn Disord. 1999;10:319–324. doi: 10.1159/000017162. [DOI] [PubMed] [Google Scholar]
- 26.Eikelenboom P, Hoogendijk WJ, Jonker C, van Tilburg W. Immunological mechanisms and the spectrum of psychiatric syndromes in Alzheimer’s disease. J Psychiatr Res. 2002;36:269–280. doi: 10.1016/s0022-3956(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 27.Kronfol Z, Remick DG. Cytokines and the brain: implications for clinical psychiatry. Am J Psychiatry. 2000;157:683–694. doi: 10.1176/appi.ajp.157.5.683. [DOI] [PubMed] [Google Scholar]
- 28.Willard LB, Hauss-Wegrzyniak B, Wenk GL. Pathological and biochemical consequences of acute and chronic neuroinflammation within the basal forebrain cholinergic system of rats. Neuroscience. 1999;88:193–200. doi: 10.1016/s0306-4522(98)00216-4. [DOI] [PubMed] [Google Scholar]
- 29.Broadhurst C, Wilson K. Immunology of delirium: new opportunities for treatment and research. Br J Psychiatry. 2001;179:288–289. doi: 10.1192/bjp.179.4.288. [DOI] [PubMed] [Google Scholar]
- 30.Stefano GB, Bilfinger TV, Fricchione GL. The immune-neuro-link and the macrophage: postcardiotomy delirium, HIV-associated dementia and psychiatry. Prog Neurobiol. 1994;42:475–488. doi: 10.1016/0301-0082(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 31.Venters HD, Tang Q, Liu Q, VanHoy RW, Dantzer R, Kelley KW. A new mechanism of neurodegeneration: a proinflammatory cytokine inhibits receptor signaling by a survival peptide. Proc Natl Acad Sci U S A. 1999;96:9879–9884. doi: 10.1073/pnas.96.17.9879. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Lindesay J. The concept of delirium. Dement Geriatr Cogn Disord. 1999;10:310–314. doi: 10.1159/000017160. [DOI] [PubMed] [Google Scholar]
- 33.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson LS. The nature of interactions involving prefrontal and striatal dopamine systems. J Psychopharmacol. 1997;11:143–150. doi: 10.1177/026988119701100207. [DOI] [PubMed] [Google Scholar]
- 35.Platt MM, Breitbart W, Smith M, Marotta R, Weisman H, Jacobsen PB. Efficacy of neuroleptics for hypoactive delirium. J Neuropsychiatry Clin Neurosci. 1994;6:66–67. doi: 10.1176/jnp.6.1.66. [DOI] [PubMed] [Google Scholar]
- 36.Mrzljak L, Goldman-Rakic PS. Acetylcholinesterase reactivity in the frontal cortex of human and monkey: contribution of AChE-rich pyramidal neurons. J Comp Neurol. 1992;324:261–281. doi: 10.1002/cne.903240208. [DOI] [PubMed] [Google Scholar]
- 37.Broderick PA, Gibson GE. Dopamine and serotonin in rat striatum during in vivo hypoxic-hypoxia. Metab Brain Dis. 1989;4:143–153. doi: 10.1007/BF00999391. [DOI] [PubMed] [Google Scholar]
- 38.Breitbart W, Strout D. Delirium in the terminally ill. Clin Geriatr Med. 2000;16:357–372. doi: 10.1016/s0749-0690(05)70061-6. [DOI] [PubMed] [Google Scholar]
- 39.Hirano H, Day J, Fibiger HC. Serotonergic regulation of acetylcholine release in rat frontal cortex. J Neurochem. 1995;65:1139–1145. doi: 10.1046/j.1471-4159.1995.65031139.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanda G, Carboni E, Frau R, Di Chiara G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology (Berl) 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- 41.Chan CH, Liu HC, Huang MC. Delirium associated with concomitant use of low-dose bupropion sustained release and fluoxetine. J Clin Psychopharmacol. 2006;26:677–679. doi: 10.1097/01.jcp.0000246210.18777.c2. [DOI] [PubMed] [Google Scholar]
- 42.Tassin JP. Norepinephrine-dopamine interactions in the prefrontal cortex and the ventral tegmental area: relevance to mental diseases. Adv Pharmacol. 1998;42:712–716. doi: 10.1016/s1054-3589(08)60847-9. [DOI] [PubMed] [Google Scholar]
- 43.Trzepacz PT. The neuropathophysiology of delirium. In: Lindesay JRK, Macdonald A, editors. Delirium in Old Age. Oxford: Oxford University Press; 2002. pp. 51–90. [Google Scholar]
- 44.Neef D, Walling AD. Dementia with Lewy bodies: an emerging disease. Am Fam Physician. 2006;73:1223–1229. [PubMed] [Google Scholar]
- 45.Levkoff SE, Evans DA, Liptzin B, et al. Delirium. The occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 46.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 47.Kiely DK, Jones RN, Bergmann MA, Murphy KM, Orav EJ, Marcantonio ER. Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61:204–208. doi: 10.1093/gerona/61.2.204. [DOI] [PubMed] [Google Scholar]
- 48.Yokota H, Ogawa S, Kurokawa A, Yamamoto Y. Regional cerebral blood flow in delirium patients. Psychiatry Clin Neurosci. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 49.Rakonczay Z, Horvath Z, Juhasz A, Kalman J. Peripheral cholinergic disturbances in Alzheimer’s disease. Chem Biol Interact. 2005;157–158:233–238. doi: 10.1016/j.cbi.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 50.Di Lazzaro V, Pilato F, Dileone M, et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology. 2006;66:1111–1113. doi: 10.1212/01.wnl.0000204183.26231.23. [DOI] [PubMed] [Google Scholar]
- 51.Oddo S, LaFerla FM. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J Physiol (Paris) 2006;99:172–179. doi: 10.1016/j.jphysparis.2005.12.080. [DOI] [PubMed] [Google Scholar]
- 52.Ely EW, Girard TD, Shintani AK, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med. 2007;35:112–117. doi: 10.1097/01.CCM.0000251925.18961.CA. [DOI] [PubMed] [Google Scholar]
- 53.Lehtovirta M, Soininen H, Helisalmi S, et al. Clinical and neuro-psychological characteristics in familial and sporadic Alzheimer’s disease: relation to apolipoprotein E polymorphism. Neurology. 1996;46:413–419. doi: 10.1212/wnl.46.2.413. [DOI] [PubMed] [Google Scholar]
- 54.Lehtovirta M, Partanen J, Kononen M, et al. Spectral analysis of EEG in Alzheimer’s disease: relation to apolipoprotein E polymorphism. Neurobiol Aging. 1996;17:523–526. doi: 10.1016/0197-4580(96)00024-3. [DOI] [PubMed] [Google Scholar]
- 55.Kowall NW, Beal MF, Busciglio J, Duffy LK, Yankner BA. An in vivo model for the neurodegenerative effects of beta amyloid and protection by substance P. Proc Natl Acad Sci U S A. 1991;88:7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozturk A, DeKosky ST, Kamboh MI. Genetic variation in the choline acetyltransferase (CHAT) gene may be associated with the risk of Alzheimer’s disease. Neurobiol Aging. 2006;27:1440–1444. doi: 10.1016/j.neurobiolaging.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soininen H, Lehtovirta M, Helisalmi S, Linnaranta K, Heinonen O, Riekkinen P. Increased acetylcholinesterase activity in the CSF of Alzheimer patients carrying apolipoprotein epsilon4 allele. Neuroreport. 1995;6:2518–2520. doi: 10.1097/00001756-199512150-00017. [DOI] [PubMed] [Google Scholar]
- 58.Liptzin B, Laki A, Garb JL, Fingeroth R, Krushell R. Donepezil in the prevention and treatment of post-surgical delirium. Am J Geriatr Psychiatry. 2005;13:1100–1106. doi: 10.1176/appi.ajgp.13.12.1100. [DOI] [PubMed] [Google Scholar]
- 59.Sampson EL, Raven PR, Ndhlovu PN, et al. A randomized, double-blind, placebo-controlled trial of donepezil hydrochloride (Aricept) for reducing the incidence of postoperative delirium after elective total hip replacement. Int J Geriatr Psychiatry. 2007;22:343–349. doi: 10.1002/gps.1679. [DOI] [PubMed] [Google Scholar]
- 60.Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJ. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry. 2007;22:688–694. doi: 10.1002/gps.1732. [DOI] [PubMed] [Google Scholar]
- 61.Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop D, Inouye SK. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61:1281–1286. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 62.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 63.Singh VK, Guthikonda P. Circulating cytokines in Alzheimer’s disease. J Psychiatr Res. 1997;31:657–660. doi: 10.1016/s0022-3956(97)00023-x. [DOI] [PubMed] [Google Scholar]
- 64.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 65.Nordberg A, Wahlstrom G. Cholinergic mechanisms in physical dependence on barbiturates, ethanol and benzodiazepines. J Neural Transm Gen Sect. 1992;88:199–221. doi: 10.1007/BF01244733. [DOI] [PubMed] [Google Scholar]
- 66.Kikuchi T, Wang Y, Shinbori H, Sato K, Okumura F. Effects of ketamine and pentobarbitone on acetylcholine release from the rat frontal cortex in vivo. Br J Anaesth. 1997;79:128–130. doi: 10.1093/bja/79.1.128. [DOI] [PubMed] [Google Scholar]
- 67.Meltzer HY. Serotonin receptors and antipsychotic drug action. Psychopharmacol Ser. 1993;10:70–81. doi: 10.1007/978-3-642-78010-3_7. [DOI] [PubMed] [Google Scholar]
- 68.Papassotiropoulos A, Bagli M, Jessen F, et al. A genetic variation of the inflammatory cytokine interleukin-6 delays the initial onset and reduces the risk for sporadic Alzheimer’s disease. Ann Neurol. 1999;45:666–668. doi: 10.1002/1531-8249(199905)45:5<666::aid-ana18>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 69.Nicoll JA, Mrak RE, Graham DI, et al. Association of interleukin-1 gene polymorphisms with Alzheimer’s disease. Ann Neurol. 2000;47:365–368. [PMC free article] [PubMed] [Google Scholar]
- 70.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]