Abstract
Pediatric low-grade gliomas encompass a heterogeneous set of tumors of different histologies. Cerebellar pilocytic astrocytomas occur most frequently followed by supratentorial diffuse fibrillary astrocytomas. Recent research has implicated activation of the RAS/RAF/MEK pathway in tumorigenesis of these tumors. Surgery is the mainstay of therapy. Overall survival rates for patients whose tumors are completely resected are 90% or greater, 10 years from diagnosis. Conversely, most optic pathway/hypothalamic, deep midline, and brain stem gliomas have minimal potential for resection; these tumors can be difficult to treat and deserve special attention. Combination chemotherapy is currently recommended as front-line adjuvant treatment for progressive or recurrent tumors. Second-line radiotherapy can also improve overall survival but is associated with more frequent and significant neurocognitive, endocrine, and other long-term toxicities.
Keywords: low-grade, glioma, astrocytoma, pilocytic, fibrillary
Pediatric low-grade gliomas are a heterogeneous set of tumors. They encompass tumors of astrocytic, oligodendroglial, and mixed glial-neuronal histology. Although their clinical behavior can vary, the majority of low-grade gliomas are indolent and do not undergo malignant transformation. Case reports have even described spontaneous regression of some tumors.1,2 This is in contrast to adult low-grade gliomas that have a more aggressive phenotype. One reason for the differences between the 2 populations may be the different frequencies of histological subtypes. Pilocytic astrocytomas infrequently occur in adults but are the leading histology in children. Conversely, diffuse gemistocytic astrocytomas, which have been associated with an increased tendency toward malignant progression, are rarely found in children.3 Low-grade gliomas are estimated to account for anywhere from 30% to 50% of central nervous system tumors in children.4-6
Discussion of gliomas can be confusing as it is a descriptive and not a pathological term. Tumors are classified according to the World Health Organization (WHO) criteria, most recently published in 2007, which describes their histological features and also provides a grading or “malignancy scale.”3 Low-grade gliomas encompass grade 1 and grade 2 tumors (Table 1).3 Grading is based on a number of factors including presence of necrosis, giant cells, mitosis, endothelial proliferation, hyperchromatic nuclei, and pleomorphic cells. These findings can be subjective, and retrospective studies have documented significant interpathologist disagreement on grading.7 Moreover, it can be difficult to distinguish a low-grade from a high-grade glioma.8
Table 1.
Key Histological Features of Pediatric Low-Grade Gliomasa
| Astrocytic tumors |
| Grade 1 |
| Pilocytic astrocytoma—biphasic pattern, rosenthal fibers, microcysts, eosinphilic granular bodies |
| Subependymal giant cell astrocytoma—large gangliod astrocytes |
| Grade 2 |
| Diffuse astrocytoma (fibrillary,b gemistocytic, or protoplasmic)—nuclear atypia with very rare or absent mitoses, microcysts containing mucinous fluid |
| Pilomyxoid astrocytoma—prominent mucoid matrix, angiocentric arrangement of monomorphous, bipolar tumor cells, rosenthal fibers, eosinophilic granular bodies |
| Pleomorphic xanthoastrocytoma—pleomorphic and lipidized cells, surrounding reticulin network, eosinophilic granular bodies |
| Oligodendroglial tumors |
| Grade 2 |
| Oligodendroglial—monomorphic cells, uniform round nuclei, perinuclear halos, microcalcifications, mucoid/cystic degeneration, dense network of branching capillaries |
| Neuronal and mixed neuronal-glial tumors |
| Grade 1 |
| Ganglioglioma—combination of neoplastic, mature ganglion cells, and neoplastic glial cells |
| Gangliocytoma—irregular groups of large, multipolar neurons with dysplastic features |
| Desmoplastic infantile ganglioglioma—prominent desmoplastic stroma, poorly differentiated neuroepithelial cells, deeply basophilic nuclei |
| Dysembryoplastic neuroepithelial tumor—hallmark “specific glioneuronal element” |
WHO Classification of Tumors of the CNS. 3rd ed.3
Most diffuse astrocytomas in children are fibrillary.
Pediatric low-grade gliomas can be difficult to categorize as they can occur anywhere in the central nervous system and comprise multiple different tumor histologies. Historically, the cerebellum is the most prevalent location, and cerebellar low-grade gliomas account for 15% to 25% of all pediatric central nervous system tumors. They are followed by hemispheric (cerebral) gliomas (10%-15%), gliomas of the deep midline structures (10%-15%), optic pathway gliomas (5%), and brain stem gliomas (2%-4%; Figure 1).4 Children with neurofibromatosis type 1 account for the majority (over 70%) of the optic pathway/hypothalamic gliomas.9 In fact, 15% to 20% of children with neurofibromatosis type 1 will develop an optic pathway/hypothalamic glioma. Fortunately, only about half of them will become symptomatic and require treatment, usually before the age of 5.10-13 Low-grade brain stem gliomas include the dorsally exophytic, cervicomedullary, and focal brain stem gliomas and are to be distinguished from the more aggressive diffuse intrinsic brain stem gliomas.
Figure 1.
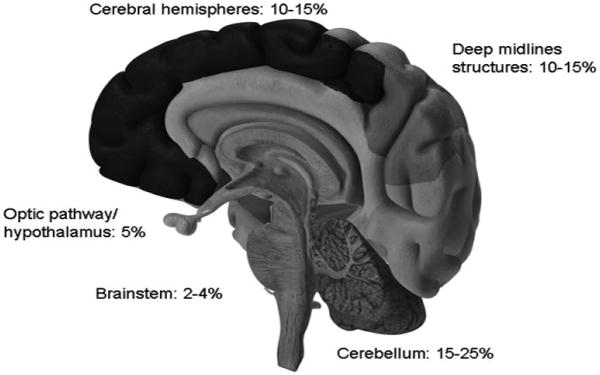
Distribution by location of pediatric low-grade gliomas. Shown are the approximate frequencies of low-grade gliomas compared with all pediatric central nervous system tumors.
The 2 most common low-grade glioma histologies in children are the pilocytic (WHO grade 1) and diffuse fibrillary astrocytoma (WHO grade 2). The former occurs mainly in children aged 5 to 19 years with a peak incidence in the 5- to 9-year-old age range.14 The age distribution for diffuse fibrillary astrocytomas is older, with only 10% occurring below the age of 20 years.15 Pilocytic astrocytomas can arise anywhere in the central nervous system; however, they predominate in the cerebellum,16-18 optic pathway,19,20 and dorsally exophytic brain stem.21 Conversely, diffuse fibrillary astrocytomas are more frequent in the supratentorial region, deep midline structures, and the cervicomedullary region.3,22
Other, less common, low-grade glioma histologies in children include pilomyxoid astrocytoma, pleomorphic xanthoastrocytoma, ganglioglioma, subependymal giant cell astrocytoma, and oligodendroglioma. The term “pilomyxoid” astrocytoma was first introduced in 1999 to describe a subset of pilocytic tumors with more aggressive clinical behavior and has since been included in the most recent World Health Organization classification of central nervous system tumors.23 Pilomyxoid astrocytomas (WHO grade 2) tend to occur in very young children (mean age 18 months) and localize in the hypothalamic region.24 Pleomorphic xanthoastrocytomas (WHO grade 2) have a propensity for the supratentorial region, most often the temporal lobe, but their age distribution and clinical phenotype are more similar to pilocytic astrocytoma. Gangliogliomas (WHO grade 2) localize in the temporal lobes and have a mean age at diagnosis of 9.5 years.25 Intraventricular subependymal giant cell astrocytomas (WHO grade 1) occur almost exclusively in children with tuberous sclerosis syndrome. Oligodendrogliomas (WHO grade 2) account for only 2% of brain tumors in children <14 years of age; the most common site of occurrence is the frontal lobe. This review will focus on pilocytic astrocytoma and diffuse fibrillary astrocytoma, as they account for the majority of pediatric low-grade gliomas and approximately one quarter of all primary central nervous system tumors in children.15
Clinical Presentation
The clinical presentation for children with low-grade gliomas, regardless of histology, can be grouped into generalizing and localizing symptoms. Almost 50% of children will have had 6 months or longer symptom duration prior to the eventual diagnosis.26 Generalizing symptoms are due to increased intracranial pressure from obstruction of the ventricles and include headaches (particularly in the morning), nausea, vomiting, and lethargy. Physical examination findings include decreased upward gaze, sixth cranial nerve palsies, and papilledema. These are most often caused by tumors located in the cerebellum, optic chiasm/hypothalamus, dorsally exophytic brain stem (which arise from the floor of the fourth ventricle and grow into the ventricle), and tectum (which obstruct the cerebral aqueduct).
Localizing symptoms are dictated by tumor location and include focal neurological findings, seizures and endocrinopathies. In particular, cerebellar tumors are associated with ataxia and dysmetria. The presentation of tumors of the cerebral hemisphere depends upon which lobe is involved and includes seizures, hemiparesis, and behavioral changes. Children with tumors of the hypothalamus and/or pituitary gland can suffer from obesity, failure to thrive, diabetes insipidus, other endocrine dysfunction, and visual field deficits from compression of the optic chiasm. Optic pathway gliomas may arise anywhere along the visual pathway (Figure 2). Children with sporadic optic pathway gliomas are more likely to present with chiasmal and postchiasmal involvement, whereas multifocal and bilateral optic nerve involvement is seen almost exclusively in children with neurofibromatosis type 1.10,27 Children can present with decreased visual acuity, optic nerve atrophy, proptosis, or strabismus. Low-grade gliomas of the brain stem are indolent by nature and often have a long clinical course (months to years) before diagnosis. Although they do not significantly infiltrate the brain stem, both the dorsally exophytic and cervicomedullary tumors can cause lower cranial nerve deficits (dysphagia, dysarthria, abnormal breathing) and long tract signs (hemiparesis, spasticity, hyperreflexia, Babinski's sign). The cervicomedullary gliomas also present with torticollis, long tract signs, and sensory loss from involvement of the upper cervical cord. Focal brain stem tumors are predominantly tectal in location and therefore present principally with hydrocephalus, although cranial nerve deficits, hemiparesis, and Parinaud syndrome are rarely seen.
Figure 2.
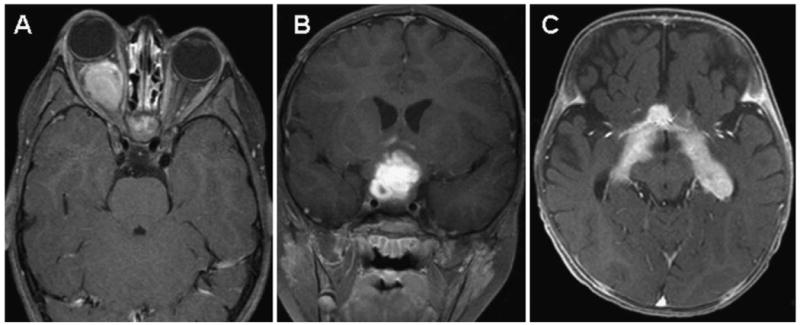
Postgadolinium magnetic resonance (MR) images of (A) optic nerve glioma (axial), (B) optic chiasm/hypothalamic glioma (coronal), and (C) optic tract/radiation tumor (axial).
Diagnosis
Pediatric low-grade gliomas share similar characteristics on neuroimaging. On magnetic resonance imaging (MRI), they tend to be hypointense on T1-weighted, hyperintense on T2-weighted, and have varying degrees of enhancement on postgadolinium sequences. Pilocytic astrocytomas usually appear as well-circumscribed tumors, often with a large cystic component and an enhancing mural nodule (Figure 3). Diffuse fibrillary astrocytomas are less circumscribed and typically do not enhance to a large extent (Figure 4).
Figure 3.
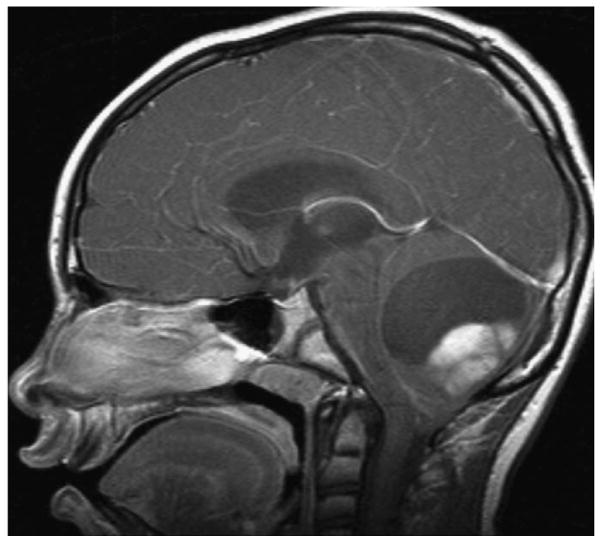
Sagittal postgadolinium magnetic resonance image of a pilocytic astrocytoma of the cerebellum.
Figure 4.
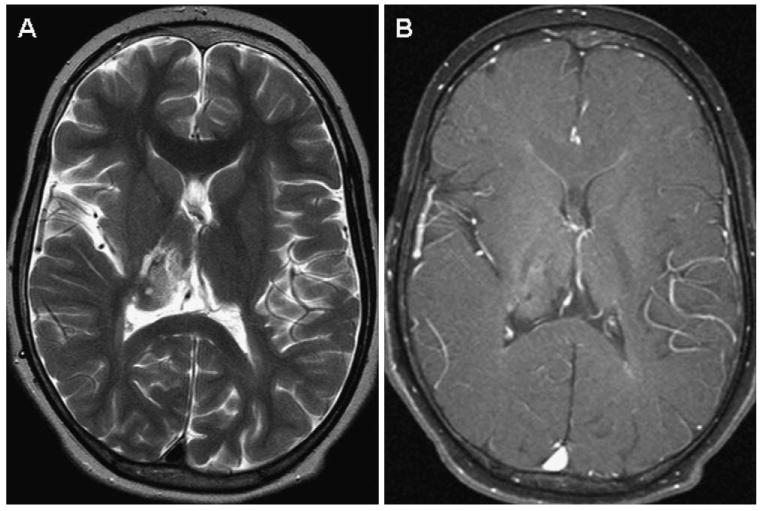
Axial magnetic resonance images of a right thalamic fibrillary astrocytoma status postbiopsy. A, Heterogeneous and hyperintense on T2-weighted images. B, Minimal enhancement on postgadolinium imaging. The patient had left hemiparesis. Because of its location, the tumor was treated with chemotherapy.
Surgical biopsy, and total tumor resection when feasible, is recommended to verify tumor histology. To preserve optic nerve function, many children with optic pathway/hypothalamic gliomas do not undergo diagnostic biopsy if the MRI characteristics are consistent with low-grade glioma, especially if there is a prior diagnosis of neurofibromatosis type 1. Biopsies of deep midline and brain stem tumors are also to be pursued cautiously, especially if they are asymptomatic and have not progressed on serial MRI evaluations.
Staging for the majority of tumors includes postoperative MRI of the tumor resection site to determine extent of resection. This should be performed within 24 to 48 hours after surgery to best distinguish residual tumor versus postoperative changes. Dissemination and leptomeningeal involvement are uncommon; however, if suspected, MRI of the entire spine and cerebrospinal fluid sampling for cytology should also be considered.18
Biology
The tumorigenesis of pediatric low-grade gliomas is not well understood. One of the limiting factors has been failure to identify common molecular abnormalities in tumor specimens. Traditional karyotype analysis has been unrevealing in multiple studies, with chromosome 7 gain the only consistent finding, but present in a minority of tumors.28-32 Conventional genomic hybridization and the first generation of single nucleotide polymorphism arrays also failed to identify consistent molecular abnormalities.33,34 More recently, multiple groups have reported a small nonrandom duplication in the 7q34 region in the majority of pilocytic astrocytomas, identified using high-resolution single nucleotide polymorphism arrays.35-39 The 7q34 duplication involves a known oncogene, BRAF, and appears to result in upregulation of the RAS/RAF/MEK pathway (Figure 5). Studies looking at adult tumors have also shown that the RAS/RAF/MEK pathway is activated in gliomas.40 Other biological mechanisms under investigation in low-grade gliomas include angiogenesis and the tumor microenvironment, telomere maintenance, and glioma-associated antigens.41-43
Figure 5.
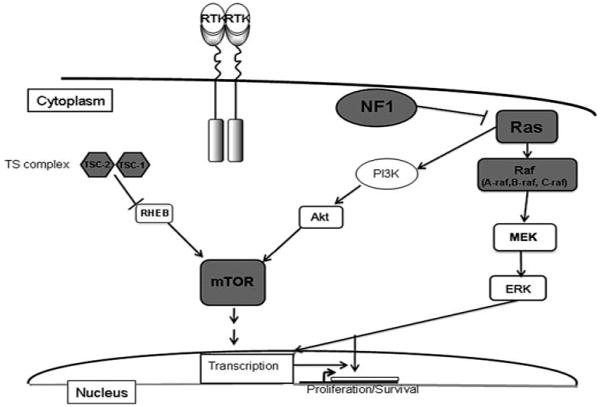
Pediatric low-grade glioma tumorigenesis. BRAF, an oncogene implicated in malignant melanoma and other carcinomas, may be involved in the tumorigenesis of sporadic low-grade gliomas. A BRAF fusion gene with increased kinase activity upregulates the downstream MEK/ERK pathway and results in increased transcriptional activity and cellular proliferation. Low-grade gliomas in children with susceptibility syndromes also reveal some of the pathways leading to tumorigenesis; inactivation of the tumor suppressor neurofibromin in neurofibromatosis type 1 results in RAS activation and upregulation of the RAS/RAF/MEK pathway; in tuberous sclerosis, mutation in 1 of 2 tumor suppressor genes, TSC1 or TSC2, can lead to direct activation of the mammalian target of rapamycin pathway. Neurofibromatosis type 1 tumors also reveal increased mammalian target of rapamycin expression, albeit by different mechanisms.
Pediatric low-grade gliomas do not harbor the same molecular abnormalities as their adult counterparts. For example, adult low-grade gliomas show frequent TP53 mutations, present in up to 88% of gemistocytic and 53% of diffuse fibrillary astrocytomas. TP53 mutations in children appear to be restricted to 5% to 10% of low-grade gliomas that undergo malignant transformation.44,45 Loss of heterozygosity of chromosomes 1p36 and 19q13, and in particular codeletion at both loci, is a favorable prognostic factor in adults with oligodendrogliomas (WHO grade 2) but has not been replicated in pediatric tumors.46
Tumors in children with genetic susceptibility syndromes such as neurofibromatosis type 1 and tuberous sclerosis syndrome provide some insight into the signal transduction pathways involved in development of low-grade gliomas. The neurofibromatosis type 1 model shows that inactivation of the tumor suppressor neurofibromin results in upregulation of the RAS family of proteins, involved in a number of oncogenic signal transduction pathways in astrocytes.47 Attempts to modulate this upregulation by the use of farnesyl transfererase inhibitors have been largely unsuccessful. Mutations in 1 of 2 tumor suppressor genes, TSC1 and TSC2, result in tuberous sclerosis syndrome. These 2 genes form an intracellular complex (called the tuberous sclerosis complex) that is also involved in the RAS pathway and contains a guanosine triphosphatase–activating property for a small G protein, Ras homologue enriched in brain. This homologue can directly activate mammalian target of rapamycin.48 Interestingly, neurofibromatosis type 1 tumors have also been shown to have increased mammalian target of rapamycin expression, albeit by a different mechanism (Figure 5).49 Inhibitors of mammalian target of rapamycin, such as rapamycin, are effective in causing regression of subependymal giant cell astrocytomas in patients with tuberous sclerosis syndrome50 and trials are currently being considered for refractory low-grade gliomas in patients with neurofibromatosis type 1.
Treatment and Outcomes
Surgery remains the mainstay of therapy for pediatric low-grade gliomas, and gross total resection is the most consistent prognostic factor for prolonged progression-free and overall survival.26,51-55 As such, treatment decisions for children with low-grade gliomas can be stratified into 3 main groups as follows: (1) children with tumors that have been fully resected, (2) children with tumors that have undergone subtotal resection, and (3) children with tumors that have undergone biopsy only or no surgical approach is feasible. The latter group deserves special attention and usually involves tumors in the deep midline supratentorial region, optic pathway/hypothalamus, and brain stem.
Low-grade gliomas in the cerebellum and superficial cerebrum are most amenable to resection. Children in whom a gross total resection has been achieved, confirmed by surgeon's report and/or postoperative brain MRI within 24 to 48 hours of surgery, often do not need any further therapy. In several series, complete resection was associated with 10-year overall survival rates of 90% or greater and rare tumor recurrences.18,26,51,53,56,57 Therefore, postoperative management is directed toward close clinical and radiographic follow-up, especially if the tumor histology has concerning features. Reports are conflicting because of the difficulty distinguishing the prognostic effect of histology from that of extent of resection, but tumor histology does appear to be an independent predictor of progression. Nonpilocytic, and specifically diffuse fibrillary, histology is more highly associated with progression, recurrence, and anaplastic transformation, although the latter is rare in children.8,26,51,54,55 Children have better survival than adults,58-61 but the role of age as a prognostic factor within children is unclear.18,55,62-64
The treatment decision for children with subtotal resection has been controversial. If the likelihood of functional impairment is minimal and the neurosurgeon thinks it is feasible, a repeat surgery can be attempted to remove the residual tumor.65,66 Others advocate a “wait and see approach” with follow-up brain MRI at 3- to 6-month intervals.67-69 Because the tumors tend to be indolent by nature, the decision for a repeat resection or adjuvant treatment can be postponed until there is either measurable progression by neuroimaging or clinical symptoms. This interval may last several years and some tumors may never progress. In 1 series looking at 128 children with subtotal resection of low-grade gliomas, 58% had no evidence of tumor progression 7 years from diagnosis.57 A prospective intergroup study between Children's Cancer Group and Pediatric Oncology Group (prior to the creation of the Children's Oncology Group) of 660 children reported a 5-year progression-free survival of 45% to 65% for residual tumor of any size.68 In addition, the value of postoperative adjuvant radiotherapy for residual low-grade glioma is unclear. Although it may improve progression-free survival,51,52,70 studies suggest this does not necessarily translate into improved overall survival, especially in children.51,57,58 Other studies show no advantage in progression-free survival with immediate irradiation.26,57 Thus, adjuvant therapy should be reserved for when tumors progress and re-resection is not feasible. The role of radiotherapy and chemotherapy in this setting will be discussed below.
Low-grade gliomas in the supratentorial midline areas are often not amenable to initial resection (Figure 4). For this cohort of children, observation until radiographic progression has been an accepted approach if there are minimal clinical symptoms. When progression occurs, adjuvant therapy should be considered. Historically, focal radiotherapy was used in this setting with standard doses of 45 to 54 Gy.71-75 Although the data looking at the impact of radiotherapy on overall survival are conflicting, retrospective studies combining adults and children reveal improved survival associated with administration of conventional radiotherapy.59,76,77 Other studies focused on children reveal the value of radiotherapy, particularly for optic pathway/hypothalamic gliomas (10-year progression-free survival of 65%-90%).73,74,78,79 In addition, it appears that almost half of irradiated low-grade gliomas in children have at least a 25% reduction in tumor size.79,80 Despite these potential benefits, the risks of radiotherapy to the developing nervous system have been well documented.81-86 Radiotherapy of low-grade gliomas of the cerebral hemisphere is associated with both hormone deficits and cognitive impairment.51,87,88 Treatment of optic pathway/hypothalamic gliomas can result in endocrine dysfunction,73,74,89 cerebrovascular disease,90,91 secondary malignant neoplasms,92 and neurocognitive deficits,20,79,93 particularly in young children.
To defer radiotherapy and its adverse effects, especially in infants and young children, chemotherapy is now the front-line adjuvant therapy for children with progressive low-grade gliomas. The combination of carboplatin and vincristine has been shown to result in tumor reduction or stable disease and a 3-year PFS of 68%.94 Unfortunately, up to 40% of children experience hypersensitivity reactions with carboplatin, more common with increased number and frequency of doses.95,96 An alternative regimen of 6-thioguanine, procarbazine, lomustine (CCNU), dibromodulcitol, and vincristine has also been shown to be effective, with a 3-year PFS of 45%.97 Because of side effects and drug availability, dibromodulcitol was not included in a randomized Children's Oncology Group protocol comparing thioguanine/procarbazine/CCNU/vincristine (TPCV), with the combination of carboplatin and vincristine. Preliminary results of this study show a trend for improved event-free survival for the TPCV regimen, but this is not significant.98
Initial management of optic pathway/hypothalamic gliomas is usually observation with serial MRI scans and ophthalmologic examinations.27,99 Most tumors remain indolent, and when they progress, it is often slow in pace.100,101 A concurrent diagnosis of neurofibromatosis type 1 appears to be associated with a favorable progression-free survival and lower likelihood of visual morbidity.10,63,102-104 Location is also important; tumors in the anterior visual pathway (optic nerves and chiasm) have a better visual outcome than tumors involving the hypothalamus and optic tracts/radiations.105,106 Treatment is reserved for patients with a documented decline in visual acuity or significant tumor progression on MRI scan with associated symptoms and signs. Rarely, treatment is advocated at diagnosis for patients with severe visual impairment, extensive tumor, and/or involvement of the posterior visual pathway. Surgical resection is rarely pursued because of the risk of further visual decline as well as the endocrinologic and cerebrovascular risks. Exceptions include unilateral optic nerve tumors with absent or severe impairment of vision and painful or disfiguring proptosis. Tumor debulking is sometimes undertaken for large chiasmal/hypothalamic tumors that cause hydrocephalus via obstruction of the third ventricle or exert mass effect on surrounding structures. Although radiotherapy for optic pathway/hypothalamic glioma is associated with excellent, long-term, progression-free survival, its use is rarely advocated because of the profound risk of unacceptable morbidity (as described above), particularly in patients with neurofibromatosis type 1. Therefore, chemotherapy, specifically carboplatin with vincristine, has become the mainstay of initial therapy for optic pathway/hypothalamic gliomas.27 To date, this regimen is associated with the best progression-free survival, especially in patients with neurofibromatosis type 1, and allows radiotherapy to be deferred until patients are older.94,107 Other regimens, such as TPCV, are avoided in patients with neurofibromatosis type 1 because of their underlying leukemia predisposition108-111 and the risk of secondary leukemia associated with lomustine and procarbazine.112-114
Brain stem gliomas also constitute a unique category of low-grade gliomas, and specific location provides guidance for treatment. The primary treatment for dorsally exophytic brain stem gliomas is surgical (Figure 6A). However, aggressive surgery to achieve a gross total resection is not advocated, due to the risks associated with this approach and because the majority of patients remain progression free after near total resection.21,115 Tumors that recur can be controlled with repeat resection and/or radiotherapy. Chemotherapy is primarily used to delay radiotherapy in young patients. Cervicomedullary gliomas tend to grow slowly and are often managed conservatively with close observation for an extended period of time (Figure 6B). Evidence of radiographic or clinical progression should prompt referral to neurosurgery. Gross total resections are possible, but more often the tumor cannot be removed completely without unacceptable risk. For tumors that progress following surgery, adjuvant therapy is recommended. Five-year progression-free survival after initial surgical management exceeds 60%.22,116 Tectal gliomas also tend to be indolent and rarely cause functional impairment (Figure 6C). Progression occurs in 15% to 25% of tumors.117 Therefore, management is directed toward resolution of the hydrocephalus via shunting or endoscopic third ventriculostomy. Patients are then observed and biopsy and adjuvant therapy reserved for patients with radiographic and clinical progression.117-120
Figure 6.
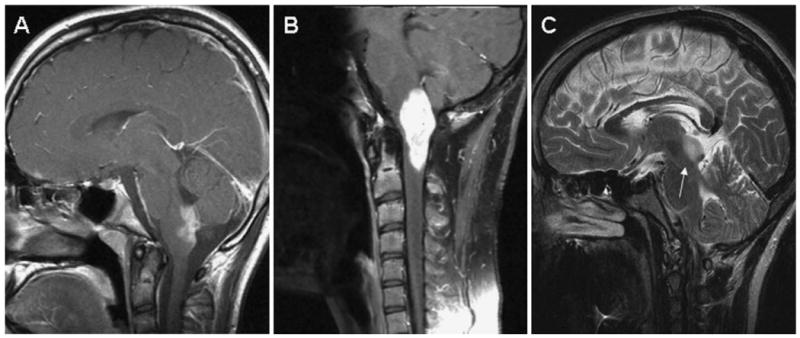
Magnetic resonance (MR) images of low-grade gliomas of the brain stem. A, Postgadolinium sagittal image of a dorsally exophytic glioma. B, Postgadolinium sagittal image of a cervicomedullary glioma. C, T2-weighted sagittal image of a tectal glioma.
Future Directions
Despite the excellent overall survival for these patients, the management of pediatric low-grade gliomas remains a challenge. Because a number of these tumors will be indolent, the treatment must not be worse than the disease. The focus of therapy is to provide long-term survival with as few potential long-term sequelae as possible. Fortunately, complete resection is able to provide cure for many children. When this is not possible, front-line chemotherapy regimens, such as the combinations of carboplatin and vincristine, or TPCV can result in stable disease or even tumor regression for an extended period of time. Unfortunately, there are few, proven, effective, second-line chemotherapy regimens for refractory or recurrent low-grade gliomas. Cisplatin plus etoposide has been evaluated in a small group of children with unresectable low-grade gliomas with a reported 3-year progression-free survival of 78%;121 however, this approach should be taken cautiously given the risk of moderate-to-severe hearing loss and secondary leukemia with these agents.122-124 Weekly vinblastine is promising with initial studies indicating stable disease in the majority of children who were switched to it because of carboplatin sensitivity125 or recurrent/refractory disease.126 Temozolomide as a single agent has shown to be active in pediatric low-grade gliomas127-129 and is currently being explored in combination with other agents. More recently, the combination of bevacuzimab and irinotecan, a regimen used to treat high-grade gliomas, has been shown to result in objective durable responses.130 Additionally, as we learn more about the signal transduction pathways that lead to pediatric low-grade glioma tumorigenesis, molecularly targeted drugs may also be developed as therapeutic options.
Radiotherapy should also be considered for refractory or recurrent tumors, especially in older children. In considering the use of this modality, careful consideration of the long-term morbidity of radiotherapy, particularly the potential neurocognitive effects, must be weighed. Multiple studies confirm the adverse impact of whole brain irradiation on intelligence quotient (IQ) in survivors of pediatric brain tumors.86,131,132 Focal radiotherapy to tumors of the cerebral hemispheres and optic pathway can also affect IQ79,85,93 and is associated with the child's need for special education.20,51 Other neurocognitive effects of radiotherapy to the brain include memory impairment and attention problems, and these effects are not limited to young children.133-135
Newer approaches to minimize the long-term morbidity of radiotherapy are being evaluated. Conformal radiotherapy with a tighter margin around the tumor achieved a 2-year actuarial event-free survival of 88% in 38 children with low-grade astrocytoma.136 More sophisticated techniques, such as intensity-modulated, proton andstereotactic radiotherapy, have been developed that enable delivery of targeted therapy to the tumor while limiting exposure to surrounding normal brain tissue.137-140 One large study of 50 children with low-grade gliomas treated with stereotactic radiotherapy reported a 5-year progression-free survival of 82.5%.140 Although the short-term progression-free survival for this and other studies appear equivalent to conventional radiotherapy,137-140 the long-term toxicity, particularly neurocognitive, has yet to be carefully evaluated.
Despite these advances in therapy, it is becoming increasingly recognized that a number of pediatric long-term survivors have significant adverse outcomes that are not always associated with radiotherapy. Cerebral hemisphere location, younger age at diagnosis, and hydrocephalus requiring a shunt have all been found to be significant predictors of lower cognitive performance on IQ scales.135 Children with infratentorial tumors can have significant language, cognitive, behavioral, and social dysfunctions.141-144 A combined analysis of Children's Cancer Group (CCG-9891) and Pediatric Oncology Group (POG-9130) data of 103 children with low-grade cerebellar astrocytomas treated with surgery alone revealed an elevated risk of cognitive and adaptive-behavioral impairment that was not associated with complications of the tumor or surgery.145 Similar deficits were found in 93 children with extracerebellar tumors treated with surgery alone.146
Therefore, even in the absence of radiotherapy, parents of children with low-grade gliomas must be properly counseled not only on the risk of tumor progression or recurrence but also the risk of neurocognitive and behavioral impairments. Physicians caring for children with low-grade glioma should consider early referral to neuropsychologists for baseline evaluations that may identify potential deficits in learning and processing, so that appropriate interventions can be put in place. Outcomes research needs to focus not just on the effects of radiotherapy and IQ but more on specific cognitive domains associated with the regions of brain affected by the tumor and treatments. In addition, a better understanding of the adaptive, social, and behavioral outcomes of survivors of pediatric low-grade glioma is essential.
Acknowledgments
The authors would like to thank Jane E. Minturn, MD, PhD, for her help with figure design.
Footnotes
The authors have no conflicts of interest to disclose with regard to this article.
For reprints and permissions queries, please visit SAGE's Web site at http://www.sagepub.com/journalsPermissions.nav
References
- 1.Rozen WM, Joseph S, Lo PA. Spontaneous regression of low-grade gliomas in pediatric patients without neurofibromatosis. Pediatr Neurosurg. 2008;44(4):324–328. doi: 10.1159/000134925. [DOI] [PubMed] [Google Scholar]
- 2.Parsa CF, Hoyt CS, Lesser RL, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119(4):516–529. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- 3.Louis D. WHO Classification of Tumors of the CNS. 4th. World Health Organization; 2007. [Google Scholar]
- 4.Freeman CR, Farmer JP, Montes J. Low-grade astrocytomas in children: evolving management strategies. Int J Radiat Oncol Biol Phys. 1998;41(5):979–987. doi: 10.1016/s0360-3016(98)00163-1. [DOI] [PubMed] [Google Scholar]
- 5.Blaney SM, Kun LE, Hunter J, et al. Tumors of the Central Nervous System. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5th. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 786–864. [Google Scholar]
- 6.Pollack IF. The role of surgery in pediatric gliomas. J Neurooncol. 1999;42(3):271–288. doi: 10.1023/a:1006107227856. [DOI] [PubMed] [Google Scholar]
- 7.Gilles FH, Tavare CJ, Becker LE, et al. Pathologist interobserver variability of histologic features in childhood brain tumors: results from the CCG-945 study. Pediatr Dev Pathol. 2008;11(2):108–117. doi: 10.2350/07-06-0303.1. [DOI] [PubMed] [Google Scholar]
- 8.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children's Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98(6):1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 9.Listernick R, Charrow J, Tomita T, Goldman S. Carboplatin therapy for optic pathway tumors in children with neurofibromatosis type-1. J Neurooncol. 1999;45(2):185–190. doi: 10.1023/a:1006338322266. [DOI] [PubMed] [Google Scholar]
- 10.Czyzyk E, Jozwiak S, Roszkowski M, Schwartz RA. Optic pathway gliomas in children with and without neurofibromatosis 1. J Child Neurol. 2003;18(7):471–478. doi: 10.1177/08830738030180070401. [DOI] [PubMed] [Google Scholar]
- 11.Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63–66. doi: 10.1016/s0022-3476(94)70122-9. [DOI] [PubMed] [Google Scholar]
- 12.Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114(5):788–792. doi: 10.1016/s0022-3476(89)80137-4. [DOI] [PubMed] [Google Scholar]
- 13.Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type 1. Am J Med Genet A. 2004;127A(3):224–229. doi: 10.1002/ajmg.a.20650. [DOI] [PubMed] [Google Scholar]
- 14.Central Brain Tumor Registry of the United States. Table 8: Number of childhood cases by major histology groupings, histology and age at diagnosis, CBTRUS, 2000-2004, 16 states combined. CBTRUS 2005-2006 Statistical Report Tables. 2000-2004 (Years of Data Collected) [October 2008]; from http://www.cbtrus.org.
- 15.Central Brain Tumor Registry of the United States. CBTRUS 2007-2008 Primary brain tumors in the United States statistical report, 2000-2004 (years of data collected) [November 2008]; from http://www.cbtrus.org.
- 16.Hayostek CJ, Shaw EG, Scheithauer B, et al. Astrocytomas of the cerebellum. A comparative clinicopathologic study of pilocytic and diffuse astrocytomas. Cancer. 1993;72(3):856–869. doi: 10.1002/1097-0142(19930801)72:3<856::aid-cncr2820720335>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez C, Figarella-Branger D, Girard N, et al. Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery. 2003;53(3):544–553. doi: 10.1227/01.neu.0000079330.01541.6e. discussion 554-545. [DOI] [PubMed] [Google Scholar]
- 18.Gajjar A, Sanford RA, Heideman R, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15(8):2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman HJ, Humphreys RP, Drake JM, et al. Optic pathway/hypothalamic gliomas: a dilemma in management. Pediatr Neurosurg. 1993;19(4):186–195. doi: 10.1159/000120729. [DOI] [PubMed] [Google Scholar]
- 20.Sutton LN, Molloy PT, Sernyak H, et al. Long-term outcome of hypothalamic/chiasmatic astrocytomas in children treated with conservative surgery. J Neurosurg. 1995;83(4):583–589. doi: 10.3171/jns.1995.83.4.0583. [DOI] [PubMed] [Google Scholar]
- 21.Khatib ZA, Heideman RL, Kovnar EH, et al. Predominance of pilocytic histology in dorsally exophytic brain stem tumors. Pediatr Neurosurg. 1994;20(1):2–10. doi: 10.1159/000120759. [DOI] [PubMed] [Google Scholar]
- 22.Weiner HL, Freed D, Woo HH, Rezai AR, Kim R, Epstein FJ. Intra-axial tumors of the cervicomedullary junction: surgical results and long-term outcome. Pediatr Neurosurg. 1997;27(1):12–18. doi: 10.1159/000121219. [DOI] [PubMed] [Google Scholar]
- 23.Tihan T, Fisher PG, Kepner JL, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58(10):1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Komotar RJ, Burger PC, Carson BS, et al. Pilocytic and pilomyxoid hypothalamic/chiasmatic astrocytomas. Neurosurgery. 2004;54(1):72–79. doi: 10.1227/01.neu.0000097266.89676.25. discussion 79-80. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JHJ, Hariharan S, Berman J, et al. Clinical outcome of pediatric gangliomas: ninety-nine cases over 20 years. Pediatr Neurosurg. 1997;27(4):203–207. doi: 10.1159/000121252. [DOI] [PubMed] [Google Scholar]
- 26.Fisher PG, Tihan T, Goldthwaite PT, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51(2):245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 27.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White FV, Anthony DC, Yunis EJ, Tarbell NJ, Scott RM, Schofield DE. Nonrandom chromosomal gains in pilocytic astrocytomas of childhood. Hum Pathol. 1995;26(9):979–986. doi: 10.1016/0046-8177(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharjee MB, Armstrong DD, Vogel H, Cooley LD. Cytogenetic analysis of 120 primary pediatric brain tumors and literature review. Cancer Genet Cytogenet. 1997;97(1):39–53. doi: 10.1016/s0165-4608(96)00330-5. [DOI] [PubMed] [Google Scholar]
- 30.Bigner SH, McLendon RE, Fuchs H, McKeever PE, Friedman HS. Chromosomal characteristics of childhood brain tumors. Cancer Genet Cytogenet. 1997;97(2):125–134. doi: 10.1016/s0165-4608(96)00404-9. [DOI] [PubMed] [Google Scholar]
- 31.Roberts P, Chumas PD, Picton S, Bridges L, Livingstone JH, Sheridan E. A review of the cytogenetics of 58 pediatric brain tumors. Cancer Genet Cytogenet. 2001;131(1):1–12. doi: 10.1016/s0165-4608(01)00483-6. [DOI] [PubMed] [Google Scholar]
- 32.Orr LC, Fleitz J, McGavran L, Wyatt-Ashmead J, Handler M, Foreman NK. Cytogenetics in pediatric low-grade astrocytomas. Med Pediatr Oncol. 2002;38(3):173–177. doi: 10.1002/mpo.1305. [DOI] [PubMed] [Google Scholar]
- 33.Jones DT, Ichimura K, Liu L, Pearson DM, Plant K, Collins VP. Genomic analysis of pilocytic astrocytomas at 0.97 Mb resolution shows an increasing tendency toward chromosomal copy number change with age. J Neuropathol Exp Neurol. 2006;65(11):1049–1058. doi: 10.1097/01.jnen.0000240465.33628.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong KK, Tsang YT, Chang YM, et al. Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res. 2006;66(23):11172–11178. doi: 10.1158/0008-5472.CAN-06-2438. [DOI] [PubMed] [Google Scholar]
- 35.Sievert A, Jackson E, Gai X, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19(3):449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones DT, Kocialkowski S, Liu L, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68(21):8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfister S, Janzarik WG, Remke M, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118(5):1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deshmukh H, Yeh TH, Yu J, et al. High-resolution, dual-platform aCGH analysis reveals frequent HIPK2 amplification and increased expression in pilocytic astrocytomas. Oncogene. 2008;27(34):4745–4751. doi: 10.1038/onc.2008.110. [DOI] [PubMed] [Google Scholar]
- 39.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 40.Jeuken J, van den Broecke C, Gijsen S, Boots-Sprenger S, Wesseling P. RAS/RAF pathway activation in gliomas: the result of copy number gains rather than activating mutations. Acta Neuropathol. 2007;114(2):121–133. doi: 10.1007/s00401-007-0239-0. [DOI] [PubMed] [Google Scholar]
- 41.Bartels U, Hawkins C, Jing M, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg. 2006;104(5 suppl):314–320. doi: 10.3171/ped.2006.104.5.314. [DOI] [PubMed] [Google Scholar]
- 42.Tabori U, Vukovic B, Zielenska M, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8(2):136–142. doi: 10.1593/neo.05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88(3):245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Felix C, Slavc I, Dunn M, et al. p53 gene mutations in pediatric brain tumors. Med Pediatr Oncol. 1995;25(6):431–436. doi: 10.1002/mpo.2950250603. [DOI] [PubMed] [Google Scholar]
- 45.Broniscer A, Baker SJ, West AN, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25(6):682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 46.Mariani L, Deiana G, Vassella E, et al. Loss of heterozygosity 1p36 and 19q13 is a prognostic factor for overall survival in patients with diffuse WHO grade 2 gliomas treated without chemotherapy. J Clin Oncol. 2006;24(29):4758–4763. doi: 10.1200/JCO.2006.05.9238. [DOI] [PubMed] [Google Scholar]
- 47.Gutmann DH. Using neurofibromatosis-1 to better understand and treat pediatric low-grade glioma. J Child Neurol. 2008;23(10):1186–1194. doi: 10.1177/0883073808321061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9(1):73–79. doi: 10.1016/S1470-2045(07)70411-4. [DOI] [PubMed] [Google Scholar]
- 49.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 50.Franz DN, Leonard J, Tudor C, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59(3):490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 51.Pollack IF, Claassen D, al-Shboul Q, Janosky JE, Deutsch M. Low-grade gliomas of the cerebral hemispheres in children: an analysis of 71 cases. J Neurosurg. 1995;82(4):536–547. doi: 10.3171/jns.1995.82.4.0536. [DOI] [PubMed] [Google Scholar]
- 52.Griffin TW, Beaufait D, Blasko JC. Cystic cerebellar astrocytomas in childhood. Cancer. 1979;44(1):276–280. doi: 10.1002/1097-0142(197907)44:1<276::aid-cncr2820440147>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 53.Pencalet P, Maixner W, Sainte-Rose C, et al. Benign cerebellar astrocytomas in children. J Neurosurg. 1999;90(2):265–273. doi: 10.3171/jns.1999.90.2.0265. [DOI] [PubMed] [Google Scholar]
- 54.Smoots DW, Geyer JR, Lieberman DM, Berger MS. Predicting disease progression in childhood cerebellar astrocytoma. Childs Nerv Syst. 1998;14(11):636–648. doi: 10.1007/s003810050290. [DOI] [PubMed] [Google Scholar]
- 55.Desai KI, Nadkarni TD, Muzumdar DP, Goel A. Prognostic factors for cerebellar astrocytomas in children: a study of 102 cases. Pediatr Neurosurg. 2001;35(6):311–317. doi: 10.1159/000050443. [DOI] [PubMed] [Google Scholar]
- 56.Sutton LN, Cnaan A, Klatt L, et al. Postoperative surveillance imaging in children with cerebellar astrocytomas. J Neurosurg. 1996;84(5):721–725. doi: 10.3171/jns.1996.84.5.0721. [DOI] [PubMed] [Google Scholar]
- 57.Fisher BJ, Leighton CC, Vujovic O, Macdonald DR, Stitt L. Results of a policy of surveillance alone after surgical management of pediatric low grade gliomas. Int J Radiat Oncol Biol Phys. 2001;51(3):704–710. doi: 10.1016/s0360-3016(01)01705-9. [DOI] [PubMed] [Google Scholar]
- 58.Kandil A, Khafaga Y, ElHusseiny G, Allam A, Jamshed A, Schultz H. Low-grade astrocytoma—a retrospective analysis of 102 patients. Acta Oncol. 1999;38(8):1051–1056. doi: 10.1080/028418699432356. [DOI] [PubMed] [Google Scholar]
- 59.Laws ER, Jr, Taylor WF, Clifton MB, Okazaki H. Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg. 1984;61(4):665–673. doi: 10.3171/jns.1984.61.4.0665. [DOI] [PubMed] [Google Scholar]
- 60.North CA, North RB, Epstein JA, Piantadosi S, Wharam MD. Low-grade cerebral astrocytomas. Survival and quality of life after radiation therapy. Cancer. 1990;66(1):6–14. doi: 10.1002/1097-0142(19900701)66:1<6::aid-cncr2820660103>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 61.Lote K, Egeland T, Hager B, et al. Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol. 1997;15(9):3129–3140. doi: 10.1200/JCO.1997.15.9.3129. [DOI] [PubMed] [Google Scholar]
- 62.Ahn Y, Cho BK, Kim SK, et al. Optic pathway glioma: outcome and prognostic factors in a surgical series. Childs Nerv Syst. 2006;22(9):1136–1142. doi: 10.1007/s00381-006-0086-7. [DOI] [PubMed] [Google Scholar]
- 63.Opocher E, Kremer LC, Da Dalt L, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42(12):1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 64.Palma L, Guidetti B. Cystic pilocytic astrocytomas of the cerebral hemispheres. Surgical experience with 51 cases and long-term results. J Neurosurg. 1985;62(6):811–815. doi: 10.3171/jns.1985.62.6.0811. [DOI] [PubMed] [Google Scholar]
- 65.Khan RB, Sanford RA, Kun LE, Thompson SJ. Morbidity of second-look surgery in pediatric central nervous system tumors. Pediatr Neurosurg. 2001;35(5):225–229. doi: 10.1159/000050426. [DOI] [PubMed] [Google Scholar]
- 66.Palma L, Celli P, Mariottini A. Long-term follow-up of childhood cerebellar astrocytomas after incomplete resection with particular reference to arrested growth or spontaneous tumour regression. Acta Neurochir (Wien) 2004;146(6):581–588. doi: 10.1007/s00701-004-0257-9. discussion 588. [DOI] [PubMed] [Google Scholar]
- 67.Benesch M, Eder HG, Sovinz P, et al. Residual or recurrent cerebellar low-grade glioma in children after tumor resection: is retreatment needed? A single center experience from 1983 to 2003. Pediatr Neurosurg. 2006;42(3):159–164. doi: 10.1159/000091859. [DOI] [PubMed] [Google Scholar]
- 68.Shaw EG, Wisoff JH. Prospective clinical trials of intracranial low-grade glioma in adults and children. Neuro Oncol. 2003;5(3):153–160. doi: 10.1215/S1152-8517-02-00060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saunders DE, Phipps KP, Wade AM, Hayward RD. Surveillance imaging strategies following surgery and/or radiotherapy for childhood cerebellar low-grade astrocytoma. J Neurosurg. 2005;102(2 suppl):172–178. doi: 10.3171/jns.2005.102.2.0172. [DOI] [PubMed] [Google Scholar]
- 70.Garcia DM, Marks JE, Latifi HR, Kliefoth AB. Childhood cerebellar astrocytomas: is there a role for postoperative irradiation? Int J Radiat Oncol Biol Phys. 1990;18(4):815–818. doi: 10.1016/0360-3016(90)90402-6. [DOI] [PubMed] [Google Scholar]
- 71.Kortmann RD, Timmermann B, Taylor RE, et al. Current and future strategies in radiotherapy of childhood low-grade glioma of the brain. Part I: treatment modalities of radiation therapy. Strahlenther Onkol. 2003;179(8):509–520. doi: 10.1007/s00066-003-9104-9. [DOI] [PubMed] [Google Scholar]
- 72.Flickinger JC, Torres C, Deutsch M. Management of low-grade gliomas of the optic nerve and chiasm. Cancer. 1988;61(4):635–642. doi: 10.1002/1097-0142(19880215)61:4<635::aid-cncr2820610403>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 73.Grabenbauer GG, Schuchardt U, Buchfelder M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)—radiographic response, vision and late toxicity. Radiother Oncol. 2000;54(3):239–245. doi: 10.1016/s0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 74.Horwich A, Bloom HJ. Optic gliomas: radiation therapy and prognosis. Int J Radiat Oncol Biol Phys. 1985;11(6):1067–1079. doi: 10.1016/0360-3016(85)90052-5. [DOI] [PubMed] [Google Scholar]
- 75.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 76.Shibamoto Y, Kitakabu Y, Takahashi M, et al. Supratentorial low-grade astrocytoma. Correlation of computed tomography findings with effect of radiation therapy and prognostic variables. Cancer. 1993;72(1):190–195. doi: 10.1002/1097-0142(19930701)72:1<190::aid-cncr2820720134>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 77.Leibel SA, Sheline GE, Wara WM, Boldrey EB, Nielsen SL. The role of radiation therapy in the treatment of astrocytomas. Cancer. 1975;35(6):1551–1557. doi: 10.1002/1097-0142(197506)35:6<1551::aid-cncr2820350612>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 78.Jenkin D, Angyalfi S, Becker L, et al. Optic glioma in children: surveillance, resection, or irradiation? Int J Radiat Oncol Biol Phys. 1993;25(2):215–225. doi: 10.1016/0360-3016(93)90342-s. [DOI] [PubMed] [Google Scholar]
- 79.Cappelli C, Grill J, Raquin M, et al. Long-term follow up of 69 patients treated for optic pathway tumours before the chemotherapy era. Arch Dis Child. 1998;79(4):334–338. doi: 10.1136/adc.79.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fisher BJ, Bauman GS, Leighton CE, Stitt L, Cairncross JG, Macdonald DR. Low-grade gliomas in children: tumor volume response to radiation. Neurosurg Focus. 1998;4(4):e5. doi: 10.3171/foc.1998.4.4.8. [DOI] [PubMed] [Google Scholar]
- 81.Meadows AT, Gordon J, Massari DJ, Littman P, Fergusson J, Moss K. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;2(8254):1015–1018. doi: 10.1016/s0140-6736(81)91216-2. [DOI] [PubMed] [Google Scholar]
- 82.Muirhead SE, Hsu E, Grimard L, Keene D. Endocrine complications of pediatric brain tumors: case series and literature review. Pediatr Neurol. 2002;27(3):165–170. doi: 10.1016/s0887-8994(02)00402-2. [DOI] [PubMed] [Google Scholar]
- 83.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10(9):1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 84.Halberg FE, Kramer JH, Moore IM, Wara WM, Matthay KK, Ablin AR. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 1992;22(1):13–16. doi: 10.1016/0360-3016(92)90976-o. [DOI] [PubMed] [Google Scholar]
- 85.Reimers TS, Ehrenfels S, Mortensen EL, et al. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003;40(1):26–34. doi: 10.1002/mpo.10211. [DOI] [PubMed] [Google Scholar]
- 86.Packer RJ, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70(5):707–713. doi: 10.3171/jns.1989.70.5.0707. [DOI] [PubMed] [Google Scholar]
- 87.West CG, Gattamaneni R, Blair V. Radiotherapy in the treatment of low-grade astrocytomas. I. A survival analysis. Childs Nerv Syst. 1995;11(8):438–442. doi: 10.1007/BF00334960. [DOI] [PubMed] [Google Scholar]
- 88.Chadderton RD, West CG, Schuller S, Quirke DC, Gattamaneni R, Taylor R. Radiotherapy in the treatment of low-grade astrocytomas. II. The physical and cognitive sequelae. Childs Nerv Syst. 1995;11(8):443–448. doi: 10.1007/BF00334961. [DOI] [PubMed] [Google Scholar]
- 89.Pierce SM, Barnes PD, Loeffler JS, McGinn C, Tarbell NJ. Definitive radiation therapy in the management of symptomatic patients with optic glioma. Survival and long-term effects. Cancer. 1990;65(1):45–52. doi: 10.1002/1097-0142(19900101)65:1<45::aid-cncr2820650111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 90.Grill J, Couanet D, Cappelli C, et al. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann Neurol. 1999;45(3):393–396. doi: 10.1002/1531-8249(199903)45:3<393::aid-ana17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 91.Kestle JR, Hoffman HJ, Mock AR. Moyamoya phenomenon after radiation for optic glioma. J Neurosurg. 1993;79(1):32–35. doi: 10.3171/jns.1993.79.1.0032. [DOI] [PubMed] [Google Scholar]
- 92.Sharif S, Ferner R, Birch JM, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24(16):2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 93.Lacaze E, Kieffer V, Streri A, et al. Neuropsychological outcome in children with optic pathway tumours when first-line treatment is chemotherapy. Br J Cancer. 2003;89(11):2038–2044. doi: 10.1038/sj.bjc.6601410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 95.Yu DY, Dahl GV, Shames RS, Fisher PG. Weekly dosing of carboplatin increases risk of allergy in children. J Pediatr Hematol Oncol. 2001;23(6):349–352. doi: 10.1097/00043426-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Lafay-Cousin L, Sung L, Carret AS, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112(4):892–899. doi: 10.1002/cncr.23249. [DOI] [PubMed] [Google Scholar]
- 97.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32(3):235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 98.Ater J, Holmes E, Zhou T, et al. Abstracts from the thirteenth international symposium on pediatric neuro-oncology: results of COG protocol A9952—a randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. Neuro Oncol. 2008;10(3):451. [Google Scholar]
- 99.Astrup J. Natural history and clinical management of optic pathway glioma. Br J Neurosurg. 2003;17(4):327–335. doi: 10.1080/02688690310001601216. [DOI] [PubMed] [Google Scholar]
- 100.King A, Listernick R, Charrow J, Piersall L, Gutmann DH. Optic pathway gliomas in neurofibromatosis type 1: the effect of presenting symptoms on outcome. Am J Med Genet A. 2003;122A(2):95–99. doi: 10.1002/ajmg.a.20211. [DOI] [PubMed] [Google Scholar]
- 101.Thiagalingam S, Flaherty M, Billson F, North K. Neurofibromatosis type 1 and optic pathway gliomas: follow-up of 54 patients. Ophthalmology. 2004;111(3):568–577. doi: 10.1016/j.ophtha.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Deliganis AV, Geyer JR, Berger MS. Prognostic significance of type 1 neurofibromatosis (von Recklinghausen Disease) in childhood optic glioma. Neurosurgery. 1996;38(6):1114–1118. doi: 10.1097/00006123-199606000-00010. discussion 1118-1119. [DOI] [PubMed] [Google Scholar]
- 103.Chateil JF, Soussotte C, Pedespan JM, Brun M, Le Manh C, Diard F. MRI and clinical differences between optic pathway tumours in children with and without neurofibromatosis. Br J Radiol. 2001;74(877):24–31. doi: 10.1259/bjr.74.877.740024. [DOI] [PubMed] [Google Scholar]
- 104.Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001;22(10):1963–1969. [PMC free article] [PubMed] [Google Scholar]
- 105.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28(4):262–270. doi: 10.1016/s0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 106.Balcer LJ, Liu GT, Heller G, et al. Visual loss in children with neurofibromatosis type 1 and optic pathway gliomas: relation to tumor location by magnetic resonance imaging. Am J Ophthalmol. 2001;131(4):442–445. doi: 10.1016/s0002-9394(00)00852-7. [DOI] [PubMed] [Google Scholar]
- 107.Ater J, Mazewski C, Roberts W, et al. ISPNO 2006 abstract—Phase 3 randomized study of two chemotherapy regimens for treatment of progressive low-grade glioma in young children: preliminary report from the children's oncology group protocol A9952. Neuro Oncol. 2006;9(2):169–221. [Google Scholar]
- 108.Maris JM, Wiersma SR, Mahgoub N, et al. Monosomy 7 myelodysplastic syndrome and other second malignant neoplasms in children with neurofibromatosis type 1. Cancer. 1997;79(7):1438–1446. [PubMed] [Google Scholar]
- 109.Stiller CA, Chessells JM, Fitchett M. Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study. Br J Cancer. 1994;70(5):969–972. doi: 10.1038/bjc.1994.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsui I, Tanimura M, Kobayashi N, Sawada T, Nagahara N, Akatsuka J. Neurofibromatosis type 1 and childhood cancer. Cancer. 1993;72(9):2746–2754. doi: 10.1002/1097-0142(19931101)72:9<2746::aid-cncr2820720936>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 111.Shannon KM, O'Connell P, Martin GA, et al. Loss of the normal NF1 allele from the bone marrow of children with type 1 neurofibromatosis and malignant myeloid disorders. N Engl J Med. 1994;330(9):597–601. doi: 10.1056/NEJM199403033300903. [DOI] [PubMed] [Google Scholar]
- 112.Leone G, Mele L, Pulsoni A, Equitani F, Pagano L. The incidence of secondary leukemias. Haematologica. 1999;84(10):937–945. [PubMed] [Google Scholar]
- 113.Devereux S, Selassie TG, Vaughan Hudson G, Vaughan Hudson B, Linch DC. Leukaemia complicating treatment for Hodgkin's disease: the experience of the British National Lymphoma Investigation. BMJ. 1990;301(6760):1077–1080. doi: 10.1136/bmj.301.6760.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Perry JR, Brown MT, Gockerman JP. Acute leukemia following treatment of malignant glioma. J Neurooncol. 1998;40(1):39–46. doi: 10.1023/a:1006175831785. [DOI] [PubMed] [Google Scholar]
- 115.Pollack IF, Hoffman HJ, Humphreys RP, Becker L. The long-term outcome after surgical treatment of dorsally exophytic brain-stem gliomas. J Neurosurg. 1993;78(6):859–863. doi: 10.3171/jns.1993.78.6.0859. [DOI] [PubMed] [Google Scholar]
- 116.Robertson PL, Allen JC, Abbott IR, Miller DC, Fidel J, Epstein FJ. Cervicomedullary tumors in children: a distinct subset of brainstem gliomas. Neurology. 1994;44(10):1798–1803. doi: 10.1212/wnl.44.10.1798. [DOI] [PubMed] [Google Scholar]
- 117.Stark AM, Fritsch MJ, Claviez A, Dorner L, Mehdorn HM. Management of tectal glioma in childhood. Pediatr Neurol. 2005;33(1):33–38. doi: 10.1016/j.pediatrneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 118.Pollack IF, Pang D, Albright AL. The long-term outcome in children with late-onset aqueductal stenosis resulting from benign intrinsic tectal tumors. J Neurosurg. 1994;80(4):681–688. doi: 10.3171/jns.1994.80.4.0681. [DOI] [PubMed] [Google Scholar]
- 119.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40(2):265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 120.Bowers DC, Georgiades C, Aronson LJ, et al. Tectal gliomas: natural history of an indolent lesion in pediatric patients. Pediatr Neurosurg. 2000;32(1):24–29. doi: 10.1159/000028893. [DOI] [PubMed] [Google Scholar]
- 121.Massimino M, Spreafico F, Cefalo G, et al. High response rate to cisplatin/etoposide regimen in childhood low-grade glioma. J Clin Oncol. 2002;20(20):4209–4216. doi: 10.1200/JCO.2002.08.087. [DOI] [PubMed] [Google Scholar]
- 122.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26(10):649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 123.Lewis MJ, Dubois SG, Fligor B, Li X, Goorin A, Grier HE. Ototoxicity in children treated for osteosarcoma. Pediatr Blood Cancer. 2009;52(3):387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 124.Le Deley MC, Vassal G, Taibi A, Shamsaldin A, Leblanc T, Hartmann O. High cumulative rate of secondary leukemia after continuous etoposide treatment for solid tumors in children and young adults. Pediatr Blood Cancer. 2005;45(1):25–31. doi: 10.1002/pbc.20380. [DOI] [PubMed] [Google Scholar]
- 125.Lafay-Cousin L, Holm S, Qaddoumi I, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103(12):2636–2642. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 126.Bouffet E, Jakacki R, Goldman S, et al. Abstracts from the thirteenth international symposium on pediatric neuro-oncology: phase II study of weekly vinblastine in recurrent/refractory pediatric low-grade gliomas. Neuro Oncol. 2008;10(3):450. [Google Scholar]
- 127.Kuo DJ, Weiner HL, Wisoff J, Miller DC, Knopp EA, Finlay JL. Temozolomide is active in childhood, progressive, unresectable, low-grade gliomas. J Pediatr Hematol Oncol. 2003;25(5):372–378. doi: 10.1097/00043426-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 128.Khaw SL, Coleman LT, Downie PA, Heath JA, Ashley DM. Temozolomide in pediatric low-grade glioma. Pediatr Blood Cancer. 2007;49(6):808–811. doi: 10.1002/pbc.21270. [DOI] [PubMed] [Google Scholar]
- 129.Gururangan S, Fisher MJ, Allen JC, et al. Temozolomide in children with progressive low-grade glioma. Neuro Oncol. 2007;9(2):161–168. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent pediatric low-grade gliomas to bevacizumab and irnotecan. Pediatr Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 131.Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 132.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 133.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 134.Armstrong CL, Hunter JV, Ledakis GE, et al. Late cognitive and radiographic changes related to radiotherapy: initial prospective findings. Neurology. 2002;59(1):40–48. doi: 10.1212/wnl.59.1.40. [DOI] [PubMed] [Google Scholar]
- 135.Reimers TS, Mortensen EL, Schmiegelow K. Memory deficits in long-term survivors of childhood brain tumors may primarily reflect general cognitive dysfunctions. Pediatr Blood Cancer. 2007;48(2):205–212. doi: 10.1002/pbc.20818. [DOI] [PubMed] [Google Scholar]
- 136.Merchant TE, Zhu Y, Thompson SJ, Sontag MR, Heideman RL, Kun LE. Preliminary results from a Phase II trail of conformal radiation therapy for pediatric patients with localised low-grade astrocytoma and ependymoma. Int J Radiat Oncol Biol Phys. 2002;52(2):325–332. doi: 10.1016/s0360-3016(01)01807-7. [DOI] [PubMed] [Google Scholar]
- 137.Hug EB, Muenter MW, Archambeau JO, et al. Conformal proton radiation therapy for pediatric low-grade astrocytomas. Strahlenther Onkol. 2002;178(1):10–17. doi: 10.1007/s00066-002-0874-2. [DOI] [PubMed] [Google Scholar]
- 138.Landy HJ, Schwade JG, Houdek PV, Markoe AM, Feun L. Long-term follow-up of gliomas treated with fractionated stereotactic irradiation. Acta Neurochir Suppl. 1994;62:67–71. doi: 10.1007/978-3-7091-9371-6_14. [DOI] [PubMed] [Google Scholar]
- 139.Combs SE, Schulz-Ertner D, Moschos D, Thilmann C, Huber PE, Debus J. Fractionated stereotactic radiotherapy of optic pathway gliomas: tolerance and long-term outcome. Int J Radiat Oncol Biol Phys. 2005;62(3):814–819. doi: 10.1016/j.ijrobp.2004.12.081. [DOI] [PubMed] [Google Scholar]
- 140.Marcus KJ, Goumnerova L, Billett AL, et al. Stereotactic radiotherapy for localized low-grade gliomas in children: final results of a prospective trial. Int J Radiat Oncol Biol Phys. 2005;61(2):374–379. doi: 10.1016/j.ijrobp.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 141.Aarsen FK, Paquier PF, Reddingius RE, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106(2):396–402. doi: 10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]
- 142.Turkel SB, Shu Chen L, Nelson MD, et al. Case series: acute mood symptoms associated with posterior fossa lesions in children. J Neuropsychiatry Clin Neurosci. 2004;16(4):443–445. doi: 10.1176/jnp.16.4.443. [DOI] [PubMed] [Google Scholar]
- 143.Pollack IF. Posterior fossa syndrome. Int Rev Neurobiol. 1997;41:411–432. doi: 10.1016/s0074-7742(08)60362-1. [DOI] [PubMed] [Google Scholar]
- 144.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 145.Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23(22):5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 146.Ris MD, Beebe DW, Armstrong FD, et al. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children's Oncology Group. J Clin Oncol. 2008;26(29):4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]


