Abstract
Five resveratrol sulfate metabolites were synthesized and assessed for activities known to be mediated by resveratrol: inhibition of tumor necrosis factor (TNF)-α-induced NFκB activity, cylcooxygenases (COX-1 and COX-2), aromatase, nitric oxide production in endotoxin-stimulated macrophages, and proliferation of KB or MCF7 cells, induction of quinone reductase 1 (QR1), accumulation in the sub-G1 phase of the cell cycle, and quenching of 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical. Two metabolites showed activity in these assays; the 3-sulfate exhibited QR1 induction, DPPH free radical scavenging, and COX-1 and COX-2 inhibitory activities, and the 4′-sulfate inhibited NFκB induction, as well as COX-1 and COX-2 activities. Resveratrol, as well as its 3′-sulfate and 4-sulfate, inhibit NO production by NO scavenging and down-regulation of iNOS expression in RAW 264.7 cells. Resveratrol sulfates displayed low antiproliferative activity and negligible uptake in MCF7 cells.
Introduction
Resveratrol (1, 3,5,4′-trihydroxystilbene) is a naturally occurring phytoalexin produced by various plants in response to environmental stress or pathogenic attack. It is present in peanuts, mulberries, blueberries, and grapes1–3 and possesses numerous biological activities that result in antioxidant,4 anti-inflammatory,5–7 anti-ischemic,8–10 neuroprotective,11,12 anti-aging,13–15 anti-obesity,16 antiviral,17 cardioprotective,18–20 anticancer,21 and cancer chemopreventive effects.1,22–24 As a cancer chemopreventive agent, resveratrol has been shown to interfere with or inhibit all three stages of carcinogenesis: initiation, promotion, and progression.1 Interestingly, it is apparent that resveratrol can elicit these effects even though serum concentrations are low.25 Although resveratrol is efficiently absorbed on oral administration, rapid metabolism leads to the production of sulfates and glucuronides.25–34 These facts cast doubt on the physiological relevance of the high resveratrol concentrations typically used for in vitro studies, and suggest at least some, if not most, of the biological effects elicited by resveratrol may be attributed to resveratrol metabolites.
Several resveratrol absorption and metabolism studies have been performed in rodent models. Initially, an isolated rat small intestine perfusion model was used.29 Kuhnle and coworkers reported that orally administered resveratrol is mainly converted to glucuronide conjugates.29 The metabolism of resveratrol was also investigated by Yu et al., who carried out oral and intraperitoneal injections with rats and mice.31 Using synthetic standards, they identified trans-resveratrol-3-O-glucuronide and trans-resveratrol-3-sulfate in mouse serum.31 Similar to previous findings, the glucuronide and sulfate conjugates of resveratrol were detected in rat urine and mouse serum, with minimal amounts of unchanged resveratrol.31 Wenzel et al. established that all five possible resveratrol sulfate metabolites, as well as the 3-O-β-D-glucuronide, are produced in rats.25
Resveratrol metabolism studies with human beings have produced similar results to those obtained with rodent models. De Santi et al. reported the sulfation and glucuronidation of resveratrol in human liver samples,26,27 and also observed sulfation in a human duodenum preparation.28 Goldberg and collaborators demonstrated that after oral or iv injection, the majority of resveratrol detected in serum and urine was glucuronide and sulfate conjugates.35 Following that study, comparable data were reported by Meng et al., who found that no more than 2.3% of the administered resveratrol is unchanged.33 Walle et al. investigated the absorption, bioavailability, and metabolism of resveratrol by administering 14C-resveratrol to human subjects, confirming the findings that resveratrol is metabolized quickly and extensively.34 All of these in vivo studies support the idea of resveratrol being predominantly converted to its glucuronic acid and sulfate conjugates after oral, ip, or iv administration.25,29,32–36
Based on resveratrol metabolism studies, it is reasonable to suggest the in vitro data obtained using high concentrations of resveratrol lack direct in vivo relevance. Although administration of resveratrol has led to responses such as anticancer21 and cancer chemopreventive37–39 activities in animal models, it remains a fact that rapid and extensive metabolism leads to glucuronides and sulfates. Accordingly, response data could be explained by 1) local chemopreventive effects in the GI tract before metabolism occurs;37,39 2) the conversion of resveratrol sulfates and glucuronides back to resveratrol in target organs such as the liver;25,32 3) enterohepatic recirculation involving biliary secretion of resveratrol metabolites followed by deconjugation by gut microflora and then reabsorption;36 and 4) the possible biological activities of the resveratrol metabolites themselves. The latter has been suggested for other compounds, such as quercetin, (−)-epicatechin, and (+)-catechin.40–42
Selective chemical syntheses of glucuronide conjugates have been reported,43,44 and syntheses of sulfated resveratrol are known as well. However, these are non-regioselective syntheses that requires HPLC separation of mono-, di-, and tri-sulfated conjugates.25,45 As such, these procedures impede the preparation of sufficient quantities of sulfates required for a systematic investigation of their biological activities. In order to address this limitation, we have synthesized the previously identified resveratrol sulfate metabolites. All five metabolites have been prepared and isolated as their salts, and the biological effects of each metabolite have been investigated in a set of assays that are associated with cancer chemopreventive activity.
Results and Discussion
Chemistry
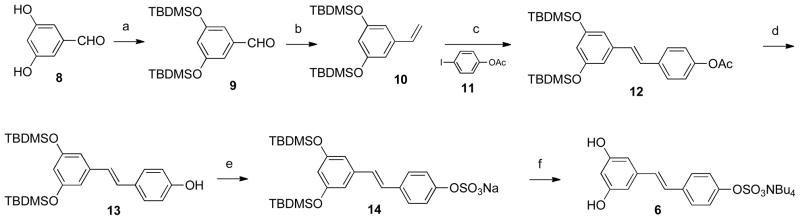
The synthesis of the five resveratrol sulfate metabolites is complicated by the fact that there are two different mono- and two different disulfated resveratrol compounds. This means that, regardless of the type of synthetic scheme that is utilized, there needs to be a way to selectively protect the three hydroxyl groups present in the resveratrol structure. Thus, the 4′-sulfate 6 was chosen as the first target due to the ease of selective protection of the 3,5-hydroxyl groups.
Compounds 10 and 11 were selected as the two reactants to undergo the Heck coupling. TBDMS and acetate groups were chosen in order to increase the yield46 and enable selective cleavage under different conditions (Scheme 1). As reported previously,46 acetyl migrations have been observed during the Heck reaction; nevertheless, successful formation of compound 12 was confirmed. Following this, a catalytic amount of NaOMe was used to cleave the acetate group to generate 13. The sulfation of 13 posed several problems. Since intermediate 14 is a sulfate sodium salt, it was not practical to perform organic extraction to remove inorganic impurities. It was therefore purified by applying the concentrated reaction mixture to a silica gel column, using EtOAc and MeOH as the solvent system. Deprotection of 14 with TBAF provided the tetra-n-butylammonium salt 6.
Scheme 1.
aReagents and conditions: (a) TBDMSCl, DMF, 80%; (b) NaNH2, MePPh3Br, Et2O, 72%; (c) Pd(OAc)2, Et3N, PPh3, MeCN, 40%; (d) NaOMe, MeOH, 92%; (e) SO3-pyridine, pyridine; (f) TBAF, MeOH, 15% (for e and f).
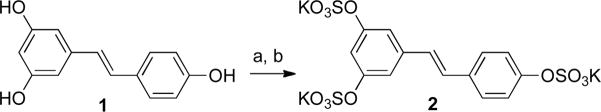
The synthesis of 6 made it apparent that it is not easy to prepare and purify sulfated resveratrol metabolites. The published literature correctly states that the multiple sulfation reaction is “a synthetic nightmare.”47 For that reason the synthesis of trisulfated resveratrol 2 was attempted next in order to optimize the sulfation reaction, sulfate salt formation, and sulfate salt purification steps using commercially available resveratrol as the starting material (Scheme 2).
Scheme 2.
aReagents and conditions: (a) SO3-NMe3, MeCN, Et3N, reflux, 21%; (b) Dowex 50WX8-200 ion exchange column, K+ form.
The first difficulty encountered in the process of synthesizing 2 was the method used to monitor the progress of the sulfation reaction. Switching the sulfation reagent from SO3·pyridine complex to SO3·NMe3 facilitated the TLC monitoring of the reaction and made the work-up more convenient. Since trisulfated resveratrol is a very polar compound, the types of TLC plates used were changed from normal-phase to reversed-phase.
After driving the sulfation reaction to completion, the next challenge in the synthesis of tri-sulfated resveratrol metabolite 2 was forming the sulfate salts. Initial attempts using excess Na2CO3 made it clear that removal of inorganic salt from the product was very difficult because of the highly polar nature of the desired product. Use of a Dowex 50X8-200 column that had been converted to the K+ form enabled formation of the tripotassium salt while limiting the introduction of excess K+ into the reaction mixture. The final traces of inorganic impurities were removed by size exclusion chromatography.
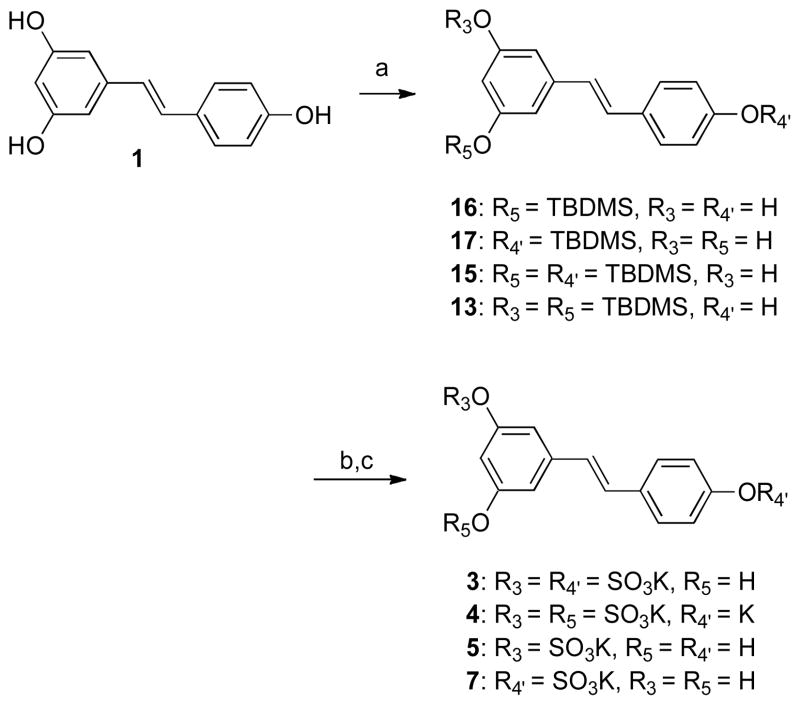
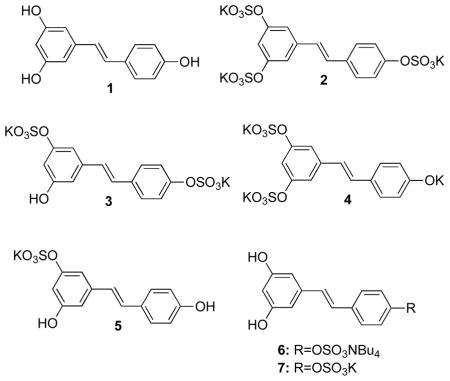
The synthesis of mono- and di-sulfated resveratrol metabolites requires the preparation of four different precursors (Scheme 3). These precursors require selective protection of three hydroxyl groups that are present on the stilbene framework. The protected intermediates necessary to synthesize mono- and di-sulfated resveratrol were prepared and separated (Scheme 3; compounds 13, 15-17) following the procedure established by Zhang et al.43 Using the previously determined reaction conditions, each of the four TBDMS-protected resveratrol compounds was sulfated. At this point, these sulfated intermediates were not converted to potassium sulfates due to observation that not forming potassium salts before the deprotection step enhanced the solubilities of the intermediate with both non-polar TBDMS groups and polar sulfate groups. Although the difference appears minimal, not forming potassium salts greatly improved the solubilities of the intermediates, and made it possible to effectively remove organic impurities from the reaction mixture before the TBDMS deprotection reaction. The TBDMS deprotection reactions were carried out with KF instead of TBAF in order to avoid formation of the tetra-n-butylammonium salts. By combining these optimization efforts, four different potassium salts 3–5 and 7 of sulfated resveratrol metabolites were successfully formed as shown in Scheme 3.
Scheme 3.
aReagents and conditions: (a) TBDMSCl, imidazole, DMF, 12–33%; (b) SO3-NMe3, MeCN, Et3N; (c) KF, MeOH/H2O, 15–26% (for b and c).
The NMR peak assignments for all possible sulfated resveratrol metabolites are compared in Table 1. As expected, the signals move downfield as sulfates are added. The 1H NMR chemical shifts and coupling constants, along with the mass spectrometry data, allowed the unambiguous assignments of the structures of all five metabolites. In particular, the equivalence or non-equivalence of the protons attached to C-2 and C-6 was diagnostic.
Table 1.
1H NMR (in D2O) Shifts (ppm) for Sulfated Resveratrols
 proton assignment | |||||||
|---|---|---|---|---|---|---|---|
| compound | 4 | 2 | 6 | 7 | 8 | 2′ | 3′ |
| Resveratrol | 5.41 | 5.68 | 5.68 | 6.60 | 6.33 | 6.94 | 6.20 |
| 2 | 7.03 | 7.26 | 7.26 | 7.14 | 7.03 | 7.49 | 7.15 |
| 3 | 6.38 | 6.65 | 6.71 | 7.05 | 6.94 | 7.46 | 7.14 |
| 4 | 6.94 | 7.18 | 7.18 | 7.01 | 6.83 | 7.31 | 6.70 |
| 5 | 6.54 | 6.77 | 6.88 | 7.02 | 6.84 | 7.34 | 6.74 |
| 6 | Not soluble in D2O. | ||||||
| 7 | 6.13 | 6.47 | 6.47 | 6.97 | 6.84 | 7.40 | 7.11 |
Biological Results
One of the most extensively studied biological activities of resveratrol investigated during the past few years has been its cancer-chemopreventive potential.48 This stilbene has been demonstrated to block the multistep process of carcinogenesis at the various stages of initiation, promotion, and progression. Some possible mechanisms involve down regulation of the inflammatory response through inhibition of synthesis and release of pro-inflammatory mediators, modification of eicosanoid synthesis, inhibition of activated immune cells, or inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) via modulation of NFκB. To explore the activities of the synthetic resveratrol sulfate derivatives, they were tested in a set of assays indicative of chemoprevention, including inhibition of TNF-α-induced NFκB activity, COX-1 and COX-2 inhibition, inhibition of nitric oxide production by iNOS in LPS-induced macrophage cells, aromatase inhibition, QR1 induction, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical quenching, and cytotoxicity in KB and MCF7 cells. The results are summarized in Table 2.
Table 2.
Biological Evaluation of Resveratrol and Resveratrol Sulfates in Bioassays Indicative of Cancer Chemoprevention
| compound tested |
||||||
|---|---|---|---|---|---|---|
| assay | Resveratrol | 2 | 3 | 4 | 5 | 7 |
| NFκB | ||||||
| % inhibitiona | 75.7 ± 2.12 | 36.7 ± 9.4 | 53.4 ± 2.9 | 42.5 ± 5.23 | 33.0 ± 4.81 | 64.0 ± 2.26 |
| IC50 (μM) | 0.173±0.05 | – | – | – | – | 18.2 ± 0.99 |
| COX-1 | ||||||
| % inhibitiona | 75.2 ± 4.53 | 37.8 ± 0.71 | 23.3 ± 0.98 | 30.9 ± 2.69 | 74.3 ± 0.99 | 63.2 ± 3.39 |
| IC50 (μM) | 6.65 ± 2.5 | – | – | – | 3.60 ± 0.8 | 5.55 ± 1.73 |
| COX-2 | ||||||
| % inhibitiona | 72.2±4.67 | 2.4 ± 2.0 | 16.5 ± 2.69 | 25.5 ± 5.52 | 62.0 ± 1.7 | 65.8 ± 7.64 |
| Nitric Oxide | ||||||
| % inhibitiona | 71.8 ± 3.5 | 24.8 ± 3.1 | 41.7 ± 5.5 | 4.8 ± 4.5 | 41.0 ± 0.7 | 56.8 ± 5.9 |
| IC50 (μM) | 15.0 ± 2.6 | – | – | – | – | – |
| % SubG1a,b | 24.9 ± 0.149 | – | – | – | – | 2.22 ± 0.012 |
| Cytotoxicityc | ||||||
| KB % survival | 47.8 ± 5.2 | 78.1 ± 7.8 | 99.3 ± 15.0 | 84.2 ± 13.1 | 70.3 ± 8.8 | 108.5 ± 10.3 |
| MCF7 % survival | 38.6 ± 3.5 | 106.7 ± 9.6 | 100.0 ± 11.3 | 97.2 ± 5.2 | 51.7 ± 0.2 | 103.4 ± 8.8 |
| Aromatase | ||||||
| % inhibitiona | 34.8 ± 1.21 | 28.5 ± 0.2 | 22.5 ± 0.64 | 20.5 ± 0.43 | 28.2 ± 1.12 | 30.4 ± 0.56 |
| QR1 | ||||||
| CD (μM)d | 21 ± 0.46 | > 11.7 | > 10.1 | > 10.1 | 2.6 ± 0.38 | > 6.9 |
| DPPH | ||||||
| % inhibitione | 65.2 ± 2.0 | 0.4 ± 1.1 | 6.8 ± 1.0 | 14.5 ± 4.2 | 68.0 ± 1.9 | 42.8 ± 2.5 |
| IC50 (μM) | 178.5 ± 9.3 | – | – | – | 219.2 ± 3.1 | – |
Determined at a compound concentration of 34 μM. The compounds were not cytotoxic at this concentration in HEK-293 cells.
The percentage of subG1 HL-60 cells, characteristic of apoptosis. The distribution in subG1 of vehicle-treated cells was 2.2 ± 0.0%.
Determined at a concentration of 20 μg/mL.
Concentration to double the expression of QR1.
Determined at a compound concentration of 340 μM.
The role of NFκB in many cellular processes is well studied. Deregulated activity of the NFκB pathway has been observed and linked to the progression of cancer and several human ailments. Our test system assesses inhibition of NFκB induction by TNF-α in a stably transfected 293/NFκB-Luc human embryonic kidney cell line.49 Presumably, the sulfate metabolites could be transported intact into the kidney cells by organic anion transporters.50–53 All of the metabolites retained some level of activity in this assay, but potency was reduced, relative to resveratrol. The most active metabolite was the 4′-sulfate 7, and the two least active were 2 and 4. In general, the ability of these stilbenes to inhibit the induction of NFκB is surprisingly insensitive to the substituents present, or to their arrangement.
The involvement of prostaglandins (PG) and other eicosanoids in the development of human cancer is well known.54 Importantly, an increase in PG synthesis may influence tumor growth in human beings and experimental animals. Cleaved from membrane phospholipids by phospholipases, arachidonic acid (AA) is converted through the cyclooxygenase (COX) pathway to produce PGs.55 Therefore, inhibition of COX and the subsequent reduction of PG synthesis provides a viable strategy for the prevention of cancer.56–60 Accordingly, resveratrol and resveratrol sulfates were tested for inhibition of both COX-1 and COX-2. Resveratrol inhibited COX-1 and -2 with IC50 values of 6.65 and 0.75 μM, respectively. The 3-sulfate 5 and the 4′-sulfate 7 inhibited COX-1 with IC50 values comparable to resveratrol (3.60 and 5.55 μM, respectively). In COX-2 inhibition assays, the 3-sulfate 5 demonstrated an IC50 of 7.53 μM and the 4′-sulfate 7 had an IC50 of 8.95 μM.
Nitric oxide (NO), a product of nitric oxide synthase (NOS), mediates diverse physiological processes (e.g., vasodilation, immune response) as a signaling molecule. However, continuous and excessive production of NO by inducible nitric oxide synthase (iNOS) causes pathophysiological problems such as chronic inflammatory diseases and cancer development.61 In addition, up-regulation of nitric oxide (NO) synthesis may contribute to tumor growth by facilitating angiogenesis.62 Inhibitors of inducible nitric oxide synthase (iNOS) may have chemopreventive activity due to antiproliferative effects,56 and resveratrol has been reported to function in this capacity.63 Therefore, the abilities of resveratrol and resveratrol sulfates to inhibit the production of NO by iNOS in macrophage cells were determined. With an IC50 value of 15 μM, resveratrol was the most potent inhibitor of nitric oxide synthase. Modest activity was observed with 4′-sulfate 7, followed by the 3,4′-disulfate 3 and the 3-sulfate 5, which were equipotent. The least potent nitric oxide synthase inhibitor was the 3,5-disulfate 4, which was actually less potent than the trisulfate 2.
To determine whether resveratrol sulfates induce nitric oxide production by themselves, these compounds were tested under LPS-free circumstances. None of the resveratrol sulfates showed significant enhancement of NO production when tested at a concentration of 34 μM (data not shown).
Several publications have indicated that polyphenols like resveratrol64 and epigallocatechin gallate (EGCG)65 inhibit NO and peroxynitrite formation due to antioxidant activity.66 Therefore, NO scavenging activity of each resveratrol sulfate was measured using the NO generating reagent sodium nitroprusside (SNP). At a concentration of 60 μM, compounds 1, 5 and 7 showed slight NO scavenging activity, with 17.4 ± 4.3, 24.7 ± 1.2, 10.3 ± 4.3% inhibition, while compounds 2, 3, and 4 were not active, demonstrating 1.6 ± 3.8, 0.0 ± 3.3, and 1.2 ± 4.9% inhibition, respectively
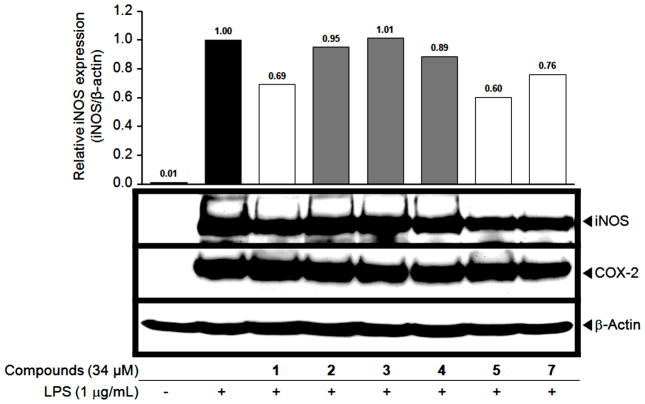
Several polyphenols, including 6-gingerol, epigallocatechin gallate (EGCG), indole-3-carbinol, and oroxylin A, as well as resveratrol itself, have been reported to inhibit iNOS expression in LPS-treated RAW 264.7 cell lines.67 We performed Western blot analyses to determine if compounds 1, 5, 7 inhibit nitrite production via down-regulation of iNOS expression. As shown in Figure 1, LPS increased the protein expression of iNOS in comparison with untreated RAW 264.7 cells. Under the same conditions, resveratrol sulfates 1, 5, 7 moderately suppressed the expression of iNOS compared to LPS-treated control. In sum, compounds 1, 5, 7 showed moderate inhibition in NO production by NO scavenging activity and down-regulation of iNOS protein expression. Although these responses are not strong, since up-regulation of iNOS is correlated with activation of upstream NFκB pathways,61 and some inhibitors of NO production function through NFκB regulation,68 a compound such as 7 could possibly mediate a stronger response in a broader biological milieu.
Figure 1.
Effects of resveratrol and its sulfate derivatives on iNOS and COX-2 expression in LPS-stimulated RAW 264.7 cells. RAW 264.7 cells were treated with compounds 15 min prior to LPS (1 μg/mL) stimulation and further incubated for 18 h. Cells were lysed, and protein (30 μg) was subjected to 8% SDS-PAGE. The level of iNOS and COX-2 protein expression was examined by immunoblot analysis. β-Actin was used as an internal control. The experiment was performed in duplicate. The density of each band was measured by using an image analyzer system. Average values of relative iNOS protein expression are shown in comparison with the LPS-treated control (black bar). Compounds 1, 5 and 7 (open bars) showed suppressive effects on the expression of iNOS protein with relative values of 0.62, 0.54 and 0.67, respectively. Compounds 2, 3, and 4 were not active, and COX-2 expression was not altered.
Using the same experimental approach simultaneously we investigated protein expression of COX-2 (Figure 1). None of tested compounds, at the concentration 34 μM, inhibited COX-2 expression, which is consistent with a previous report.69
Resveratrol is known to induce programmed cell death (apoptosis) in a variety of cell lines, including lung, colon, prostate, and breast.70–73 Since a subG1 cell population is an indicator of cell death by apoptosis or necrosis, we explored the potential of resveratrol and resveratrol sulfates to induce accumulation of HL-60 human acute leukemia cells in the subG1 compartment. Consistent with previous results,74 resveratrol was active in this process, but the sulfate metabolites were inactive.
The lack of cytotoxicity of the sulfates 2–4 and 7 in MCF7 and KB cells (Table 2), as well as low activity in other cell-based assays, suggests a lack of uptake as well as a lack of hydrolysis. To test for cellular uptake, the 3,5-disulfate 4 and resveratrol were incubated separately with MCF7 cells, and LC-MS-MS was used to measure intracellular levels of resveratrol and its various sulfate conjugates. After incubation with resveratrol as a control, MCF7 cells were found to contain resveratrol and the disulfate 4, but not the 4′-sulfate 7. This indicated that resveratrol entered MCF7 cells and was metabolized to form 7. When MCF7 cells were incubated with the 4′-sulfate 7, no absorption of 7 was detected, and neither resveratrol 1 nor the disulfate 4 were detected intracellularly. These results indicate lack of hydrolysis and uptake of the 4′-sulfate 7 by MCF7 cells. Based on the cytotoxicity results (Table 2), the other sulfates are probably also not hydrolyzed or absorbed to an appreciable extent by MCF7 cells.
To test the stability of resveratrol and its 4′-sulfate 7, each compound was incubated separately for 24 h at 37 °C in the cell culture medium used for MCF7 cellular uptake studies. Based on LC-MS-MS analyses, the 4′-sulfate 7 was stable under these incubation conditions. On the other hand, resveratrol 1 degraded approximately 20% during this time period. Therefore, metabolic sulfation of resveratrol forms stable derivatives that can be excreted in bile or urine. Since enterohepatic recirculation of resveratrol occurs,36 resveratrol sulfates will be deconjugated by gut microflora, and then resveratrol will be reabsorbed to prolong its anticancer effects.
CYP19 (aromatase) converts C19 androgens to aromatic C18 estrogens through three consecutive hydroxylation reaction steps.75 Aromatase transcription is mediated by IκB kinaseβ (IKKβ), a kinase previously known for cancer-promoting activity.76 Under some situations (e.g., post menopause), aromatase is a key player in estrogen production, and inhibitors have been shown to function as chemopreventive agents. Based on an in vitro test system, resveratrol and its sulfates were found to be relatively weak inhibitors. The most active of the metabolites was the 4′-sulfate 7, which produced 30% inhibition at a concentration of 34 μM.
Induction of NAD(P)H:quinone reductase 1 (QR1) is a well established mechanism for cancer chemoprevention.77–80 Induction of QR1 commonly coincides with the induction of other phase II detoxifying enzymes.81 Therefore, a rapid and sensitive QR1 cellular assay82 was used to evaluate resveratrol and resveratrol metabolites. The results summarized in Table 2 include the induction ratio (IR), which is the ratio of the observed QR1 activity resulting from treatment with 34 μM of the test compound vs. DMSO control. In addition, the concentrations to double the activity of QR1 (CD) are listed. The 3-sulfate 5 was more potent than resveratrol in this assay, while the other sulfate metabolites were all less potent. However, all of the sulfates retained some degree of activity.
The cancer chemopreventive effects of resveratrol and related phenolic natural products may be due, in part, to quenching unstable free radicals and reducing damage to DNA by reactive oxygen species (ROS).83–85 The free-radical scavenging activities of the test compounds were examined by measuring ability to quench the DPPH radical. The activity of the 3-sulfate 5 was comparable to that of the parent compound 1, while the activity of the 4′-sulfate 7 was somewhat lower. The remaining disulfates 3 and 4 were much less active as free radical scavengers and, as expected from the absence of any phenolic hydroxyl groups, the trisulfate 2 was inactive.
Conclusion
Resveratrol exerts chemopreventive activity and a host of targets have been established.86 We selected a variety of in vitro and cell-based targets (Table 2) to determine the activity displayed by resveratrol relative to sulfate metabolites. Overall, the sulfate metabolites are less active than resveratrol, with some exceptions, such as resveratrol 3-sulfate (5), which mediates comparable or even greater QR1 induction, DPPH radical scavenging, and COX-1 inhibition. Not surprisingly, in general, the activities of the sulfate metabolites decrease as the degree of sulfation increases, although there are exceptions (e.g., the activities of 2 vs. 4 on inhibition of nitric oxide synthase). Since serum concentrations of sulfated metabolites are higher than the serum concentrations of resveratrol, the ability of the metabolites to typically retain some degree of activity may be of relevance.
Experimental Section
Melting points were determined using capillary tubes with a Mel-Temp apparatus and are uncorrected. The proton nuclear magnetic resonance spectra were recorded using an ARX300 300 MHz Bruker NMR spectrometer. IR spectra were recorded using a Perkin-Elmer 1600 series FTIR spectrometer. Flash and gravity chromatographic purification were performed using 230–400 mesh silica gel unless otherwise noted. Chemicals and solvents were reagent grade and obtained from commercial sources without further purification. Synthetic compounds were analyzed at the Purdue University Campus-Wide Mass Spectrometry Center using a Finnigan MAT LCQ Classic mass spectrometer system equipped with electrospray. Combustion microanalyses were performed at the Purdue University Microanalysis Laboratory using a Perkin-Elmer Series II CHNS/O model 2400 analyzer and all reported values are within 0.4% of calculated values. These elemental analyses confirmed ≥ 95% purity.
Resveratrol Tripotassium 3,5,4′-Sulfate (2)
A mixture of SO3·NMe3 (1.097 g, 7.886 mmol) in Et3N (2.50 mL, 13.14 mmol) was added to a well-stirred mixture of 1 (0.100 g, 0.438 mmol) in anhydrous MeCN (5.0 mL) at room temperature under argon. The resulting reaction mixture was heated at reflux under argon for 120 h. The reaction mixture was cooled to room temperature, decanted and concentrated under reduced pressure. Water (5.0 mL) was added to the reaction mixture and the resulting mixture was stirred for 30 min at room temperature. The water layer was concentrated to approximately 2.0 mL and applied to a column of cation-exchange resin (Dowex 50WX8-200, H+ form, 9 g, 2 × 19 cm) prepared by eluting solvents in the following order: water (300 mL), saturated K2CO3 solution (400 mL), and water (300 mL). The crude product was eluted with water and fractions containing the desired intermediate were combined and concentrated and applied to a column of reversed-phase C-18 silica gel (eluent: 0-20% MeOH-H2O, reversed-phase C-18 silica gel, 6 g, 2 × 11 cm). Fractions containing the desired compound were combined and concentrated. The crude product was applied to a size-exclusion chromatography column (eluent: H2O, Sephadex® G-10, 14 g, 2 × 20 cm) to afford 2 as a white solid (0.054 g, 21%: mp >350 °C. 1H NMR (300 MHz, D2O) δ 7.49 (d, J = 8.7 Hz, 2 H), 7.26 (d, J = 2.1 Hz, 2 H), 7.15 (d, J = 8.7 Hz, 2 H), 7.14 (d, J = 16.5 Hz, 1 H), 7.03 (d, J = 16.5 Hz, 1 H), 6.99 (t, J = 2.1 Hz, 1 H); positive ESIMS m/z (rel intensity) 621 (MK+, 100). Anal. Calcd for C14H9K3O12S3·1.25H2O: C, 27.78; H, 1.92; S, 15.89. Found: C 27.54; H, 1.77; S, 15.62.
Resveratrol Dipotassium 3,4′-Sulfate (3)
SO3·NMe3 (0.487 g, 3.504 mmol) and Et3N (0.81 mL, 5.83 mmol) were added to a well-stirred mixture of 16 (0.100 g, 0.292 mmol) in anhydrous MeCN (5.0 mL) at room temperature under argon. The resulting reaction mixture was heated to reflux under argon for 48 h. The reaction mixture was cooled to room temperature, decanted and concentrated under reduced pressure and applied to a column of reversed-phase C-18 silica gel (eluent: 10–25 % CH3CN-H2O, 5 g, 2 × 10 cm). Fractions containing the desired compound and some impurities were combined, concentrated and dissolved in 30% aqueous MeOH (10 mL). KF (0.051 g, 0.875 mmol) was added to the solution, which was stirred vigorously at room temperature under argon for 12 h and concentrated under reduced pressure. The crude product was applied to a column of cation-exchange resin (Dowex 50WX8-200, H+ form, 9 g, 2 × 19 cm) prepared by eluting solvents in the following order: water (300 mL), saturated K2CO3 solution (400 mL), and water (300 mL). The crude product was eluted with water and fractions containing the desired intermediate were combined, concentrated, and applied to a column of reversed-phase C-18 silica gel (eluent: 0–20% MeOH-H2O, 6 g, 2 × 11 cm). Fractions containing the desired compound and some impurities were combined, concentrated, and applied to a size-exclusion chromatography column (eluent: H2O, Sephadex® G-10, 14 g, 2 × 20 cm) to afford 3 as a white solid (0.015 g, 26%): mp >300 °C. 1H NMR (300 MHz, D2O) δ 7.46 (d, J = 8.4 Hz, 2 H), 7.13 (d, J = 8.1 Hz, 2 H), 7.05 (d, J = 16.5 Hz, 1 H), 6.94 (d, J = 16.5 Hz, 1 H), 6.71 (s, 1 H), 6.65 (s, 1 H), 6.38 (t, J = 2.1 Hz, 1 H); positive ESIMS m/z (rel intensity) 487 (MNa+, 100). Anal. Calcd for C14H10K2O9S2·2H2O: C, 33.59; H, 2.82; S, 12.81. Found: C, 33.42; H, 2.38; S, 12.83.
Resveratrol Tripotassium 3,5-Sulfate (4)
SO3·NMe3 (0.487 g, 3.504 mmol) and Et3N (0.81 mL, 5.83 mmol) were added to a well-stirred mixture of 17 (0.100 g, 0.292 mmol) in nhydrous MeCN (5.0 mL) at room temperature under argon. The resulting reaction mixture was heated to reflux under argon for 48 h. The reaction mixture was cooled to room temperature, decanted and concentrated under reduced pressure and applied to a column of reversed-phase C-18 silica gel (eluent: 10–25% CH3CN-H2O, 5 g, 2 × 10 cm). Fractions containing the desired compound and some impurities were combined and concentrated and dissolved in 30% aqueous MeOH (10 mL). KF (0.051 g, 0.875 mmol) was added to the solution, which was stirred vigorously at room temperature under argon for 12 h and concentrated under reduced pressure. The crude product was applied to a to a column of cation-exchange resin (Dowex 50WX8-200, H+ form, 9 g, 2 × 19 cm) prepared by eluting solvents in the following order: water (300 mL), saturated K2CO3 solution (400 mL), and water (300 mL). The crude product was eluted with water and fractions containing the desired intermediate and some impurities were combined and concentrated and applied to a column of reversed-phase C-18 silica gel (eluent: 0–20% MeOH-H2O, 6 g, 2 × 11 cm). Fractions containing the desired compound and some impurities were combined and concentrated and applied to a size-exclusion chromatography column (eluent: H2O, Sephadex® G-10, 14 g, 2 × 20 cm) to afford 4 as a white solid (0.015 g, 20%): mp >300 °C. 1H NMR (300 MHz, D2O) δ 7.31 (d, J = 8.7 Hz, 2 H), 7.18 (d, J = 2.1 Hz, 2 H), 7.01 (d, J = 16.2 Hz, 1 H), 6.94 (t, J = 2.1 Hz, 1 H), 6.83 (d, J = 16.5 Hz, 1 H), 6.70 (d, J = 8.7 Hz, 2 H); positive ESIMS m/z (rel intensity) 487 (MNa+, 100). Anal. Calcd for C14H9K3O9S2·2H2O: C, 31.22; H, 2.43; S, 11.91. Found: C, 30.94; H, 2.26; S, 11.73.
Resveratrol Potassium 3-Sulfate (5)
SO3·NMe3 (0.548 g, 3.948 mmol) and Et3N (0.92 mL, 6.56 mmol) were added to a well-stirred mixture of 15 (0.300 g, 0.656 mmol) in anhydrous MeCN (5.0 mL) at room temperature under argon. The resulting reaction mixture was heated to reflux under argon for 48 h. The reaction mixture was cooled to room temperature, decanted and concentrated under reduced pressure and applied to a column of reversed-phase C-18 silica gel (eluent: 10–30% CH3CN-H2O, 5 g, 2 × 10 cm). Fractions containing the desired compound and some impurities were combined and concentrated and dissolved in anhydrous MeOH (10 mL). KF (0.051 g, 0.875 mmol) was added to the solution, which was stirred vigorously under argon at room temperature for 12 h and concentrated under reduced pressure. The resulting crude reaction mixture was applied to a column of cation-exchange resin (Dowex 50WX8-200, H+ form, 8 g, 2 × 18 cm) prepared by eluting solvents in the following order: water (300 mL), saturated K2CO3 solution (400 mL), and water (300 mL). The crude product was eluted with water and fractions containing the desired intermediate and some impurities were combined and concentrated and applied to a column of reversed-phase C-18 silica gel (eluent: 0–30% MeOH-H2O, 6 g, 2 × 11 cm). Fractions containing the desired compound and some impurities were combined and concentrated and applied to a size-exclusion chromatography column (eluent: H2O, Sephadex® G-10, 14 g, 2 × 20 cm) to give 5 as a white solid (0.010 g, 15%): mp >300 °C. 1H NMR (300 MHz, D2O) δ7.34 (d, J = 8.7 Hz, 2 H), 7.02 (d, J = 16.5 Hz, 1 H), 6.88 (s, 1 H), 6.84 (d, J = 16.5 Hz, 1 H), 6.77 (s, 1 H), 6.74 (d, J = 8.4 Hz, 2 H), 6.54 (t, J = 2.1 Hz, 1 H); positive ESIMS m/z (rel intensity) 386 (MK+, 100). Anal. Calcd for C14H11KO6S: C, 48.54; H, 3.20; S, 9.26. Found: C, 48.24; H, 3.12; S, 8.90.
Resveratrol Tetrabutylammonium 4′-Sulfate (6)
TBAF (0.53 mL, 2.189 mmol) was added to a well-stirred mixture of 14 (0.100 g, 0.178 mmol) in MeOH (5.0 mL) at room temperature under argon. The resulting reaction mixture was stirred at room temperature under argon for 12 h. The reaction mixture was concentrated under reduced pressure and the crude product was purified by recrystallization from methanol to afford the product 6 as a white solid (0.015 g, 15%): mp 182–184 °C. 1H NMR (300 MHz, MeOH-d4) δ 7.38 (d, J = 8.7 Hz, 2 H), 7.17 (d, J = 8.4 Hz, 2 H), 6.92 (d, J = 16.5 Hz, 1 H), 6.81 (d, J = 16.2 Hz, 1 H), 6.37 (d, J = 2.1 Hz, 2 H), 6.07 (t, J = 2.1 Hz, 1 H), 3.11 (t, J = 8.4 Hz, 8 H), 1.59–1.49 (m, 8 H), 1.36–1.23 (m, 8 H), 0.90 (t, J = 7.3 Hz, 12 H); positive ESIMS m/z (rel intensity) 353 (MNa+, 100). Anal. Calcd for C30H47NO6S: C, 65.54; H, 8.62; N, 2.55; S, 5.83. Found: C, 65.47; H, 8.63; N, 2.44; S, 5.74.
Resveratrol Potassium 4′-Sulfate (7)
SO3·NMe3 (0.533 g, 3.829 mmol) and Et3N (0.73 mL, 5.22 mmol) were added to a well-stirred mixture of 13 (0.330 g, 0.722 mmol) in anhydrous MeCN (8.0 mL) at room temperature under argon. The resulting reaction mixture was heated to reflux under argon for 48 h. The reaction mixture was cooled to room temperature, decanted and concentrated under reduced pressure and applied to a column of reversed-phase C-18 silica gel (eluent: 10–30% CH3CN-H2O, 5 g, 2 × 10 cm). Fractions containing the desired compound and some impurities were combined and concentrated and dissolved in anhydrous MeOH (10 mL). KF (0.054 g, 0.935 mmol) was added to the solution, which was stirred vigorously under argon at room temperature for 12 h and concentrated under reduced pressure. The resulting crude reaction mixture was applied to a column of cation-exchange resin (Dowex 50WX8-200, H+ form, 8 g, 2 × 18 cm) prepared by eluting solvents in the following order: water (300 mL), saturated K2CO3 solution (400 mL), and water (300 mL). The crude product was eluted with water and fractions containing the desired intermediate and some impurities were combined, concentrated and applied to a column of reversed-phase C-18 silica gel (eluent: 0–30% MeOH-H2O, 6 g, 2 × 11 cm). Fractions containing the desired compound and some impurities were combined and concentrated and applied to a size-exclusion chromatography column (eluent: H2O, Sephadex® G-10, 14 g, 2 × 20 cm) to give 7 as a light-brown solid (0.025 g, 10%): mp >300 °C. 1H NMR (300 MHz, D2O) δ 7.40 (d, J = 8.4 Hz, 2 H), 7.11 (d, J = 8.4 Hz, 1 H), 6.97 (d, J = 16.5 Hz, 1 H), 6.84 (d, J = 16.5 Hz, 2 H), 6.47 (d, J = 2.1 Hz, 2 H), 6.13 (t, J = 2.1 Hz, 1 H); negative ESIMS m/z (rel intensity) 307 (100). Anal. Calcd for C14H11KO6S: C, 48.54; H, 3.20; S, 9.26. Found: C, 48.19; H, 3.05; S, 8.95.
3,5-bis(tert-Butyldimethylsilyloxy)benzaldehyde (9)
TBDMSCl (2.73 g, 14.5 mmol) was added to 8 (1.00 g, 7.24 mmol) in DMF (10 mL) at 0 °C under argon. The reaction mixture was stirred for 16 h at room temperature. Water (50 mL) was added to the reaction mixture and the mixture was extracted with CH2Cl2 (3 × 100 mL). The combined organic layers were dried over sodium sulfate and concentrated. The resulting crude product was purified by column chromatography (eluent: hexanes-EtOAc 9:1, silica gel) to afford the product 9 as an orange oil (2.12 g, 80%). IR (film) 3072, 2956, 2931, 2886, 1704, 1384, 1259, 1031 cm−1; 1H NMR (300 MHz, CDCl3) δ 9.86 (s, 1 H), 6.95 (d, J = 2.1 Hz, 1 H), 6.58 (t, J = 2.1 Hz, 1 H), 0.98 (s, 18 H), 0.21 (s, 12 H); 13C NMR (75 MHz, CDCl3) δ 191.6, 157.6, 138.8, 118.6, 114.6, 25.9, 18.5, −4.1; EIMS m/z (rel intensity) 366 (M+, 100), 309 (96), 267 (58), 239 (52), 84 (78), 73 (70). Anal. Calcd for C19H34O3Si2: C, 62.24; H, 9.41. Found: C, 61.90; H, 9.41.
(5-Vinyl-1,3-phenylene)bis(oxy)bis(tert-butyldimethylsilane) (10)
A reaction mixture containing MePPh3Br (2.93 g, 8.20 mmol), NaNH2 (0.319 g, 8.20 mmol), and dry ether (12.0 mL) was stirred under argon at room temperature for 20 h. The reaction mixture was cannulated into a well-stirred mixture of 9 (0.300 g, 0.820 mmol) in dry ether (1.0 mL) at −10 °C under argon. After 10 min, the ice bath was removed and the reaction mixture was stirred at room temperature for 5 h. The crude product was concentrated and purified by column chromatography (eluent: hexanes, silica gel) to provide the product 10 as a clear oil (0.21 g, 72%). IR (film) 3056, 2930, 1584, 1471, 1255, 1061 cm−1; 1H NMR (300 MHz, CDCl3) δ 6.60 (dd, J = 17.8, 10.8 Hz, 1 H), 6.55 (d, J = 2.1 Hz, 2 H), 6.28 (t, J = 2.4 Hz, 1 H), 5.68 (dd, J = 17.8, 0.9 Hz, 1 H), 5.22 (dd, J = 10.8, 0.9 Hz, 1 H), 1.00 (s, 18 H), 0.24 (s, 12 H); 13C NMR (75 MHz, CDCl3) δ 157.0, 139.8, 137.2, 114.3, 112.1, 111.9, 26.1, 18.7, −3.9; positive ESIMS m/z (rel intensity) 365 (MH+, 100). Anal. Calcd for C20H36O2Si2: C, 65.87; H, 9.95; Si, 15.40. Found: C, 65.48; H, 9.65, Si, 15.53.
4-Iodophenyl Acetate (11)
Ac2O (3.44 mL, 36.36 mmol) was added to a well-stirred mixture of 4-iodophenol (4.00 g, 18.18 mmol) in dry pyridine (15 mL) at room temperature under argon. The resulting reaction mixture was stirred at room temperature under argon for 12 h. H2O (40 mL) was added and the mixture was extracted with CHCl3 (3 × 40 mL). The combined organic layers were washed with citric acid (10% w/v) to remove extra pyridine. The resulting crude product was purified by column chromatography (eluent: CHCl3, silica gel) to provide the product as a clear oil (0.823 g, 53%). 1H NMR (300 MHz, CDCl3) δ 7.66 (d, J = 8.4 Hz, 2 H), 6.84 (d, J = 8.7 Hz, 2 H), 2.26 (s, 3 H); EIMS m/z (rel intensity) 262 (M+, 23), 220 (100).
(E)-4-(3,5-bis(tert-Butyldimethylsilyloxy)styryl)phenyl Acetate (12).46
Et3N (1.25 mL), Pd(OAc)2 (0.008 g, 0.038 mmol), and PPh3 (0.006 g, 0.025 mmol) were added to a well-stirred mixture of 10 (1.865 g, 5.114 mmol), 11 (1.00 g, 3.816 mmol), and CH3CN (10 mL) at room temperature under argon. The reaction mixture was heated to reflux under argon for 35 h. The resulting suspension was extracted with Et2O (3 × 30 mL). The combined organic layers were washed with water (1 × 20 mL) and brine (1 × 20 mL), dried over sodium sulfate, and concentrated to provide the product 12 as a clear oil (0.760 g, 40%). 1H NMR (300 MHz, CDCl3) δ 7.32 (d, J = 8.7 Hz, 2 H), 6.90 (d, J = 8.4 Hz, 2 H), 6.82 (d, J = 16.5 Hz, 1 H), 6.74 (d, J = 16.5 Hz, 1 H), 6.45 (d, J = 2.1 Hz, 2 H), 6.10 (t, J = 2.1 Hz, 1 H), 2.11 (s, 3 H), 0.81 (s, 18 H), 0.06 (s, 12 H); 13C NMR (75 MHz, CDCl3) δ 169.9, 157.2, 150.5, 139.5, 135.5, 129.3, 127.9, 122.2, 112.1, 32.1, 26.2, 18.7, −3.9; positive ESIMS m/z (rel intensity) 521 (MNa+, 100), 499 (MH+, 82).
(E)-4-[3,5-bis(tert-Butyldimethylsilyloxy)styryl]phenol (13).43
NaOMe (0.002 g, 0.038 mmol) was added to a well-stirred mixture of 12 (0.760 g, 1.52 mmol) in dry MeOH (2 mL) at room temperature. The resulting reaction mixture was stirred at room temperature for 2 h and concentrated under reduced pressure. The crude product was purified by column chromatography (eluent: ether-hexanes 2:1, silica gel) to afford the product 13 as a clear oil (0.629 g, 92%). 1H NMR (300 MHz, CDCl3) δ 7.39 (d, J = 8.7 Hz, 2 H), 6.96 (d, J = 16.2 Hz, 1 H), 6.83 (d, J = 16.2 Hz, 1 H), 6.81 (d, J = 8.7 Hz, 2 H), 6.63 (d, J = 2.1 Hz, 2 H), 6.27 (t, J = 2.1 Hz, 1 H), 5.92 (s, 1 H), 1.00 (s, 18 H), 0.24 (s, 12 H); negative ESIMS m/z (rel intensity) 455 (100).
TBDMS-protected Resveratrol Sodium 4′-Sulfate (14)
SO3·pyridine (0.349 g, 2.189 mmol) was added to a well-stirred mixture of 13 (0.500 g, 1.095 mmol) in dry pyridine (2.0 mL) at room temperature under argon. The reaction mixture was heated to reflux under argon for 15 h. A solution of Na2CO3 (0.232 g, 2.189 mmol) in H2O (2.0 mL) was added to the reaction mixture, which was stirred at 60 °C under argon for 1 h. The reaction mixture was cooled to room temperature and concentrated. The crude product was used directly for the next step, but the structure was confirmed by NMR. 1H NMR (300 MHz, MeOH-d4) δ 7.43 (d, J = 8.7 Hz, 2 H), 7.20 (d, J = 8.7 Hz, 1 H), 6.95 (d, J = 16.2 Hz, 1 H), 6.88 (d, J = 16.5 Hz, 1 H), 6.56 (d, J = 2.1 Hz, 2 H), 6.14 (t, J = 2.1 Hz, 1 H), 0.91 (s, 18 H), 0.13 (s, 12 H).
(E)-3-(tert-Butyldimethylsilyloxy)-5-[4-(tert-butyldimethylsilyloxy)styryl] Phenol (15).43
TBDMSCl (0.347 g, 2.300 mmol) was added to a well-stirred mixture of resveratrol (1, 0.500 g, 2.190 mmol) and imidazole (0.186 g, 2.738 mmol) in anhydrous DMF (2.0 mL) at −10 °C under argon. The reaction mixture was warmed to ambient temperature. After 12 h, additional imidazole (0.186 g, 2.738 mmol) and TBDMSCl (0.347 g, 2.300 mmol) were added to the reaction mixture and stirring was continued for 12 h. The resulting reaction mixture was diluted with EtOAc (80 mL) and washed with H2O (3 × 30 mL). The combined organic layers were dried with sodium sulfate and concentrated. The resulting crude product was purified by flash column chromatography (eluent: 10–25% hexanes-EtOAc, silica gel) to afford the product 15 as an orange oil (0.296 g, 33%). 1H NMR (300 MHz, CDCl3) δ 7.35 (d, J = 8.4 Hz, 2 H), 6.95 (d, J = 16.2 Hz, 1 H), 6.83–6.78 (m, 3 H), 6.57 (t, J = 1.5 Hz, 1 H), 6.53 (t, J = 1.8 Hz, 1 H), 6.24 (t, J = 2.1 Hz, 1 H), 5.24 (s, 1 H), 0.98 (s, 18 H), 0.20 (s, 12 H); negative ESIMS m/z (rel intensity) 455 (100).
(E)-3-(tert-Butyldimethylsilyloxy)-5-(4-hydroxystyryl)phenol (16).43
Continuation of the column chromatography of 15 afforded 16 as an orange oil (0.113 g, 15%). 1H NMR (300 MHz, CDCl3) δ 7.37 (d, J = 8.4 Hz, 2 H), 6.95 (d, J = 16.2 Hz, 1 H), 6.83–6.78 (m, 3 H), 6.56 (t, J = 1.5 Hz, 1 H), 6.53 (t, J = 1.8 Hz, 1 H), 6.23 (t, J = 2.1 Hz, 1 H), 4.83 (s, 1 H), 4.71 (s, 1 H), 0.98 (s, 9 H), 0.20 (s, 6 H).
(E)-5-[4-(tert-Butyldimethylsilyloxy)styryl]benzene-1,3-diol (17).43
Continuation of the column chromatography of 16 afforded 17 as a clear oil (0.090 g, 12%). 1H NMR (300 MHz, CDCl3) δ 7.32 (d, J = 8.7 Hz, 2 H), 6.94 (d, J = 16.2 Hz, 1 H), 6.81–6.76 (m, 3 H), 6.52 (d, J = 2.1 Hz, 2 H), 6.26 (t, J = 2.1 Hz, 1 H), 6.02 (s, 2 H), 0.98 (s, 9 H), 0.20 (s, 6 H); negative ESIMS m/z (rel intensity) 341 (100).
NFκB Luciferase Assay
Human embryonic kidney cells 293 were used to monitor any changes occurring along the NFκB pathway. This cell line contains chromosomal integration of a luciferase reporter construct regulated by the NFκB response element. Transcription factors can bind to the response element when stimulated by certain agents, allowing transcription of the luciferase gene. Following an incubation period of 48 h with TNFα and test compounds, cells were analyzed for luciferase activity using the Luc assay system from Promega.49 Results were expressed as a percentage, relative to control (TNFα-treated) samples, and dose–response curves were constructed for the determination of IC50 values, which were generated from the results of five serial dilutions of test compounds and were the mean of two different experiments.
COX-1 and -2 Assays
COX-1 from sheep seminal vesicles and recombinant human COX-2 was purchased from Cayman Chemical, Ann Arbor, Michigan. The effect of test compounds on COX-1 and COX-2 was determined by measuring PGE2 production produced in the COX reaction via an enzyme immunoassay. The reaction was initiated by adding arachidonic acid (AA), the mixture incubated for 10 min at room temperature, and terminated with 27.8 μM indomethecin. PGE2 was quantitated by an ELISA method. Diluted samples of the reaction mixture were transferred to a 96-well plate (Nunc-Immuno Plate Maxisorp, Fisher Scientific, Pittsburgh, PA) coated with goat anti-mouse IgG (Jackson Immuno Research Laboratories, West Grove, PA). The tracer (PGE2-acetylcholinesterase, Cayman Chemical, Ann Arbor, MI) and primary antibody (mouse anti-PGE2, Monsanto, St. Louis, MO) were added. PGE2 was determined by the spectrophotometric method at 412 nm using Elman’s reagent. A standard curve with PGE2 (Cayman Chemical, Ann Arbor, MI) was generated on the same plate, which was used to quantify the PGE2 levels produced in the sample-treated wells. Results were expressed as a percentage, relative to control (solvent-treated) samples, and dose–response curves were generated for the determination of IC50 values.87
Measurement of Nitric Oxide (NO) Production in LPS-stimulated Macrophages
This assay was performed as previously described.88 Briefly, RAW 264.7 cells (1 × 105 cells/well) were incubated in 96-well culture plates for 24 h. The cells were treated with serially diluted compounds dissolved in phenol red-free DMEM for 30 min, followed by treatment with or without LPS (1 μg/mL) for an additional 20 h. NO is an unstable molecule and subsequently oxidized to a stable end product nitrite, therefore the amount of NO was estimated by the measurement of nitrite. After 20 h, nitrite released in the media was reacted with Griess reagent, and absorbance was measured at 540 nm. A standard curve was created by using known concentrations of sodium nitrite.
NO-Scavenging Activity
Compounds were diluted ten times with PBS and 20 μL of the diluted solution of each compound was incubated with 100 μL of 6 mM SNP in PBS for 3 h at room temperature. The final concentration of compounds and SNP were 60 μM and 5 mM, respectively. The Griess reaction was performed to estimate the amount of nitrite. Briefly, 180 μL of Griess reagent was added in each well and the absorbance was measured at 540 nm.89 The results are expressed as average of % inhibition of triplicate determinations ± standard deviation.
Western Blot Analysis
RAW 264.7 cells were pretreated with samples for 15 min before 1 μg/mL LPS for 18 h to examine the expression of iNOS protein. Cells were lysed with lysis buffer. Total protein (30 μg) in each cell lysate was resolved using 8% SDS-PAGE, and electrotransferred to PVDF membranes. The membranes were incubated with 5% skimmed milk in 0.1% Tween 20 containing TBS (TBST) for 1 h at room temperature. Then, membranes were incubated with corresponding primary antibodies in 3% skimmed milk in TBS for 1 h at 37 °C. After washing with TBST for 5 min, three times, membranes were incubated with horse radish peroxidase (HRP)-conjugated secondary antibodies for 1 h at 37 °C. Chemiluminescence (ECL) detection kit from Amersham Bioscience (Piscataway, NJ) was employed for the visualization according to the manufacturer’s instructions.
Cell Cycle Analysis
HL-60 cells (2 × 105 cells/well) were treated with samples for 24 h. The media was discarded and nuclear isolation medium 4′,6-diamidino-2-phenylindole (NIM-DAPI; Beckman Coulter) solution was added just before the measurement using Cell Lab Quanta™ SC (Beckman Coulter) flow cytometer. NIM-DAPI-stained cells were analyzed after excitation with UV light source. The distribution of cells in each phase of cell cycle was exhibited in a DNA histogram and percentage in subG1 was analyzed.
Cytotoxicity with Cultured Cells
The effect of compounds on cancer cell proliferation was evaluated using the sulforhodamine B (SRB) method.90 Briefly, KB or MCF7 cells were plated in 96-well plates containing samples and incubated at 37 °C in a humidified atmosphere with 5% CO2. After 72 h of incubation, cells were fixed with 10% trichloroacetic acid solution for 1 h and stained with 0.4% SRB in 1% acetic acid solution. Stained cells were suspended in 10 mM Tris buffer. The effect of compounds on cell viability was quantified by measuring absorbance at 515 nm.
Tandem Mass Spectrometry of Resveratrol 4′-Sulfate
Negative ion electrospray mass spectrometry was used for the analysis of resveratrol 4′-sulfate (7) using a high resolution Waters Synapt QqTOF mass spectrometer. The deprotonated molecules of resveratrol and resveratrol 4′-sulfate (7) were abundant at m/z 227 and m/z 307, respectively, and were used as precursor ions for product ion tandem mass spectrometry. Resveratrol 4′-sulfate anion eliminated SO3 to form a base peak of m/z 227 corresponding to resveratrol anion. Other abundant ions in the tandem mass spectra of resveratrol 4′-sulfate (7) and resveratrol were observed at m/z 185 and m/z 143. The transition of m/z 307 to m/z 227 was used during LC-MS-MS (using a Thermo Finnigan Quantum triple quadrupole mass spectrometer) with selected reaction monitoring (SRM) for the quantitative analysis of resveratrol 4′-sulfate (7). The quantitative analysis of resveratrol in these studies was carried using SRM of the transition m/z 227 to m/z 185 as described previously.31 Naringenin was used as an internal standard and measured by monitoring the SRM transition m/z 271 to m/z 151.
Stabilities of Resveratrol and Resveratrol 4′-Sulfate
The stabilities of resveratrol and resveratrol 4′-sulfate (7) were investigated for 24 h at 37 °C in RPMI 1640 (Invitrogen, Carlsbad, CA) cell culture medium which was used for the MCF-7 cellular uptake studies. These experiments were carried out three times. Resveratrol-4′-sulfate (7) was stable for 24 h (0 h, 100 ± 2.2%; 12 h, 95.1 ± 3.0%; 24 h, 98.8 ± 3.1%). However, resveratrol degraded ~50% during 24 h under these conditions (0 h, 100 ± 0.3%); 12 h, 71.4 ± 7.2%; 24 h, 46.8 ± 8.7%).
Cellular Uptake Studies of Resveratrol and Resveratrol 4′-Sulfate
MCF7 human breast cancer cells (4.5 × 105 cells/well in a 96-well plate) were incubated with 50 μM of resveratrol-4′-sulfate (7) at 37 °C for 24 h. The RPMI 1640 cell culture medium was removed, and the cells were rinsed three times with equal volumes of PBS. The cells in each well were harvested, treated with 120 μL lysis buffer for 30 s with mixing, and then sonicated for 5 s. Acetonitrile (370 μL) and 10 μL of naringenin (internal standard; 20 μM) were added, and the cell lysate was vortex mixed for 30 s. After centrifugation at 10,000 g at 4 °C for 15 min, the supernatant from each sample was removed, evaporated to dryness under a stream of nitrogen, and reconstituted in 100 μL methanol/water (1:4) for analysis using LC-MS-MS. MCF-7 cells treated with resveratrol 4′-sulfate contained no resveratrol 4′-sulfate, resveratrol 3,5-disulfate, or resveratrol after 24 h. A negative control experiment in which the cells were treated with only buffer also showed no resveratrol or resveratrol sulfates in the cells. MCF-7 cells treated with resveratrol (positive control) were found to contain resveratrol (54.7 ± 19.0 pmol/million cells) and resveratrol 3,5-disulfate (1028.0 ± 166.6 pmol/million cells) but no resveratrol 4′-sulfate.
Inhibition of Aromatase
A high-throughput enzyme assay was used to screen samples for aromatase inhibition.91 This assay employs dibenzylfuorescein as a substrate, and the level fluorescence due to the resultant fluorescein indicates the level of enzyme activity.
Determination of QR Activity in Cell Culture
Quinone reductase was assessed using Hepa 1c1c7 murine hepatoma cells as previously reported.81 Quinone reductase activity was measured as a function of the NADPH-dependent menadiol-mediated reduction of 3-(4,5-dimetylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to a blue formazan. Protein content was determined via crystal violet staining of identical plates. Specific activity is defined as nmol of formazan formed per mg protein per min. The induction ratio (IR) of QR activity represents the specific enzyme activity of agent-treated cells compared with a DMSO-treated control. The concentration to double activity (CD) was determined through a dose-response assay for active substances (IR >2).
Evaluation of Antioxidant Capacity
To evaluate antioxidant capacity, 1,1-diphenyl-2-picrylhydrazyl (DPPH) free-radical scavenging was performed according to the method of Lee et al.92 Briefly, 95 μL of DPPH radical solution (316 μM) was added in a 96-well plate containing 5 μL of each compound dissolved in 100% DMSO, and incubated for 30 min at 37 °C. The absorbance of each well was measured at 515 nm using a microplate reader. The DPPH radical scavenging activity of each sample was evaluated by calculating % of inhibition as follows: % inhibition = (1-Asample/Acontrol) × 100.
Acknowledgments
This work was supported by program project grant P01 CA48112 awarded by the National Cancer Institute.
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- DIPEA
diisopropylethylamine
- DMSO
dimethylsulfoxide
- DMEM
Dulbecco’s Modified Eagle’s Medium
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FTIR
Fourier transform infrared spectroscopy
- LPS
lipopolysaccharide
- QR1
quinone reductase-1
- SNP
sodium nitroprusside
- TBAF
tetra-n-butylammonium fluoride
- TBDMS
tert-butyldimethylsilyl
- TBDMSCl
tert-butyldimethylsilyl chloride
- TNF-α
tumor necrosis factor alpha
References
- 1.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CWW, Fong HHS, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Mezzuto JM. Cancer Chemopreventive Activity of Resveratrol, a Natural product Derived from Grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 2.Bhat KPL, Pezzuto JM. Cancer Chemopreventive Activity of Resveratrol. Ann NY Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 3.Pervaiz S. Resveratrol: from Grapevines to Mammalian Biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 4.Kensler T, Guyton K, Egner P, McCarthy T, Lesko S, Akman S. Role of Reactive Intermediates in Tumor Promotion and Progression. Prog Clin Biol Res. 1995;391:103–16. [PubMed] [Google Scholar]
- 5.Khanduja KL, Bhardwaj A, Kaushik G. Resveratrol Inhibits N-nitrosodiethylamine-induced Ornithine Decarboxylase and Cyclooxygenase in Mice. J Nutr Sci Vitaminol. 2004;50:61–65. [PubMed] [Google Scholar]
- 6.Kang SS, Cuendet M, Endringer DC, Croy VL, Pezzuto JM, Lipton MA. Synthesis and Biological Evaluation of a Library of Resveratrol Analogues as Inhibitors of COX-1, COX-2 and NFκB. Bioorg Med Chem. 2009;17:1044–1054. doi: 10.1016/j.bmc.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 7.Murias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, Szekeres T, Jager W. Resveratrol Analogues as Selective Cyclooxygenase-2 Inhibitors: Synthesis and Structure-Sctivity Relationship. Bioorg Med Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu S, Ishida S, Hara M, Takahashi N, Yoshimatsu H, Sakata T, Korthuis RJ. Resveratrol, a Red Wine Constituent Polyphenol, Prevents Superoxide-Dependent Inflammatory Responses Induced by Ischemia/Reperfusion, Platelet-Activating Factor, or Oxidants. Free Radic Biol Med. 2003;34:810–817. doi: 10.1016/s0891-5849(02)01430-2. [DOI] [PubMed] [Google Scholar]
- 9.Ray PS, Maulik G, Cordis GA, Bertelli AAE, Bertelli A, Das DK. The Red Wine Antioxidant Resveratrol Protects Isolated Rat Hearts from Ischemia Reperfusion Injury. Free Radic Biol Med. 1999;27:160–169. doi: 10.1016/s0891-5849(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang YJ, He F, Li XL. The Neuroprotection of Resveratrol in the Experimental Cerebral Ischemia. Zhonghua Yi Xue Za Zhi. 2003;83:534–536. [PubMed] [Google Scholar]
- 11.Lu KT, Ko MC, Chen BY, Huang JC, Hsieh CW, Lee MC, Chiou RYY, Wung BS, Peng CY, Yang YL. Neuroprotective Effects of Resveratrol on MPTP-Induced Neuron Loss Mediated by Free Radical Scaenging. J Agric Food Chem. 2008;56:6910–6913. doi: 10.1021/jf8007212. [DOI] [PubMed] [Google Scholar]
- 12.Bureau G, Longpre F, Martinoli MG. Resveratrol and Quercetin, Two Natural Polyphenols, Reduce Apoptotic Neuronal Cell Death Induced by Neuroinflammation. J Neurosci Res. 2008;86:403–410. doi: 10.1002/jnr.21503. [DOI] [PubMed] [Google Scholar]
- 13.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang HY, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small Molecule Activators of SIRT1 as Therapeutics for the Treatment of Type 2 Diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small Molecule Sctivators of Sirtuins Extend Saccharomyces cerevisiae Lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 15.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol Prolongs Lifespan and Retards the Onset of Age-Related Markers in a Short-Lived Vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Rayalam S, Yang J-Y, Ambati S, Della-Fera MA, Baile CA. Resverarol Induces Apoptosis and Inhibits Adipogenesis in 3T3-L1 Adipocytes. Phytother Res. 2008 doi: 10.1002/ptr.2503. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia GJ, Azuine MA, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, Nishino H. Chemopreventive Effect of Resveratrol, Sesamol, Sesame Oil and Sunflower Oil in the Epstein-Barr Virus Early Antigen Activation Assay and the Mouse Skin Two-Stage Carcinogenesis. Pharmacol Research. 2002;45:499–505. doi: 10.1006/phrs.2002.0992. [DOI] [PubMed] [Google Scholar]
- 18.Seya K, Kanemaru K, Sugimoto C, Suzuki M, Takeo T, Motomura S, Kitahara H, Niwa M, Oshima Y, Furukawa KI. Opposite Effects of Two Resveratrol (trans-3,5,4′ Trihydroxystilbene) Tetramers, Vitisin A and Hopeaphenol, on Apoptosis of Myocytes Isolated from Adult Rat Heart. J Pharmacol Exp Ther. 2009;328:90–98. doi: 10.1124/jpet.108.143172. [DOI] [PubMed] [Google Scholar]
- 19.Dudley JI, Lekli I, Mukherjee S, Das M, Bertelli AAA, Das DK. Does White Wine Qualify for French Paradox? Comparison of the Cardioprotective Effects of Red and White Wines and Their Constituents: Resveratrol, Tyrosol, and Hydroxytyrosol. J Agric Food Chem. 2008;56:9362–9373. doi: 10.1021/jf801791d. [DOI] [PubMed] [Google Scholar]
- 20.Fremont L. Minireview - Biological Effects of Resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- 21.Carbó N, Costelli P, Baccino FM, López-Soriano FJ, Argilés JM. Resveratrol, a Natural Product Present in Wine, Decreases Tumour Growth in a Rat Tumour Model. Biochem Biophys Res Commun. 1999;254:739–743. doi: 10.1006/bbrc.1998.9916. [DOI] [PubMed] [Google Scholar]
- 22.Roy P, Kalra N, Prasad S, George J, Shukla Y. Chemopreventive Potential of Resveratrol in Mouse Skin Tumors Through Regulation of Mitochondrial and PI3K/AKT Signaling Pathways. Pharm Res. 2009;26:211–217. doi: 10.1007/s11095-008-9723-z. [DOI] [PubMed] [Google Scholar]
- 23.Aziz MH, Kumar R, Ahmad N. Cancer Chemoprevention by Resveratrol: In vitro and In Vivo Studies and the Underlying Mechanisms (Review) Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- 24.Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-Induced Cellular Apoptosis and Cell Cycle Arrest in Neuroblastoma Cells and Antitumor Effects on Neuroblastoma in Mice. Surgery. 2004;136:57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Wenzel E, Soldo T, Erbersdobler H, Somoza V. Bioactivity and Metabolism of trans-Resveratrol Orally Administered to Wistar Rats. Mol Nutr Food Res. 2005;49:482–494. doi: 10.1002/mnfr.200500003. [DOI] [PubMed] [Google Scholar]
- 26.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of Resveratrol, a Natural product Present in Grapes and Wine, in the Human Liver. Xenobiotica. 2000;30:609–617. doi: 10.1080/004982500406435. [DOI] [PubMed] [Google Scholar]
- 27.De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulfation of Resveratrol, a Natural Compound Present in Wine, and Its Inhibition by Natural Flavonoids. Xenobiotica. 2000;30:857–866. doi: 10.1080/004982500433282. [DOI] [PubMed] [Google Scholar]
- 28.De Santi C, Pietrabissa A, Mosca F, Pacifici GM. Glucuronidation of Resveratrol, a Natural Product Present in Grape and Wine, in the Human Liver. Xenobiotica. 2000;30:1047–1054. doi: 10.1080/00498250010002487. [DOI] [PubMed] [Google Scholar]
- 29.Kuhnle G, Spencer JPE, Chowrimootoo G, Schroeter H, Debnam ES, Srai SKS, Rice-Evans C, Hahn U. Resveratrol Is Absorbed in the Small Intestine as Resveratrol Glucuronide. Biochem Biophys Res Commun. 2000;272:212–217. doi: 10.1006/bbrc.2000.2750. [DOI] [PubMed] [Google Scholar]
- 30.Dyke SF, Sainsbury M, Brown DW, Palfreyman MN, Wiggins DW. 1,2-Dihydroisoquinolines-XVI Indeno[1,2-c]isoquinoline Derivatives. Tetrahedron. 1971;27:281–289. [Google Scholar]
- 31.Yu CW, Shin YG, Chow A, Li YM, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, Rat, and Mouse Metabolism of Resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 32.Vitrac X, Desmouliere A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Merillon JM. Distribution of [C-14]-trans-Resveratrol, a Cancer Chemopreventive Polyphenol, in Mouse Tissues After Oral Administration. Life Sci. 2003;72:2219–2233. doi: 10.1016/s0024-3205(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 33.Meng XF, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and Plasma Levels of Resveratrol and Quercetin in Humans, Mice, and Rats After Ingestion of Pure Compounds and Grape Juice. J Agric Food Chem. 2004;52:935–942. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 34.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High Absorption but Very Low Bioavailability of Oral Resveratrol in Humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 35.Goldberg DA, Yan J, Soleas GJ. Absorption of Three Wine-Related Polyphenols in Three Different Matrices by Healthy Subjects. Clin Biochem. 2003;36:79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 36.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and Disposition of Resveratrol in Rats: Extent of Absorption, Glucuronidation, and Enterohepatic Recirculation Evidenced by a Linked-Rat Model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 37.Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. Suppression of N-Nitrosomethylbenzylamine (NMBA)-Induced Esophageal Tumorigenesis in F344 Rats by Resveratrol. Carcinogenesis. 2002;23:1531–1536. doi: 10.1093/carcin/23.9.1531. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-Dimethylbenz(a)anthracene-induced Mammary Carcinogenesis in Rats by Resveratrol: Role of NuclearFactor-kappa B, Cyclooxygenase 2, and Matrix Metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 39.Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol Depresses the Growth of Colorectal Aberrant Crypt Foci by Affecting bax and p21(CIP) Expression. Carcinogenesis. 2000;21:1619–1622. [PubMed] [Google Scholar]
- 40.Terao J. Dietary Flavonoids as Antioxidants in vivo: Conjugated Metabolites of (−)-Epicatechin, and Quercetin Participate in Antioxidative Defense on Blood Plasma. J Med Invest. 1999;46:159–168. [PubMed] [Google Scholar]
- 41.Moon J, Tsushida T, Nakahara K, Terao J. Identification of Quercetin 3-O-beta-D-Glucoronide as an Antioxidative Metabolite in Rat Plasma after Oral Administration of Quercetin. Free Rad Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 42.Koga T, Meydani M. Effect of Plasma Metabolites of (+)-Catechin and Quercetin on Monocyte Adhesion to Human Aortic Endothelial Cells. Am J Clin Nutr. 2001;73:941–948. doi: 10.1093/ajcn/73.5.941. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, Yu B, Schmidt RR. Synthesis of Mono- and Di-O-β-D-glucopyranoside Conjugaes of (E)-Resveratrol. Synthesis. 2006:1301–1306. [Google Scholar]
- 44.Orsini F, Pelizzoni F, Verotta L, Aburjai T, Rogers CB. Isolation, Synthesis, and Antiplatelet Aggregation Activity of Resveratrol 3-O-β-D-glucopyranoside and Related Compounds. J Nat Prod. 1997;60:1082–1087. doi: 10.1021/np970069t. [DOI] [PubMed] [Google Scholar]
- 45.Yu C, Shin YG, Kosmeder JW, Pezzuto JM, van Breemen RB. Liquid Chromatography/Tandem Mass Spectrometric Determination of Inhibition of Human Cytochrome P450 Isozymes by Resveratrol and Resveratrol-3-sulfate. Rapid Commun Mass Spectrom. 2003;17:307–313. doi: 10.1002/rcm.918. [DOI] [PubMed] [Google Scholar]
- 46.Farina A, Ferranti C, Marra C. An Improved Synthesis of Resveratrol. Nat Prod Res. 2006;20:247–252. doi: 10.1080/14786410500059532. [DOI] [PubMed] [Google Scholar]
- 47.Raghuraman A, Riaz M, Hindle M, Desai UR. Rapid and Efficient Microwave-Assisted Synthesis of Highly Sulfated Organic Scaffolds. Tetrahedron Lett. 2007;48:6754–6758. doi: 10.1016/j.tetlet.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pezzuto JM. Resveratrol as an Inhibitor of Carcinogenesis. Pharmaceutical Biology. 2008;46:443–573. [Google Scholar]
- 49.Homhual S, Bunyapraphatsara N, Kondratyuk T, Herunsalee A, Chaukul W, Pezzuto JM, Fong HHS, Zhang HJ. Bioactive Dammarane Triterpenes from the Mangrove Plant Bruguiera gymnorrhiza. J Nat Prod. 2006;69:421–424. doi: 10.1021/np058112x. [DOI] [PubMed] [Google Scholar]
- 50.Kauffman FC, Whittaker M, Anundi I, Thurman RG. Futile Cycling of a Sulfate Conjugate by Isolated Hepatocytes. Mol Pharmacol. 1991;39:414–420. [PubMed] [Google Scholar]
- 51.Nozawa T, Suzuki M, Yabuuchi H, Irokawa M, Tsuji A, Tamai I. Suppression of Cell Proliferation by Inhibition of Estrone-3-sulfate Transporter in Estrogen-Dependent Breast Cancer Cells. Pharm Res. 2005;22:1634–1641. doi: 10.1007/s11095-005-7096-0. [DOI] [PubMed] [Google Scholar]
- 52.Kwak JO, Kim HW, Oh KJ, Ko CB, Park H, Cha SH. Characterization of Mouse Rrganic Anion Transporter 5 As a Renal Steroid Sulfate Transporter. Journal of Steroid Biochemistry and Molecular Biology. 2005;97:369–375. doi: 10.1016/j.jsbmb.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Mizuno N, Takahashi T, Iwase Y, Kusuhara H, Niwa T, Sugiyama Y. Human Organic Anion Transporters 1 (hOAT1/SLC22A6) and 3 (hOAT3/SLC22A8) Transport Edaravone (MCI-186; 3-Methyl-1-phenyl-2-pyrazolin-5-one) and Its Sulfate Conjugate. Drug Metab Dispos. 2007;35:1429–1434. doi: 10.1124/dmd.106.013912. [DOI] [PubMed] [Google Scholar]
- 54.Pezzuto JM, Kosmeder J, Park EJ, Lee SK, Cuendet M, Gills JJ, Bhat K, Grubjesic S, Park HS, Mata-Greenwood E, Tan Y, Yu R, Lantvit DD, Kinghorn AD. Characterization of Natural Product Chemopreventive Agents. Vol. 2. Humana Press, Inc; Totowa, NJ: 2005. pp. 3–37. [Google Scholar]
- 55.Waffo-Teguo P, Hawthorne ME, Cuendet M, Merillon JM, Kinghorn AD, Pezzuto JM, Mehta RG. Potential Cancer-Chemopreventive Activities of Wine Stilbenoids and Flavans Extracted from Grape (Vitis vinifera) Cell Cultures. Nutr Cancer. 2001;40:173–179. doi: 10.1207/S15327914NC402_14. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, GoodTargets for Chemoprevention of Colon Cancer. Biofactors. 2000;12:129–133. doi: 10.1002/biof.5520120120. [DOI] [PubMed] [Google Scholar]
- 57.Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ. Inhibition of Cyclooxygenase: A Novel Approach to Cancer Prevention. Proc Soc Exp Biol Med. 1997;216:201–210. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- 58.Kubatka P, Ahlers I, Ahlersova E, Adamekova E, Luk P, Bojkova B, Markova M. Chemoprevention of Mammary Carcinogenesis in Female Rats by Rofecoxib. Cancer Lett. 2003;202:131–136. doi: 10.1016/j.canlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Nelson JE, Harris RE. Inverse Association of Prostate Cancer and Non-steroidal Anti-inflammatory Dugs (NSAIDs): Results of a Case-control Study. Oncol Rep. 2000;7:169–170. doi: 10.3892/or.7.1.169. [DOI] [PubMed] [Google Scholar]
- 60.Nakatsugi S, Ohta T, Kawamori T, Mutoh M, Tanigawa T, Watanabe K, Sugie S, Sugimura T, Wakabayashi K. Chemoprevention by Nimesulide, a Selective Cyclooxygenase-2 Inhibitor, of 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-Induced Mammary Gland cCarcinogenesis in Rats. Jpn J Cancer Res. 2000;91:886–892. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the Expression of Inducible Nitric Oxide Synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 62.Jasnis MA, Giri J, Davel L. Nitric Oxide Is Involved in Stimulation of Tumor Growth. Oncol Rep. 1997;4:1107–1111. doi: 10.3892/or.4.5.1107. [DOI] [PubMed] [Google Scholar]
- 63.Wadsworth TL, Koop DR. Effects of the Wine Polyphenolics Quercetin and Resveratrol on Pro-inflammatory Cytokine Expression in RAW 264.7 Macrophages. Biochem Pharmacol. 1999;57:941–949. doi: 10.1016/s0006-2952(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 64.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of Nitric Oxide Synthase and the Down-regulation of the Activation of NF Kappa B in Macrophages by Resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan MMY, Ho CT, Huang HI. Effects of 3 Dietary Phytochemicals from Tea, Rosemary and Turmeric on Inflammation-Induced Nitrite Production. Cancer Letters. 1995;96:23–29. doi: 10.1016/0304-3835(95)03913-h. [DOI] [PubMed] [Google Scholar]
- 66.Kolodziej H, Radtke OA, Kiderlen AF. Stimulus (Polyphenol, IFN-gamma, LPS)-Dependent Nitric Oxide Production and Antileishmanial Effects in RAW 264.7 Macrophages. Phytochemistry. 2008;69:3103–3110. doi: 10.1016/j.phytochem.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal BB, Shishodia S. Molecular Targets of Dietary Agents for Prevention and Therapy of Cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 68.Song SH, Min HY, Han AR, Nam JW, Seo EK, Park SW, Lee SH, Lee SK. Suppression of Inducible Nitric Oxide Synthase by (−)-Isoeleutherin from the Bulbs of Eleutherine americana through the Regulation of NF-kappa B Activity. Int Immunopharmacol. 2009;9:298–302. doi: 10.1016/j.intimp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Djoko B, Chiou RYY, Shee JJ, Liu YW. Characterization of Immunological Activities of Peanut Stilbenoids, Arachidin-1, Piceatannol, and Resveratrol on Lipopolysaccharide-induced Inflammation of RAW 264.7 Macrophages. J Agric Food Chem. 2007;55:2376–2383. doi: 10.1021/jf062741a. [DOI] [PubMed] [Google Scholar]
- 70.Whyte L, Huang YY, Torres K, Mehta RG. Molecular Mechanisms of Resveratrol Action in Lung Cancer Cells Using Dual Protein and Microarray analyses. Cancer Res. 2007;67:12007–12017. doi: 10.1158/0008-5472.CAN-07-2464. [DOI] [PubMed] [Google Scholar]
- 71.Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol Induces Colon Tumor Cell Apoptosis Independently of p53 and Preceded by Epithelial Differentiation, Mitochondrial Proliferation and Membrane Potential Collapse. Int J Cancer. 2001;94:615–622. doi: 10.1002/ijc.1516. [DOI] [PubMed] [Google Scholar]
- 72.Aziz MH, Nihal M, Fu VX, Jarrard DF, Ahmad N. Resveratrol-Caused Apoptosis of Human Prostate Carcinoma LNCaP Cells is Mediated via Modulation of Phosphatidylinositol 3′-Kinase/Akt Pathway and Bcl-2 Family Proteins. Mol Cancer Ther. 2006;5:1335–1341. doi: 10.1158/1535-7163.MCT-05-0526. [DOI] [PubMed] [Google Scholar]
- 73.Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol Induces Growth Inhibition and Apoptosis in Metastatic Breast Cancer Cells via De Novo Ceramide Signaling. FASEB J. 2003;17:2339. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- 74.Puissant A, Grosso S, Jacquel A, Belhacene N, Colosetti P, Cassuto JP, Auberger P. Imatinib Mesylate-resistant Human Chronic Myelogenous Leukemia Cell Lines Exhibit High Sensitivity to the Phytoalexin Resveratrol. FASEB J. 2008;22:1894–1904. doi: 10.1096/fj.07-101394. [DOI] [PubMed] [Google Scholar]
- 75.Murray R. Role of Anti-aromatase Agents in Postmenopausal Advanced Breast Cancer. Cancer Chemother Pharmacol. 2001;48:259–265. doi: 10.1007/s002800100345. [DOI] [PubMed] [Google Scholar]
- 76.Ghosh S, Choudary A, Musi N, Hu YF, Li R. IKK beta Mediates Cell Shape-Induced Aromatase Expression and Estrogen Biosynthesis in Adipose Stromal Cells. Molecular Endocrinology. 2009;23:662–670. doi: 10.1210/me.2008-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 Recruits Neh2 through Binding to ETGE and DLG Motifs: Characterization of the Two-site Molecular Recognition Model. Mol Cell Biol. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang YS. Chemoprotection against Cancer by Phase 2 Enzyme Induction. Toxicol Lett. 1995;82–3:173–179. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 79.Nho CW, Jeffery E. The Synergistic Upregulation of Phase II Detoxification Enzymes by Glucosinolate Breakdown Products in Cruciferous Vegetables. Toxicol Appl Pharmacol. 2001;174:146–152. doi: 10.1006/taap.2001.9207. [DOI] [PubMed] [Google Scholar]
- 80.Kennelly EJ, Gerhauser C, Song LL, Graham JG, Beecher CWW, Pezzuto JM, Kinghorn AD. Induction of Quinone Reductase by Withanolides Isolated from Physalis philadelphica (tomatillos) J Agric Food Chem. 1997;45:3771–3777. [Google Scholar]
- 81.Song LL, Kosmeder JW, II, Lee SK, Gerhäuser C, Lantvit D, Moon RC, Moriarty RM, Pezzuto JM. Cancer Chemopreventive Activity Mediated by 4′-Bromoflavone, a Potent Inducer of Phase II Detoxication Enzymes. Cancer Res. 1999;59:578–585. [PubMed] [Google Scholar]
- 82.Prochaska HJ, Santamaria AB. Direct Measurement of Nad(P)H - Quinone Reductase from Cells Cultured in Microtiter Wells - a Screening Assay for Anticarcinogenic Enzyme Inducers. Anal Biochem. 1988;169:328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- 83.Qian YP, Cai YJ, Fan GJ, Wei QY, Yang J, Zheng LF, Li XZ, Fang JG, Zhou B. Antioxidant-Based Lead Discovery for Cancer Chemoprevention: The Case of Resveratrol. J Med Chem. 2009;52:1963–1974. doi: 10.1021/jm8015415. [DOI] [PubMed] [Google Scholar]
- 84.Saiko P, Szakmary A, Jaeger W, Szekeres T. Resveratrol and Its Analogs: Defense Against Cancer, Coronary Disease and Neurodegenerative Maladies or Just a Fad? Mutat Res. 2008;658:68–94. doi: 10.1016/j.mrrev.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 85.de la Lastra CA, Villegas I. Resveratrol as Antioxidant and Pro-oxidant Agent: Mechanism and Clinical implications. Biochem Soc Trans. 2007;35:1156–1160. doi: 10.1042/BST0351156. [DOI] [PubMed] [Google Scholar]
- 86.Kundu JK, Surh YJ. Cancer Chemopreventive and Therapeutic Potential of Resveratrol: Mechanistic Perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 87.Cuendet M, Mesecar AD, DeWitt DL, Pezzuto JM. An ELISA Method to Measure Inhibition of the COX Enzymes. Nat Protocol. 2006;1:1915–1921. doi: 10.1038/nprot.2006.308. [DOI] [PubMed] [Google Scholar]
- 88.Asolkar RN, Freel KC, Jensen PR, Fenical W, Kondratyuk TP, Park EJ, Pezzuto JM. Arenamides A-C, Cytotoxic NF kappa B Inhibitors from the Marine Actinomycete Salinispora arenicola. J Nat Prod. 2009;72:396–402. doi: 10.1021/np800617a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsai PJ, Tsai TH, Yu CH, Ho SC. Comparison of NO-Scavenging and NO-Suppressing Activities of Different Herbal Teas with Those of Green Tea. Food Chem. 2007;103:181–187. [Google Scholar]
- 90.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 91.Lee D, Bhat KPL, Fong HHS, Farnsworth NR, Pezzuto JM, Kinghorn AD. Aromatase Inhibitors from Broussonetia papyrifera. J Nat Prod. 2001;64:1286–1293. doi: 10.1021/np010288l. [DOI] [PubMed] [Google Scholar]
- 92.Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Gamez EJC, Mehta RG, Kinghorn AD, Pezzuto JM. Evaluation of the Antioxidant Potential of Natural Products. Comb Chem High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]