Abstract
Our previous work has demonstrated that human follicular lymphoma (FL) infiltrating T cells are anergic, in part due to suppression by regulatory T cells. In this study, we identify pericellular adenosine, interacting with T cell-associated G protein-coupled A2A/B adenosine receptors (AR), as contributing to FL T cell hyporesponsiveness. In a subset of FL patient samples, treatment of lymph node mononuclear cells (LNMC) with specific A2A/B AR antagonists results in an increase in IFN-γ or IL-2 secretion upon anti-CD3/CD28 Ab stimulation, as compared with that seen without inhibitors. In contrast, treatment with an A1 AR antagonist had no effect on cytokine secretion. As the rate limiting step for adenosine generation from pericellular ATP is the ecto-ATPase CD39, we next show that inhibition of CD39 activity using the inhibitor ARL 67156 partially overcomes T cell hyporesponsiveness in a subset of patient samples. Phenotypic characterization of LNMC demonstrates populations of CD39-expressing CD4+ and CD8+ T cells, which are overrepresented in FL as compared with that seen in normal or reactive nodes, or normal peripheral blood. Thirty percent of the FL CD4+CD39+ T cells coexpress CD25high and FOXP3 (consistent with regulatory T cells). Finally, FL or normal LNMC hydrolyze ATP in vitro, in a dose- and time-dependent fashion, with the rate of ATP consumption being associated with the degree of CD39+ T cell infiltration. Together, these results support the finding that the ATP-ectonucleotidase-adenosine system mediates T cell anergy in a human tumor. In addition, these studies suggest that the A2A/B AR as well as CD39 are novel pharmacological targets for augmenting cancer immunotherapy.
One of the major limitations to cancer immunotherapy is overcoming the myriad of mechanisms that tumors use to subvert the antitumor immune response (1-3). One such mechanism is thought to be mediated by extracellular adenosine derived from hypoxic tumor cells, which upon binding to the G protein-coupled A2A and A2B receptors on T cells, inhibits T cell proliferation and IL-2, TNF-α, and IFN-γ secretion (4-8). Indeed, in a CL-8 murine melanoma tumor model, pharmacological inhibition of the A2A and A2B receptors in vivo inhibits tumor growth in a CD8+ T cell-dependent fashion (9). In addition, complete tumor rejection was seen in A2AR−/− mice (9). These results suggest that adenosine may play a pivotal role in tumor-mediated immune suppression and as such, may be a pharmacological target to enhance antitumor immunity (10-12).
Pericellular adenosine is generated, in part, through a catabolic ectonucleotide cascade, whereby ATP is first hydrolyzed by the ectonucleotidase CD39 to its nucleoside monophosphate AMP, which is subsequently degraded to the nucleoside adenosine by membrane bound or soluble CD73 (13-15). In addition to generating adenosine, the elimination of pericellular ATP through hydrolysis may also lead to immune suppression by mechanisms independent of adenosine. For example, ATP itself is a “danger signal”, inducing dendritic cell chemotaxis and maturation (16-18). In addition, FoxP3+ regulatory T cells (Tregs)3 are particularly sensitive to ATP (in comparison to conventional T cells, for example), undergoing necrotic cell death mediated by ATP binding to the P2X7 receptor (19). Taken together, the hydrolysis of ATP by CD39 and CD73 could induce immune suppression through 1) adenosine-mediated inhibition of T cell proliferation; 2) loss of the ATP danger signal; and 3) protecting the viability of the immune suppressive Tregs.
We and others have recently shown that murine FoxP3+ T cells suppress T cell proliferation and cytokine production, in part, through an adenosine-dependent pathway (20, 21). As we have also shown that T cells infiltrating human B cell follicular lymphomas (FL) are hyporesponsive to anti-CD3/CD28 Ab stimulation, (as measured by proliferation and cytokine production), due in part to infiltrating Tregs, we hypothesized that ATP hydrolysis and the subsequent generation of adenosine may contribute to the profound anergy of tumor derived T cells in human FL (22). Indeed, our research demonstrates the following: 1) FL infiltrating T cell hyporesponsiveness can be partially reversed in a subset of patient samples by either blockade of the A2A and A2B adenosine receptors (AR) or inhibition of CD39 activity; 2) CD39-bearing T cells are over-represented in FL nodes, as compared with that seen in normal or reactive lymph nodes (RLN), as well as normal donor peripheral blood; 3) in contrast to what is seen and has been reported in peripheral blood, CD39 is expressed on both FOXP3+ and FOXP3− CD4+ T cells, as well as on a subset of CD8+ T cells in nodal tissue; and 4) increased proportions of CD39+ T cells is associated with increased ATP hydrolysis to AMP in vitro. These results strongly suggest that the ATP-ectonucleotidase-adenosine system contributes to T cell anergy in a human tumor.
Materials and Methods
Abs for flow cytometry
The following fluorochrome-conjugated Abs were used for surface staining: CD39-PE-Cy7 (eBioscience); CD3-allophycocyanin, CD16-PE-Cy5, CD25-allophycocyanin, CD25-allophycocyanin-Cy7, CD39-FITC, CD39-PE, CD4-Alexa Fluor 700, CD4-allophycocyanin-Cy7, CD73-PE, and 7-aminoactinomycin D for viability determination (BD Pharmingen); CD3-PE-Cy5.5, CD8-PE-Texas Red, and CD14-Tri-Color (Invitrogen). The Ab used for intracellular staining was FOXP3-Alexa Fluor 488 (eBioscience).
Flow cytometry and cell sorting
Cryopreserved samples were thawed, washed once in PBS containing 1% FBS, and surface stained on ice, using specific fluorochrome-conjugated Abs. For intracellular FOXP3 staining, surface stained cells were fixed/permeablized and stained for intracellular FOXP3 using the human Treg (FOXP3) staining kit (eBioscience), according to the manufacturer’s recommendations. Stained cells were acquired on an LSR-II Flow Cytometer (BD Biosciences) with data analysis performed using FlowJo software (version 8.8.4; Tree Star). Discrete cell populations were gated based upon fluorescence intensity of the indicated markers as compared with the appropriate fluorescence minus one controls. All specimens analyzed underwent only one freeze/thaw cycle and a fresh sample was used for each experiment. For sorting of CD39+ T cells, PBMC were stained with surface Abs, CD3-allophycocyanin, and CD39-PE (BD Biosciences) using DAPI (4′,6-diamidino-2-phenylindole; Invitrogen) as a vital stain, as described, and subjected to two-way flow cytometric sorting using a FACSAriaII cell sorter and FACSDiva software (version 6.1.1; BD Biosciences). The resultant sorted CD39+ and CD39− T cell populations were >90% CD39+ and <2% CD39+, respectively, and each was >98% CD3+ (data not shown).
Patient samples
Primary lymph nodes (malignant and nonmalignant reactive) were obtained from patients undergoing routine biopsy, whereas normal lymph nodes (NLN, nonmalignant, nonreactive) were obtained from patients undergoing vascular surgery, during which time obstructive lymph nodes are removed. Normal donor peripheral blood was obtained by venipuncture from separate individuals. Biopsy tissues were obtained from the Strong Memorial Hospital Surgical Pathology Laboratory (Rochester, NY) and maintained in sterile specimen containers on ice until processing. A histological diagnosis for each specimen received was obtained anonymously. All lymph node biopsy tissues and peripheral blood samples were obtained under an Institutional Review Board-approved protocol.
Cell isolation and separation
Lymph node biopsy tissues were mechanically separated, minced, and passed through a 70-μm nylon mesh cell strainer under sterile conditions. The resultant single cell suspensions were washed with RPMI 1640 medium, counted, and cryopreserved for future analysis. Whole blood was obtained from normal donors by venipuncture and PBMC were isolated from the whole blood using Ficoll-Paque Plus, according to the manufacturer’s instructions (Amersham Biosciences). The resultant PBMC were also cryopreserved for future analysis.
ATP consumption assay
Lymph node mononuclear cells (LNMC) or normal donor PBMC were cultured in AIM-V serum free medium in the presence of 10 μM ATP. After a 30 min or 1 h incubation at 37°C, cell-free aliquots of medium were removed from the wells and immediately assayed, in triplicate, for ATP amounts using the CellTiter-Glo Luminescent Cell Viability Assay (Promega), read in a Fluoroskan Ascent FL luminometer (Thermo-Fisher Scientific) and relative luminescent units (RLU) for each sample was assessed using Ascent software (version 2.6). ATP amounts were compared with wells containing 10 μM ATP but no cells and the percentage of ATP remaining for each condition was calculated using the following formula: (RLU of the sample – RLU of medium alone/RLU of 10 μM ATP in medium with no cells – RLU of medium alone) × 100. In the absence of cells, ATP quantities remained stable in AIM-V medium (readings at 0, 15, 30, and 60 min) with a <5% loss of ATP observed over the entire 1-h incubation period (data not shown). In addition, incubation of cells alone (at the highest concentration) for the entire 1 h of the assay, without the addition of exogenous ATP, did not result in the release of detectable levels of ATP into the medium (data not shown).
Functional assays
All functional experiments were performed in AIM-V serum-free medium to reduce the effects of adenosine deaminase present in serum. LNMC were cultured, in triplicate with exogenous ATP added (10 μM), at the cell concentrations indicated in the experiments in IFN-γ or IL-2 ELISPOT plates (MabTech) in the presence or absence of the indicated concentrations of inhibitors ARL 67156, SCH58261, CPX (Sigma-Aldrich), or ZM 241,385 (Tocris) at 37°C and 5% CO2. ELISPOT plates were developed 18 or 24 h later for IFN-γ or IL-2, respectively, according to the manufacturer’s recommendations (MabTech), and the number of cytokine-producing cells per well was enumerated on a CTL Immunospot plate reader using Immunospot software (version 3.0; Cellular Technologies).
Statistical analysis
Data were expressed in terms of their average and SD. Comparison of proportions of cells with given phenotypic expressions as measured by flow cytometry across experimental conditions was conducted using the distribution free Zc test (23). The numbers of IFN-γ- or IL-2-producing cells across experimental conditions were compared using Friedman’s distribution-free two-way ANOVA (24). Intrapatient differences in number of IFN-γ- or IL-2-producing cells were assessed using t tests with unequal variances.
Results
Blockade of A2A/A2B but not A1 AR abrogates T cell hyporesponsiveness within FL
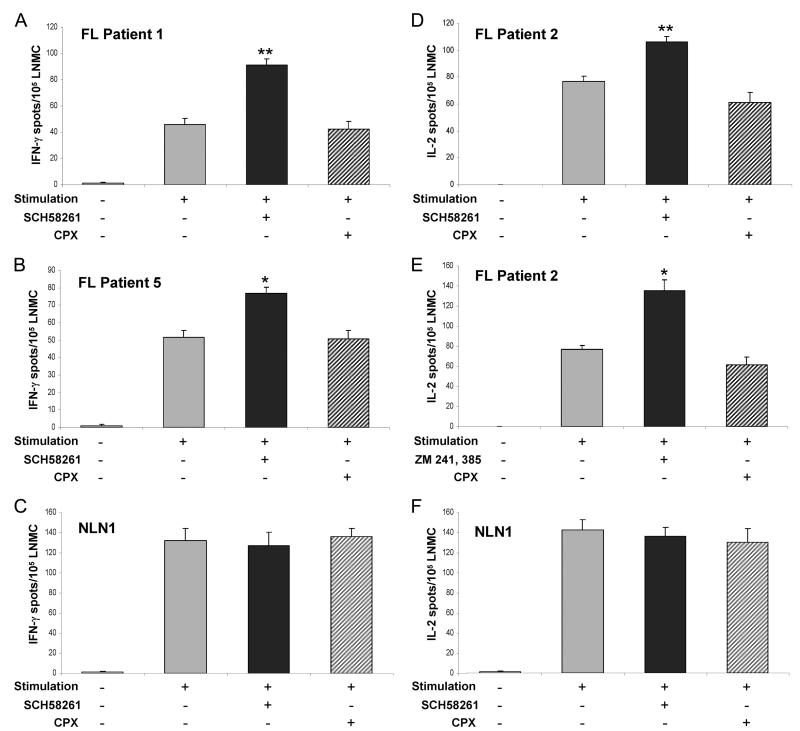
As described, adenosine-dependent suppression of T cell responses is mediated through its binding to the T cell A2A AR. In contrast, adenosine interaction with its A1 receptor has no effect on T cell proliferation or cytokine production. Therefore, if adenosine is in fact a mediator of T cell anergy in FL, we would anticipate that inhibition of adenosine binding to the A2A, but not the A1 receptor would enhance cytokine production from T cells after stimulation. To test this hypothesis, LNMC derived from patients with FL were stimulated with soluble anti-CD3 and anti-CD28 Abs after addition of exogenous ATP (see Materials and Methods) for 18 or 24 h (testing for IFN-γ or IL-2 secretion, respectively), in the presence or absence of the selective A2A antagonist SCH58261 or the selective A1 antagonist CPX. In three of the six patient samples examined, coincubation with SCH58261 resulted in a statistically significant increase in the number of IFN-γ-producing cells (patient 1 and patient 5, Fig. 1, A and B, respectively) or IL-2-producing cells (patient 2, Fig. 1D) compared with that seen in the absence of the inhibitor. In contrast, coincubation with CPX had no effect on the number of cytokine-producing cells after stimulation in each of these patient samples. To further confirm this observation, we repeated the experiment in patient 2 using a selective antagonist of both the A2A and the low affinity A2B receptors, ZM 241,385. Similar to that seen with SCH58261, coincubation with ZM 241,385 resulted in a significantly greater number of IL-2-producing cells after stimulation than the number seen in the absence of the inhibitor (Fig. 1E). Again, the selective A1 receptor antagonist CPX had no effect on cytokine secretion. Together, these results suggest that engagement of the A2A/A2B AR contributes to FL-associated T cell hyporesponsiveness in a subset of FL patient samples.
FIGURE 1.
Intratumoral FL T cell hyporesponsiveness can be partially abrogated by A2A but not A1 AR antagonists. LNMC from the indicated FL patient biopsies (A, B, D, and E) or normal LNMC (C and F) were assayed for IFN-γ (A, B, and C) or IL-2 (D, E, and F) production by ELISPOT upon stimulation with soluble anti-CD3 and anti-CD28 Abs (1 μg/ml each) in the presence or absence of the indicated AR antagonist (5 μM each). Data are presented as the average number of cytokine-producing cells (for each experimental condition performed in triplicate) per 105 LNMC ± SEM. For the normal LNMC (NLN), a representative experiment from one patient sample is shown (C), with similar results obtained using LNMC from four additional patients (n = 5). F, A representative experiment from one patient sample with similar results being obtained using LNMC from two additional patients (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Cytokine production was similarly determined from anti-CD3/anti-CD28-stimulated NLN-derived mononuclear cells in the presence or absence of the A2A receptor antagonist SCH58261. In conrast to what was seen with the stimulated FL-derived mononuclear cells, there was no increase in either IFN-γ production (5 of 5 nodes examined, Fig. 1C) or IL-2 production (3 of 3 nodes examined, Fig. 1F) in the presence of SCH58261; however, the differences between the FL and normal nodal T cells did not reach statistical significance.
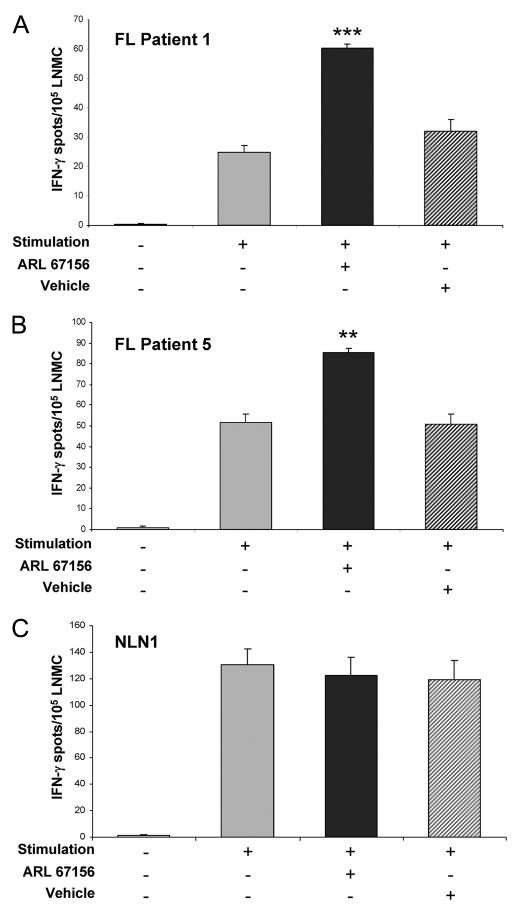
Inhibition of ATP hydrolysis abrogates T cell hyporesponsiveness within FL
If adenosine, interacting through the A2A, A2B, or both receptors, is in fact mediating FL-associated T cell hyporesponsiveness, it would be anticipated that preventing the hydrolysis of ATP by the ecto-ATPase CD39, the rate limiting step of adenosine generation from extracellular ATP, would enhance T cell cytokine production after stimulation. Therefore, the effect of the CD39 antagonist ARL 67156 on the cytokine secretion of FL LNMC stimulated for 18 h by soluble anti-CD3 and anti-CD28 Abs in the presence of exogenous ATP was next evaluated. A statistically significant increase in IFN-γ spot numbers was seen after anti-CD3 and anti-CD28 Ab stimulation of ARL 67156 treated cells relative to that seen with vehicle treated cells in two of five FL patient samples tested (Fig. 2, A and B). As we observed in the earlier experiment using the AR antagonist SCH58261, stimulation of NLN-derived mononuclear cells in the presence or absence of ARL 67156 also had no effect on the numbers of IFN-γ-producing cells (3 of 3 nodes examined, Fig. 2C). Taken together, these results further support the hypothesis that the ATP-ectonucleotidase-adenosine system is an integral part of the mechanism of tumor-associated immunosuppression in a subset of patients with FL.
FIGURE 2.
Intratumoral FL T cell hyporesponsiveness can be partially abrogated by the CD39 (ecto-ATPase) antagonist ARL 67156. FL patient 1 (A), FL patient 5 (B), or a normal LNMC (NLN) sample (C) were assayed for IFN-γ production by ELISPOT upon stimulation with soluble anti-CD3 and anti-CD28 Abs (1 μg/ml each) in the presence or absence of 125 μM ARL 67156 or an equivalent amount of DMSO (vehicle). The data are presented as the average number of cytokine-producing cells (for each experimental condition performed in triplicate) per 105 LNMC ± SEM. For the normal LNMC, a representative experiment from one patient sample is shown, with similar results obtained using LNMC from two additional patients (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
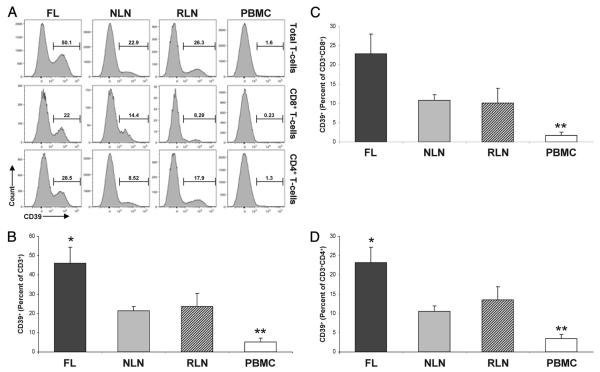
Increased proportions of CD39+ T cells infiltrate FL
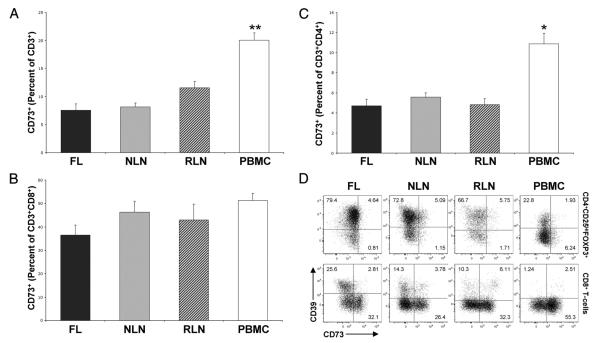
Given our findings that CD39 contributes to the functional hyporesponsiveness of T cells infiltrating FL, we next examined CD39 expression on T cell populations within nodal tissue and PBMC. As shown in Fig. 3, A and B, a significantly greater proportion of FL CD3+ T cells expressed CD39 (46.05 ± 8.38%) as compared with that seen on NLN and RLN (21.41 ± 2.17% and 23.56 ± 6.98%, respectively; p < 0.05). In addition, the proportion of nodal CD3+ T cells (FL, NLN, and RLN) expressing CD39 was significantly greater than the proportion seen of normal PBMC CD3+ T cells (5.16 ± 1.89%, p < 0.01).
FIGURE 3.
The proportion of T cells expressing CD39 is increased in FL. Single cell suspensions from FL (n = 16), NLN (n = 10), or RLN (n = 8) biopsy samples or normal donor PBMC (n = 6) were subjected to flow cytometric analysis (see Materials and Methods). A, Representative histograms highlighting CD39 expression on gated total CD3+ T cells or the CD3+CD8+ and CD3+CD4+ T cell subsets (top to bottom, respectively) within the indicated tissues. B, Frequency of CD3+ T cells expressing CD39, displayed as a percentage of the total viable T cells. Frequency of CD8+ (C) or CD4+ (D) T cells expressing CD39, displayed as a percentage of the total viable T cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
When all CD8+ T cells were evaluated (Fig. 3, A and C), a greater proportion of FL CD8+ T cells expressed CD39 (22.88 ± 5.09%) as compared with that seen on NLN or RLN (10.55 ± 1.35% and 10.07 ± 3.88%, respectively). However, this difference was not statistically significant (p = 0.09). Nevertheless, there was a greater proportion of nodal CD8+ T cells (FL, NLN, and RLN) expressing CD39 as compared with that seen on normal PBMC CD8+ T cells (1.76 ± 0.8%, p < 0.01).
A similar examination of CD39 expression on CD4+ T cells (Fig. 3, A and D) demonstrates that a significantly greater proportion of FL CD4+ T cells expressed CD39 (23.17 ± 3.97%) as compared with that seen on NLN and RLN (10.58 ± 1.38% and 13.49 ± 3.39%, respectively; p < 0.05). In addition, the proportion of nodal CD4+ T cells (FL, NLN, and RLN) that expressed CD39 was significantly greater than that seen on normal PBMC CD4+ T cells (3.40 ± 1.12%, p < 0.01). As such, the proportion of CD3+ T cells expressing CD39 is higher in nodal tissue than normal peripheral blood, and is higher in FL tissue as compared with that seen on NLN or RLN.
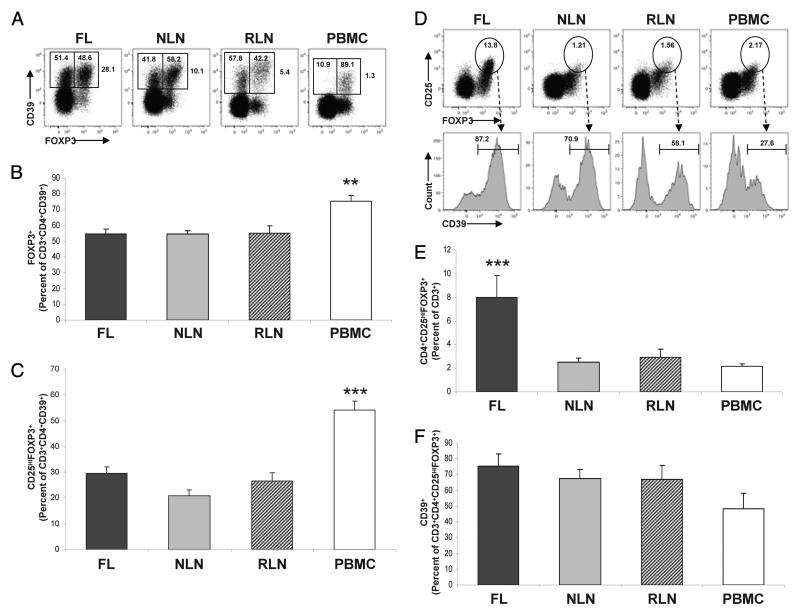
Percentage of CD3+CD4+CD39+ T cells expressing FOXP3
It has previously been shown that CD39 is expressed on both murine and human Tregs. Indeed, CD39 expression has been strongly associated with FOXP3 expression on human PBMC; however, to our knowledge the expression of CD39 on T cell populations from human secondary lymphoid tissue has not been characterized. In peripheral blood, we similarly found that CD39 expression is associated with FOXP3 expression as 75.20 ± 4.03% of the CD4+ CD39+ cells coexpressed FOXP3 (Fig. 4, A and B). In contrast, when nodal tissue was evaluated, only 54% of the CD4+CD39+ cells expressed FOXP3 (Fig. 4, A and B).
FIGURE 4.
A greater proportion of CD3+ T cells are CD4+CD25highFOXP3+ Tregs in FL and such cells express CD39. Single cell suspensions from FL (n = 16), NLN (n = 10), or RLN (n = 8) biopsy samples or normal donor PBMC (n = 6) were subjected to flow cytometric analysis (see Materials and Methods). A, Representative dot plots illustrating the frequency of CD4+CD39+ T cells expressing FOXP3. The percentage shown outside the markers represent CD4+ T cells expressing CD39 within each tissue sample. The percentage within the right and left markers represents CD4+CD39+ T cells that do or do not express FOXP3, respectively. B, The percentage of CD3+CD4+CD39+ T cells expressing FOXP3. C, The percentage of CD3+CD4+CD39+ T cells coexpressing CD25high and FOXP3. D, Representative dot plots illustrating the frequency of Tregs within each tissue type with the highlighted markers indicating the percentage of CD3+CD4+CD25highFOXP3+ T cells within the total T cell population. The histograms display CD39 expression within the gated Treg populations from the dot plots described. The percentage over the markers represent the gated CD3+CD4+CD25highFOXP3+ T cells expressing CD39. E, The percentage of CD3+ T cells coexpressing CD4, CD25high, and FOXP3. F, The percentage of CD3+CD4+CD25highFOXP3+ T cells expressing CD39. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Percentage of CD3+CD4+CD39+ T cells expressing CD25high and FOXP3
As it has been shown that FOXP3 expression alone is not a truly specific marker of human Tregs (25), we examined dual expression of CD25high and FOXP3, which more accurately identifies the Treg phenotype. Evaluation of the CD25highFOXP3+ population (Fig. 4C) also demonstrates that CD39 is more strongly associated with this Treg phenotype in peripheral blood as opposed to lymphoid tissue. Specifically, the percentage of CD4+CD39+ T cells that coexpress FOXP3 and CD25high in the FL, NLN, and RLN are 29.61 ± 2.48%, 20.92 ± 2.14%, and 26.68 ± 3.16%, respectively (p > 0.05). In contrast, the percentage of CD4+CD39+ T cells coexpressing FOXP3 and CD25high in peripheral blood is 54.15 ± 3.53%, which is significantly greater than the percentage observed within the lymphoid tissues (p < 0.01). As such, the proportion of CD4+CD39+ T cells expressing CD25 and FOXP3 is higher in the peripheral blood than in nodal tissue. However, there was no difference seen in the FL, NLN, and RLN samples.
Percentage of CD4+CD25highFOXP3+ T cells expressing CD39
We have previously shown that the percentage of CD3+ T cells that are Tregs is greater in FL as compared with that in NLN and RLN using CD25high and glucocorticoid-inducible TNF receptor to define Tregs (22). We now extend these findings using CD25high and FOXP3 to more accurately define the Tregs. As shown in Fig. 4, D and E, the proportion of CD3+ T cells that are CD4+CD25highFOXP3+ Tregs is indeed significantly greater in FL nodes as compared with that of NLN, RLN, as well as peripheral blood. Specifically, 7.96 ± 1.89% of the FL CD3+ T cells are CD4+CD25highFOXP3+ Tregs. In contrast, only 2.52 ± 0.33%, 2.92 ± 0.69% and 2.14 ± 0.22% of the NLN, RLN and PBMC CD3+ T cells, respectively, are CD4+CD25highFOXP3+ Tregs (p < 0.001). In addition, the proportion of CD4+CD25highFOXP3+ cells that expressed CD39 was similar in FL, NLN, and RLN, albeit greater than that seen on CD4+CD25highFOXP3+ cells from peripheral blood (Fig. 4F), although this difference was not statistically significant. As such, the total burden of regulatory CD4+CD25highFOXP3+ cells that express CD39 is greater in the FL microenvironment compared with that of NLN, RLN, or PBMC, partially accounting for the increased proportions of CD4+CD39+ T cells in FL as shown (Fig. 3D).
CD73 expression on T cells infiltrating nodal tissues
Although CD39 has been reported to be the rate limiting step in the extracellular nucleotide catabolic pathway, the 5′-ectonucleotidase CD73 is required for conversion of pericellular AMP (generated in part as a result of ATP hydrolysis by CD39) into adenosine (21). As such we examined CD73 expression on infiltrating T cells in nodal tissue and PBMC. As shown in Fig. 5A, there was no significant difference in the percentage of CD3+ T cells that express CD73+ in FL, NLN, or RLN. However, there is clearly a compartmental difference as there were a significantly greater proportion of CD3+ T cells expressing CD73 in the PBMC samples as compared with those within the nodal tissue. Specifically 20.03 ± 1.32% of the CD3+ T cells expressed CD73 in the PBMC, whereas only 7.55 ± 1.17%, 8.13 ± 0.70%, and 11.57 ± 1.11% of the CD3+ T cells expressed CD73 in FL, NLN, and RLN, respectively (Fig. 5A). In addition, there are nearly identical percentages of CD73-expressing CD8+ T cells (Fig. 5B) within the nodal tissues, whereas there are greater percentages, albeit nonstatistically significant, of these CD8+CD73+ T cells within PBMC (p > 0.05). There are also nearly identical percentages of CD73-expressing CD4+ T cells (Fig. 5C) within the nodal tissues, whereas there are significantly greater percentages of the CD4+CD73+ T cells within the PBMC (p < 0.05). Taken together, the increased proportions of both CD8+ and CD4+ T cells expressing CD73 within the PBMC as compared with the nodal tissues accounts for the overall higher percentage of CD73-expressing T cells observed within the PBMC.
FIGURE 5.
Proportion of CD73+ T cells within nodal tissue and PBMC. Single cell suspensions from FL (n = 16), NLN (n = 10), or RLN (n = 8) biopsy samples or normal donor PBMC (n = 6) were subjected to flow cytometric analysis (see Materials and Methods). Shown are the percentage of CD3+ T cells that express CD73 (A), percentage of CD3+CD8+ T cells that express CD73 (B), and the percentage of CD3+CD4+ T cells that express CD73 (C). D, Representative dot plots illustrating the frequency of CD3+CD4+CD25highFOXP3+ T cells (corresponding to putative Tregs, top row) or CD3+CD8+ T cells (bottom row) expressing CD39, CD73, or both. The percentage within each quadrant represents cells expressing CD39 alone (top left quadrant), CD73 alone (bottom right quadrant) or both CD39 and CD73 (upper right quadrant), within each tissue sample. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Lastly, although we show that CD73+ T cells infiltrate FL, less than 5% of human CD4+CD25highFOXP3+ Tregs (in either secondary lymphoid tissues or PBMC) coexpress CD73 and CD39 (Fig. 5D). This is in contrast to the reported findings that CD73 and CD39 are commonly coexpressed on murine Tregs (21). In addition, we also show that within the CD8+ T cell populations, coexpression of CD39 and CD73 accounts for less than 8% of the overall CD8+ T cells, and none of these CD8+ T cell populations (whether displaying single or dual expression of CD39, CD73, or both) express significant levels of FOXP3 (data not shown).
CD39+ T cells hydrolyze ATP
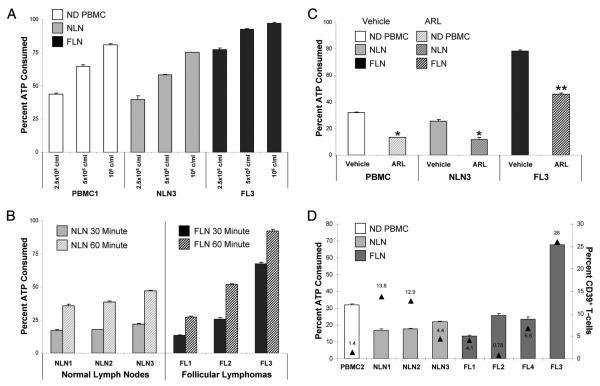
For the increased CD39 expression on FL CD3+ nodal T cells to be relevant to the FL-associated T cell hyporesponsiveness, it must be functional and hydrolyze extracellular ATP. As shown in Fig. 6, both NLN and FL LNMC, as well as normal PBMC actively consume ATP in both a cell number (Fig. 6A) and time-dependent manner (Fig. 6B). To confirm that the observed ATP consumption is in part mediated through CD39 ecto-ATPase activity, we determined whether ARL 67156, a selective ecto-ATPase antagonist, inhibited ATP consumption. As shown in Fig. 6C, ARL 67156 significantly inhibited ATP consumption by NLN and FL LNMC as well as by PBMC (p < 0.05).
FIGURE 6.
ATP consumption by LNMC and PBMC. Single cell suspensions from FL, NLN, or PBMC were assayed for ATP consumption as described in Materials and Methods. A, The indicated concentrations of LMNC or PBMC were assayed for ATP consumption for 60 min. B, The indicated LNMC (at 2.5 × 105 cells/ml) were assayed for the amount of ATP consumed at 30 or 60 min. C, The indicated LNMC or PBMC were assayed for ATP consumption for 60 min in the presence or absence of 125 μM ARL 67156 or an equivalent amount of DMSO vehicle. D, The indicated LNMC or PBMC (at 2.5 × 105 cells/ml) were assayed for ATP consumption for 30 min. The left y-axis, corresponding to data indicating the percentage of ATP consumed; the right y-axis (▲) indicates the percentage of CD39+ T cells present within each sample (as assayed previously by flow cytometry and expressed as a percentage of the total viable lymphocyte population). *, p < 0.05 and **, p < 0.01.
As is evident from Fig. 6B, an equal number of LNMC from different FL patient samples differed in the degree of ATP consumption. Therefore, we determined whether the degree of ATP consumption is associated with the percentage of the LNMC T cells expressing CD39. As shown in Fig. 6D, LNMC from FL samples exhibiting higher proportions of CD39+ T cells (measured as a percentage of total lymphocytes) consumed more ATP per LNMC, as compared with consumption seen with NLN, PBMC, or even other FL having a significantly lower proportion of LNMC T cells expressing CD39.
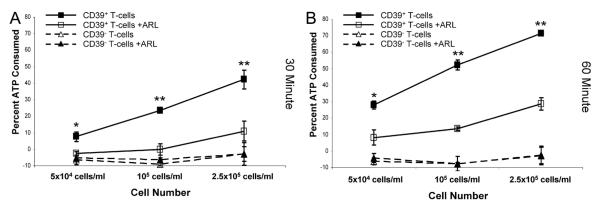
To further confirm that ATP consumption is directly associated with the proportion of T cells expressing CD39, CD39+, and CD39−, T cells were sorted from normal donor PBMC and the degree of ATP consumption was determined on a one to one cell basis. Normal donor PBMC, rather than FL LNMC, were used for these studies, due to the difficulty in obtaining a sufficient number of sorted LNMC from FL biopsy specimens. As shown in Fig. 7, CD39+ T cells consumed ATP in a time- and cell number-dependent fashion. In addition, ATP consumption by the CD39+ T cells was partially inhibited by ARL 67156 (p < 0.05), supporting the role of CD39 in the hydrolysis of ATP in this assay. In contrast, CD39− T cells demonstrated no detectable ATP consumption, regardless of cell number, at either 30 min or 1 h. Taken together these data suggest that the increased proportion of CD39-bearing T cells in FL is associated with greater ATP consumption.
FIGURE 7.
CD39+ but not CD39− T cells consume ATP. Flow cytometry sorted CD39+ or CD39− T cells from normal donor PBMC were assayed for ATP consumption using the indicated number of cells for 30 (A) or 60 (B) min. The indicated samples were also treated for the entire 30 or 60 min with 125 μM ARL 67156. Similar results were obtained using PBMC from one additional normal donor (data not shown). *, p < 0.05 and **, p < 0.01, comparing presence or absence of ARL 67156 at each cell concentration.
Discussion
Accumulating evidence over the past several years directly lends support to the concept of tumor immune surveillance (26-28). Despite this concept, antitumor effector cells coexist with the tumor within its microenvironment in such a fashion that spontaneous tumor regression is rare, a phenomenon known as the “Hellstrom Paradox” (29). The explanation for such a paradox is becoming clear as the multitude of mechanisms that tumors used to evade immune surveillance is being defined (1, 30-33).
To optimally understand such mechanisms in human malignancy, it is imperative, however, that phenotypic and functional studies be performed on cells obtained directly from the tumor microenvironment: for FL, that environment being the malignant lymph node. As many lymph node biopsies today are being performed using core biopsy needles, rather than by incisional or excisional biopsy, the amount of tumor obtained is minimal. Indeed, after the pathologist takes from this sample what is necessary to make a definitive diagnosis, the number of remaining cells available for our studies becomes significantly less. Therefore, we cannot perform all of the studies that would be of interest, or larger studies on a greater number of patient samples due to sample availability. Indeed, the importance of doing human studies to elucidate immune responses in tumor microenvironments, and the intrinsic and extrinsic limitations imposed on such studies, are eloquently discussed in Steinman and Mellman (34). Despite these limitations, however, the combination of both functional and phenotypic data obtained from the FL samples, especially when compared with that of parallel studies on reactive and normal human lymph nodes, provides important insights into the mechanisms of immune suppression within the lymphoma microenvironment.
T cells express the G protein-coupled high affinity A2A or the low affinity A2B AR (35). Engagement of the A2A receptor by adenosine results in an increase in intracellular cAMP, which antagonizes TCR signaling thereby inhibiting T cell proliferation and cytokine release (36, 37). Indeed, that TH1, TH2, and effector CD8+ T cells have a higher density of A2A receptors than naive or memory T cells, suggests that the T cell subsets directly involved in an active ongoing immune response are the most susceptible to adenosine-mediated suppression (6, 37).
We show in 3 of 6 FL samples studied, that activation of the tumor-infiltrating T cells in the presence of an A2 receptor antagonist results in a greater number of cytokine-secreting T cells than that seen in the absence of the antagonist. Given the multitude of immune suppression mechanisms elaborated within the FL microenvironment, it was surprising that blocking a single pathway, (i.e., adenosine binding to its receptor), had such a profound effect in three patient samples, suggesting that adenosine-mediated suppression is a major contributor to T cell hyporesponsiveness in these patients. In contrast, the fact that no significant effect was seen in the other three patient samples studied does not necessarily mean that adenosine played no role in immune suppression, but rather, it might not be playing a dominant role. It is of interest that the three “responding” patients (patient nos. 1, 2, and 5) had no prior treatment for their FL and the three “nonresponding” patients (patient nos. 3, 4, and 6) had prior treatment for their lymphoma (i.e., the biopsy was done at relapse or progression). Whether treatment changes the tumor microenvironment in such a fashion so to alter the immunosuppressive networks will require study of a larger number of both previously untreated and treated patient biopsy specimens.
One mechanism responsible for pericellular adenosine accumulation is by the diffusion, or transport, of intracellular adenosine to the extracellular space through as yet undefined mechanisms (15). Tumor associated hypoxia may play a role in this, as the hypoxia inducible HIF-1α inhibits adenosine kinase, resulting in an increase in intracellular adenosine and its subsequent transport to the extracellular space (38). Indeed, in murine tumor models, there exists an adenosine gradient, with the pericellular concentration of adenosine being greater in the center of the tumor than that seen in normal tissue (9, 39).
A second mechanism for pericellular adenosine generation is through the hydrolysis of extracellular ATP. Pericellular ATP is derived from cytoplasmic leakage of damaged or lysed cells, as well as upon T cell and monocyte activation in the tumor microenvironment (40, 41). The rate limiting step for adenosine generation from ATP is its hydrolysis to AMP by the ecto-ATPase activity of CD39 (21). AMP is then rapidly degraded to adenosine by CD73 (14). It is evident from our studies that ATP hydrolysis is a critical step in FL-associated immune suppression in some cases, as T cell hyporesponsiveness can be reversed in a subset of patients by inhibiting the enzymatic activity of CD39. Furthermore, our finding that the proportion of CD39-expressing cells in both CD4+ and CD8+ T cell populations is higher in FL nodes compared with that seen in other nodal tissue or peripheral blood provides further support for the relevance of adenosine as a mediator of immune suppression in FL. However, the mechanism through which CD39 is up-regulated on FL infiltrating T cells is yet to be defined. Indeed, we are further exploring whether hypoxia induces CD39 expression on T cells similar to what has been shown on endothelial cells (42). If so, perhaps tumor-related hypoxia plays a role in the modulation of CD39 expression on T cells.
To complete the enzymatic process, AMP generated by CD39 is further hydrolyzed to adenosine by the 5′-ectonucleotidase CD73. In contrast to previously reported findings that murine Tregs express both CD39 and CD73, we only find a subset of CD3+CD4+CD25highFOXP3+ Tregs that coexpress CD73 and CD39 (less than 5% on average) in human nodal tissue or peripheral blood. However, similar to what has been previously described we do show that a subset of both CD8+ and CD4+ FL-infiltrating T cells expressed CD73. We anticipate that these CD73-bearing T cells, as well as the CD73 expressed on endothelial cells, follicular dendritic cells, and several subsets of B cells, would hydrolyze AMP to adenosine. In contrast to what was found with CD39 expression, there was no significant difference in the proportion of FL T cells expressing CD73 compared with that seen in NLN or RLN. In addition, the proportion of CD3 T cells expressing CD73 was higher in normal donor peripheral blood suggesting a divergent pathway of regulation for CD39 and CD73 expression.
In addition to showing that the proportion of T cells bearing CD39 is highest in FL nodal tissue, we show that the proportion of CD39-bearing T cells is directly related to the degree of ATP hydrolysis. It is anticipated that the increased ATP hydrolysis seen in FL nodes would lead to an increase in pericellular adenosine, which we hypothesize to contribute to T cell hyporesponsiveness in a subset of FL patient samples. However, ATP is also a proinflammatory mediator that would alter the tumor microenvironment in such a way as to augment an antitumor immune response. For example, ATP induces DC chemotaxis and maturation, and induces IL-1 expression from monocytes (16-18, 40). In addition, Tregs have been shown to be more sensitive to ATP-induced cell death than any other T cell subset (19). Taken together, ATP hydrolysis by CD39 would be expected to subvert an antitumor immune response independent of its adenosine mediated immune suppression. Finally, that ATP may modulate the immune response is further supported by the recent findings that ATP may induce the differentiation of proinflammatory TH17 cells (43).
In summary, we show that inhibiting the A2A receptor, results in an increase in cytokine secretion from normally hyporesponsive FL T cells after stimulation in 50% of the patient samples studied. In contrast, we saw no evidence of augmented cytokine production from normal lymph node T cells upon stimulation. This supports the notion that adenosine is a mediator of immune suppression in FL. In further support of this notion, we show that the proportion of T cells (CD4+FOXP3−, CD8+, and CD4+FOXP3+ Tregs) bearing CD39 (which catalyzes the rate limiting step in adenosine generation) is higher in FL compared with that seen in normal lymph nodes. We further show that CD39 on FL T cells is functionally active and hydrolyzes ATP, generating AMP, which is then available for conversion to adenosine by CD73. Finally, we show that inhibition of CD39 with the CD39 antagonist ARL 67156 abrogates cytokine secretion after stimulation in 2 of 5 FL samples tested, providing further support for the functional importance of CD39 in immune suppression in a subset of patients with FL.
As we performed our studies using unfractionated cells, we cannot exclude the possibility that CD39 is present on the malignant B cells (as CD39 has been shown to be expressed on B cells (44)) and that this contributes to adenosine generation in our assay. However, in vivo it is likely that both CD39 and CD73 expressed on FL T cells, as well as on other cells in the tumor microenvironment, including the malignant B cells, act in “trans-mode” to generate adenosine from ATP/ADP.
Taken together, the data support the hypothesis that the ATP-CD39-adenosine-A2A receptor pathway is one mechanism for T cell hyporesponsiveness in FL. Indeed, to our knowledge, this is the first demonstration that this pathway is immunologically significant in any human tumor. The data also suggest that pharmacological inhibition of CD39 ecto-ATP-diphosphohydrolase activity or blockade of the adenosine-A2A receptor interaction may be rationale strategies to enhance the efficacy of immunotherapeutic treatment approaches for patients with FL.
Footnotes
This work was supported in part by Grant 5-29712 from a University of Rochester Human Immunology Core Pilot Project, by Grants R01-CA122645 and R21 CA129936 from the U.S. Public Health Service, and by Grant P50-CA130805 from a SPORE in Lymphoma.
Abbreviations used in this paper: Treg, regulatory T cell; FL, follicular lymphoma; NLN, normal lymph node; RLN, reactive lymph node; LNMC, lymph node mononuclear cell; AR, adenosine receptor; RLU, relative luminescent unit.
References
- 1.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat. Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J. Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 5.Sevigny CP, Li L, Awad AS, Huang L, McDuffie M, Linden J, Lobo PI, Okusa MD. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J. Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 6.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-γ production in murine CD4+ T cells. J. Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 7.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br. J. Pharmacol. 2008;153(Suppl. 1):S457–S464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoskin DW, Reynolds T, Blay J. Adenosine as a possible inhibitor of killer T-cell activation in the microenvironment of solid tumours. Int. J. Cancer. 1994;59:854–855. doi: 10.1002/ijc.2910590625. [DOI] [PubMed] [Google Scholar]
- 12.Merighi S, Mirandola P, Varani K, Gessi S, Leung E, Baraldi PG, Tabrizi MA, Borea PA. A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol. Ther. 2003;100:31–48. doi: 10.1016/s0163-7258(03)00084-6. [DOI] [PubMed] [Google Scholar]
- 13.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 14.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol. Rev. 1998;161:95–109. doi: 10.1111/j.1600-065x.1998.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 15.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin. Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 16.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol. Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 17.la Sala A, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Alerting and tuning the immune response by extracellular nucleotides. J. Leukocyte Biol. 2003;73:339–343. doi: 10.1189/jlb.0802418. [DOI] [PubMed] [Google Scholar]
- 18.Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, Beecroft T, Davis ID, Cebon J, Maraskovsky E. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102:613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 19.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J. Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 20.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′-adenosine monophosphate to adenosine. J. Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 21.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilchey SP, De A, Rimsza LM, Bankert RB, Bernstein SH. Follicular lymphoma intratumoral CD4+CD25+GITR+ regulatory T cells potently suppress CD3/CD28-costimulated autologous and allogeneic CD8+CD25− and CD4+CD25− T cells. J. Immunol. 2007;178:4051–4061. doi: 10.4049/jimmunol.178.7.4051. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Wu Y. K-Sample tests based on the likelihood ratio. Comput. Stat. 2007;51:4682–4691. [Google Scholar]
- 24.Hollander M, Wolfer DA. Nonparametric Statistical Methods. 2nd Ed. John Wiley & Sons; N.Y.: 1999. [Google Scholar]
- 25.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 26.Melief CJ, Toes RE, Medema JP, van der Burg SH, Ossendorp F, Offringa R. Strategies for immunotherapy of cancer. Adv. Immunol. 2000;75:235–282. doi: 10.1016/s0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- 27.Pardoll D. Does the immune system see tumors as foreign or self? Annu. Rev. Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 28.Finn OJ. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 29.Hellstrom I, Hellstrom KE, Pierce GE, Yang JP. Cellular and humoral immunity to different types of human neoplasms. Nature. 1968;220:1352–1354. doi: 10.1038/2201352a0. [DOI] [PubMed] [Google Scholar]
- 30.Hanson HL, Donermeyer DL, Ikeda H, White JM, Shankaran V, Old LJ, Shiku H, Schreiber RD, Allen PM. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 31.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 32.Rosenberg SA. Progress in the development of immunotherapy for the treatment of patients with cancer. J. Intern. Med. 2001;250:462–475. doi: 10.1046/j.1365-2796.2001.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu. Rev. Immunol. 2007;25:243–265. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 34.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 35.Koshiba M, Rosin DL, Hayashi N, Linden J, Sitkovsky MV. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells: flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol. Pharmacol. 1999;55:614–624. [PubMed] [Google Scholar]
- 36.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 37.Koshiba M, Kojima H, Huang S, Apasov S, Sitkovsky MV. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J. Biol. Chem. 1997;272:25881–25889. doi: 10.1074/jbc.272.41.25881. [DOI] [PubMed] [Google Scholar]
- 38.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 39.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 40.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1β and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc. Natl. Acad. Sci. USA. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria TH17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 44.Pulte ED, Broekman MJ, Olson KE, Drosopoulos JH, Kizer JR, Islam N, Marcus AJ. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb. Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]