Summary
Dietary restriction (DR) has been shown to robustly extend lifespan in multiple species tested so far. The pro-longevity effect of DR is often ascribed to an increase in cellular defense against somatic damage, most notably damage by reactive oxygen species (ROS), considered a major cause of aging. Especially irreversible damage to DNA, the carrier of genetic information, is considered a critical causal factor in aging. Using a recently developed transgenic Drosophila melanogaster model system harboring a lacZ-plasmid construct that can be recovered in E. coli, we measured spontaneous DNA mutation frequency in flies under DR and ad libitum conditions. Three different DR conditions, imposed by manipulating levels of different types of yeast sources, were tested in females and males of two lacZ reporter gene lines. Feeding with the ROS producer paraquat at 1 mM resulted in a rapid accumulation of somatic mutations, indicating that the frequency of mutations at the lacZ locus is a reliable marker for increased oxidative stress. However, none of the DR conditions altered the accumulation of spontaneous mutations with age. These results suggest that the beneficial effects of DR are unlikely to be linked to protection against oxidative somatic DNA damage.
Keywords: dietary restriction, Drosophila melanogaster, aging, mutation accumulation, lacZ reporter locus
Introduction
Dietary restriction (DR) has been shown to extend lifespan in species as diverse as yeast, nematodes and insects. In mice and rats DR has been demonstrated to not only increase life span but to also slow the onset of age-related diseases (Masoro 2005). Little is known about the mechanisms that underlie the DR response and their conservation between species. One possible mechanism of action proposed to explain the beneficial action by DR is a reduction of damage to macromolecules, for example by decreasing the production of reactive oxygen species (ROS) or by increasing the repair of oxidative damage (Barja 2002). Upregulating somatic maintenance at the cost of growth and reproduction may be part of an adaptive strategy during periods of food scarcity in the wild when reproduction might be costly and the chances of offspring surviving low (Harrison & Archer 1989; Holliday 1989).
Accumulation of unrepaired somatic damage has been proposed as a major cause of aging (Kirkwood 2005). DNA has been implicated as a major target for age-associated somatic damage accumulation because of its central role as the repository of genetic information and the frequently found association between defects in DNA repair and premature aging (Vijg 2007). Spontaneous DNA damage is induced with high frequency, but its steady state level is low due to efficient mechanisms for repair. However, errors during repair or replication of a damaged template lead to irreversible alterations in DNA sequence information and it has now been conclusively demonstrated that DNA mutations as well as epimutations accumulate with age in humans and mice in most tissues and cell types (Jones et al. 1995; Ramsey et al. 1995; Dollé et al. 2000; Dollé et al. 2002; Issa 2003). Using a transgenic mouse model harboring a lacZ-plasmid construct allowing accurate quantitation of spontaneous DNA mutation frequencies and spectra we have recently shown that the increased life span of Ames dwarf mice – a DR-like hormonal deficiency in growth hormone, thyroid stimulating hormone and prolactin, showing reduced levels of plasma insulin, IGF-1 and glucose (Bartke 2005) – is associated with reduced spontaneous mutation frequencies in different tissues (Garcia et al. 2008). A somewhat smaller reduction of spontaneous mutations was observed after DR. Earlier reports indicated reduced mutation frequencies under DR conditions in both mice and rats, measured as inactivation events of the X-linked Hprt gene in white blood cells (Dempsey et al. 1993; Aidoo et al. 2003).
Previously, we generated a lacZ-plasmid mutational reporter model for Drosophila melanogaster, an invertebrate model for aging research that lives much shorter than mice (Garcia et al. 2007a). Like in the mouse, somatic mutations at the lacZ locus accumulate during aging, and they do so at a higher rate at higher temperatures (Garcia et al., manuscript in preparation). Dietary restriction has been reported to extend lifespan in Drosophila by as much as 40% with strong dependence on background (Kapahi et al. 2004; Pletcher et al. 2005). Here we show that DR in Drosophila extends life span in three different dietary compositions, but does not result in reduced accumulation of somatic mutations with age. Since mutations were easily induced by paraquat, a powerful ROS producer, these results fail to support the hypothesis that DR in flies works by reducing ROS-induced damage accumulation in DNA.
Results
The generation of transgenic D. melanogaster lines carrying pUR288-S, a lacZ reporter plasmid, which can be recovered from the genome and subsequently analyzed for mutations in E. coli, has been described previously (Garcia et al. 2007a). The principles of mutation analysis in this model system, with the fly lines used, are outlined in Fig. 1 and described in detail elsewhere (Garcia et al. 2007b). Briefly, plasmids are first excised from genomic DNA using the restriction enzyme Hind III. Plasmids are then separated from the bulk of the genomic DNA by magnetic separation. Purified plasmids are then used to transform E. coli cells lacking a lacZ gene and harboring an inactivated galE gene. The latter prevents cells with intact β-galactosidase activity from growing on medium containing phenyl β-d-galactoside (p-gal). The mutation frequency is the ratio of colonies growing on the selective plate versus the total number of recovered plasmids from the DNA sample (as measured on the titer plate). Hence, mutation frequencies as determined with this system reflect a ratio and do not depend on the amount of DNA. They are expressed on a per locus basis as the number of mutant lacZ copies for a given number of lacZ copies isolated from the in vivo situation. Using this system a broad range of mutations inactivating the lacZ gene is detectable, but not everything. Most notably, copy number changes (loss of heterozygosity) and mutations that do not inactivate β-galactosidase activity to a sufficiently low level escape detection. Hence, all values reported are underestimates.
Fig. 1.
Schematic depiction of the lacZ-plasmid construct as it is integrated at loci on Drosophila chromosomes 2L and 3R. LacZ-plasmids were excised at HindIII sites, separated from the fly chromosomal DNA, circularized by ligation and used to transform E. coli ΔlacZ, galE− as described (Garcia et al. 2007a). LacZ mutants are positively selected on p-gal (red colonies) and the titer determined by plating a small fraction of the transformants on X-gal (blue colonies).
We chose lines 2 and 11 with P-element insertion sites on chromosomes 2 and 3, respectively, for our analysis. Since we demonstrated that age-related mutation accumulation at the lacZ locus in flies is much greater at elevated temperatures (Garcia et al., in preparation), we decided to conduct all experiments at 29°C. This greatly increased the power to detect a putative reduction in spontaneous mutation frequency due to DR. At this temperature maximum lifespan did not exceed 50 days for either sex. To guarantee sufficient DNA for obtaining at least several hundreds of thousands of plasmid copies rescued, we pooled 50 flies for each determination point. Mutation frequency results are always the average of at least three groups of 50 flies, indicating the experimental variation over the entire procedure, i.e., from collecting the groups of flies to DNA extraction and mutation frequency determination.
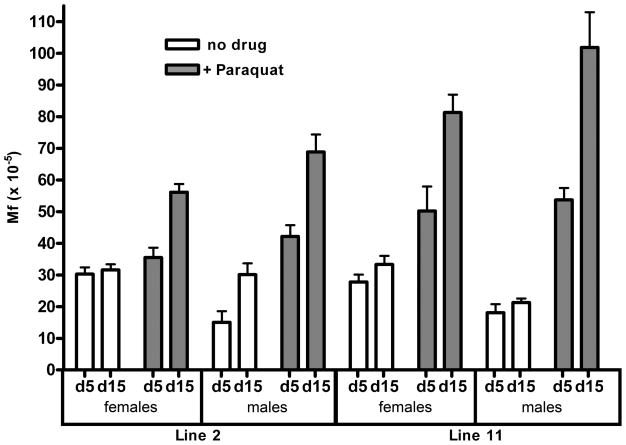
As mentioned in the introduction, alleviation of oxidative stress is often considered as a possible mechanistic basis for the beneficial effects of DR. We therefore first tested whether paraquat, a widely used herbicide that produces free radicals in cells, is able to elevate spontaneous mutation frequencies in the reporter fly model. As predicted, exposure to 1 mM paraquat led to a severe reduction in lifespan (Fig. 2A; Table 1) and a rapid rise in somatic mutation frequency (Fig. 2B) at 29 °C. In this experiment, both the life span effects and the elevation in somatic mutation frequencies were somewhat more severe in males than in females, which could be due to a sex difference in paraquat metabolism. It is noteworthy that line 2 (especially females) was significantly more resistant to paraquat, which correlated with relatively low levels of mutation induction. These results indicate that a strong oxidant easily elevates the levels of somatic mutations in flies as a function of time. Of note, albeit with overall lower mutation frequencies, similar results were obtained at 25 °C (Garcia et al., manuscript in preparation).
Fig. 2.
(A) Treatment of lines 2 and 11 with 1 mM paraquat resulted in a significant reduction of lifespan in both lines (p<0.0001). Males show a greater sensitivity to paraquat. Line 2 females exhibit marked resistance. (B) Treatment with paraquat resulted in an increase in mutation frequency at the lacZ transgene locus (p<0.0001). Mutation frequencies are lower in males than females but elevated more after paraquat exposure, which correlates positively with effects on lifespan.
Table 1.
| Line | line 2 | Line 11 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | M | ||||
| Paraquat | − | + | − | + | − | + | − | + |
| # deaths | 401 | 388 | 374 | 404 | 395 | 400 | 395 | 401 |
| med. LS | 38 | 30 | 32 | 14 | 38 | 18 | 32 | 10 |
| max. LS | 46 | 38 | 40 | 30 | 46 | 38 | 40 | 30 |
Various methods for DR have been utilized to extend lifespan in Drosophila. The diversity of diets in use by different laboratories and the potential interference by uncharacterized effects of genetic background and nonaxenic conditions complicate comparative analysis of DR. After testing several different diets we chose to vary only the yeast component of three published DR diets (Carvalho et al. 2005; Bass et al. 2007) because of the stronger effect of yeast reduction per calorie intake as opposed to sugar (Chippindale et al. 1993; Kapahi et al. 2004; Mair et al. 2005; Skorupa et al. 2008). These diets differed in yeast source, yeast concentration, and presence of cornmeal but kept glucose concentration constant (Table 2).
Table 2.
Dietary compositions.
| Units | Regular | Yeast Extract |
Brewer’s Yeast |
Whole Yeast |
||||
|---|---|---|---|---|---|---|---|---|
| 0.25% | 4% | 2% | 8% | 10% | 10% | |||
| Sugar | g/L | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Corn Meal | g/L | 85 | 85 | 85 | 85 | 85 | 0 | 0 |
| Bacto Agar | g/L | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 | 10 | 10 |
| YE | g/L | 0 | 2.5 | 40 | 0 | 0 | 0 | 0 |
| BY | g/L | 0 | 0 | 0 | 20 | 80 | 0 | 0 |
| WY | g/L | 15.6 | 0 | 0 | 0 | 0 | 100 | 200 |
| Acid Mix* | ml/L | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
Sugar - C&H Pure Cane Granulated Sugar Corn Meal - Honeyville Yellow Corn Meal Bacto Agar - Moorhead and Company Agar 700 Yeast Extract (YE) - BD Bacto Yeast Extract Brewer’s YEast (BY) - MP Biomedicals Brewer’s Yeast Whole Yeast (WY) - Saf-Instant Yeast
836 ml propionic acid + 83ml phosphoric acid/2L H20
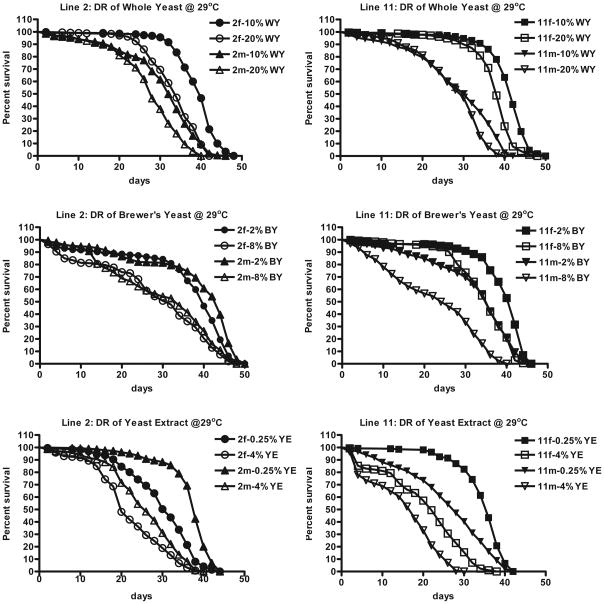
Each diet resulted in significantly different sex and line-specific responses (Fig. 3; Table 3). Use of yeast extract shortened lifespan overall compared to whole yeast or Brewer’s yeast, which may be due to limiting essential nutrients (Bass et al. 2007). Four-fold reduction of Brewer’s yeast yielded lifespan extension in the range expected based on results of Bass et al. (Bass et al. 2007), but we did not observe as large a difference in fecundity as found by these authors (results not shown). The latter could be due to the presence of cornmeal in our diet. Reduction of whole yeast from 20% to 10% elicited an optimally shaped survival curve only in females of both lines. However, median lifespan extension did not exceed 18%, significantly less than that observed by Bass et al. at the same concentrations (Bass et al. 2007) or Mair et al., at 6.5 versus 15% yeast in the presence of higher sucrose (Mair et al. 2005). Males of line 2 showed a minor extension but males of line 11 did not show a response to whole yeast DR. In general, our results are in agreement with published DR results and show increased survival associated with DR under different conditions at 29 °C. Of note, we also tested 0.25 versus 4% yeast extract at 25 °C, which also resulted in a significant increase in life span under the former condition (results not shown). As expected the elevated temperature severely shortened both median and maximum lifespan.
Fig. 3.
Effects of DR on life span at 29 °C using reduction of whole yeast, Brewer’s yeast or yeast extract. Median lifespans were significantly increased under DR conditions in all cases (p<0.0001) except for males of line 11 on whole yeast.
Table 3.
Survival characteristics under different DR conditions.
| Line | line 2 |
Line 11 |
||||||
|---|---|---|---|---|---|---|---|---|
| Sex | F |
M |
F |
M |
||||
| Diet | 10% WY | 20% WY | 10% WY | 20% WY | 10% WY | 20% WY | 10% WY | 20% WY |
| # flies | 404 | 409 | 375 | 397 | 380 | 379 | 345 | 344 |
| med. LS | 40 | 34 | 32 | 28 | 42 | 38 | 30 | 30 |
| max. LS | 48 | 42 | 46 | 40 | 50 | 48 | 42 | 40 |
| Line | line 2 |
Line 11 |
||||||
|---|---|---|---|---|---|---|---|---|
| Sex | F |
M |
F |
M |
||||
| Diet | 2% BY | 8% BY | 2% BY | 8% BY | 2% BY | 8% BY | 2% BY | 8% BY |
| # flies | 398 | 386 | 402 | 391 | 389 | 393 | 396 | 394 |
| med. LS | 40 | 32 | 44 | 34 | 42 | 36 | 36 | 25 |
| max. LS | 50 | 48 | 50 | 48 | 46 | 46 | 46 | 40 |
| Line | line 2 |
Line 11 |
||||||
|---|---|---|---|---|---|---|---|---|
| Sex | F |
M |
F |
M |
||||
| Diet | 0.25% YE | 4% YE | 0.25% YE | 4% YE | 0.25% YE | 4% YE | 0.25% YE | 4% YE |
| # flies | 392 | 385 | 390 | 389 | 399 | 392 | 392 | 397 |
| med. LS | 32 | 20 | 38 | 26 | 36 | 22 | 28 | 18 |
| max. LS | 44 | 40 | 44 | 40 | 42 | 38 | 42 | 30 |
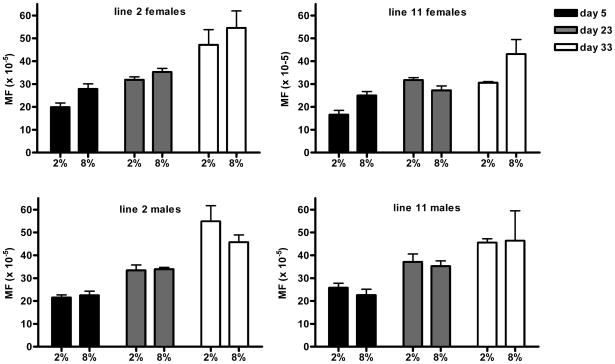
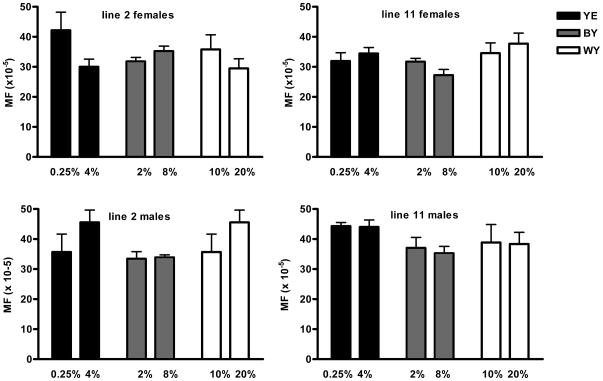
Next, we determined mutation frequencies at 2, 20 and 30 days after DR initiation on Brewer’s yeast (Fig. 4A). While an increase in the spontaneous mutation frequency was observed as a function of age (p<0.0001), there was no significant difference between 8% and 2% Brewer’s yeast. We then compared spontaneous mutation frequencies in males and females of each line after 20 days of DR for the three different diets, i.e., whole yeast, Brewer’s yeast and yeast extract, at 29°C. Since median lifespan ranges from 18 to 42 days we assumed that any significant effect of DR on mutation frequencies should be measurable after 20 days. However, in spite of the large differences in median lifespan, significant effects of DR on spontaneous mutation frequency at the lacZ reporter locus were not observed (Fig. 4B). To rule out the possibility that the lack of a DR effect was in some way related to the higher temperature, we also studied lacZ mutation at 25 °C. At that temperature, mutation do accumulate with age, albeit much slower than at the higher temperature (Garcia et al., in preparation). However, also at 25 °C there was no difference in spontaneous mutation frequency at the lacZ locus between flies at 0.25% versus 4% yeast extract (results not shown).
Fig. 4.
(A) Mutation frequencies after 2, 20, and 30 day adult onset (day 3) DR on Brewer’s yeast at 29°C; the only significant effect on mutation frequency was time. Two-way Anova of mutation frequencies in females and males of line 2 on Brewer’s yeast indicated that time accounts for 66.45% (p<0.0001; Df=2) and 67.87% (p<0.0001; Df=2) of total variation, respectively. Diet in females accounts for 6.14 % of variation (p=0.048; Df =1) and 0.99% of variation in males (not significant; Df=1). For line 11 time accounts for 42.7% (p=0.0022; Df =2) and 49.86% (p=0.0026; Df=2) of total variation, in females and males, respectively. In this line, diet accounts for 7.64% (not significant; Df=1) and 0.34% (not significant; Df=1) in females and males, respectively. (B) Mutation frequencies after 20 day DR on yeast extract, Brewer’s yeast or whole yeast at 29°C. No significant differences in mutation frequency were observed between DR and high-yeast diets.
Discussion
The beneficial effects of dietary restriction, a sheer universal mechanism to extend life span across species, are often thought based on conserved physiological and molecular mechanisms. In this respect, reduced insulin/insulin-like growth factor (IGF) signaling has been implicated as a likely trigger for the reduction of dietary nutrients to extend lifespan. However, it is uncertain if this is true for flies (Min et al. 2008; Giannakou et al. 2008). There is some evidence that dampened IGF signaling activates downstream pathways that suppress the random accumulation of DNA damage (Garinis et al. 2008). We recently demonstrated that Ames dwarfism, a hormonal defect leading to low IGF signaling, and DR both reduce spontaneous DNA mutations in the mouse (Garcia et al. 2008). Our present findings do not indicate such a protective effect of DR in Drosophila melanogaster, in spite of the fact that three different DR protocols were used, based on the optimization of yeast-reducing DR protocols by Bass et al (Bass et al. 2007). While the expected life span effects were readily observed, none of these protocols indicated a reduction in spontaneous mutation frequency.
Of note, while in these present studies we used whole flies, all our previous work on mice was done on specific organs and tissues, showing remarkable differences in both mutation frequencies and spectra (Dollé et al. 2000). Somewhat surprisingly, these results did not reveal the expected correlation between mitotic activity of a tissue and the spontaneous mutation frequency. High mitotic activity is expected to result in increased mutations due to errors in DNA replication. While we did find the highest spontaneous mutation accumulation rate in intestinal tissue, both spleen and testes showed low rates of mutation accumulation and significant mutation accumulation (higher than spleen) was shown in heart and liver. Indeed, more recently we demonstrated that while point mutations are dependent on errors made during DNA replication, large genome rearrangement mutations are not (Busuttil et al. 2007). Such mutations are frequent in both liver and heart, but much less in the intestine. Interestingly, also in whole flies almost all spontaneous mutations are genome rearrangements (Garcia et al. 2007a). This dominance of genome rearrangements in the spectrum of spontaneous mutations did not change with age (Garcia et al., in preparation) and was also not influenced by DR (results not shown). Since the fraction of point mutations, on average, is far greater in mice than in flies, it is formally possible that the contrasting results in mice and flies are due to this difference in mutation spectrum. However, we consider this unlikely since when DR would act by reducing ROS, its effects are not likely to select between different types of mutations. Indeed, ROS can induce both point mutations and genome rearrangements.
In flies the need for a sufficient amount of input DNA to accurately determine spontaneous mutation frequency essentially constrained us from separating individual tissues as in the mouse. However, we have studied different body parts, i.e., head, thorax and abdomen. Somewhat surprisingly, most mutations accumulating with age in the fly are found in the thorax with the lowest rate of accumulation in the abdomen (Garcia et al., unpublished). While in these present studies we only analyzed whole flies, it should be noted that in our previous work all three mouse tissues tested showed an effect of either Ames dwarfism or DR or both (Garcia et al. 2008). Therefore, while we cannot exclude DR effects on mutation frequency in one or few selected cell types, we are confident that our current findings are not due to a lack of power to detect changes in spontaneous mutation frequency that may not occur in all cell types.
The lack of a reduction in spontaneous DNA mutations in flies subjected to DR is surprising in light of the large number of variables affected by reduced food intake in rodents (Masoro 2005). However, DR studies in invertebrates are much more complicated than in rodents. For example, intermittent feeding is the practice in DR in rodents but does not work in flies (Piper and Partridge 2007). This is why we chose dietary yeast restriction. Hence, we cannot rule out the possibility that reductions of different food components all increase life span but do so through different mechanisms. Reducing yeast may extend life span but not through dampening genotoxic stress.
Another, interesting interpretation of our present results involves current explanations as to how reduced nutrient intake extends life span. As mentioned above, it is often assumed that DR works in a similar fashion as reduced IGF signaling, i.e., by intervening at the level of damage accumulation, through a reduction in ROS or an upregulation of cellular defense. However, it has been demonstrated that in flies DR whenever initiated immediately reduces mortality risk (Mair et al. 2003). This suggests that DR in Drosophila extends life span without changing the rate of somatic damage accumulation. Moreover, DR in Drosophila does not change the activity of CuZn SOD or Mn SOD, while SOD activity was elevated in chico, a Drosophila mutant affecting the insulin receptor substrate associated with life span extension (Kabil et al. 2007). Hence, it is possible that unlike the situation in mice, DR in flies is not based on a reduced rate of damage accumulation.
Experimental procedures
Fly Stocks
All experiments were performed with two lacZ reporter P-element insertion lines, lines 2 and 11 described elsewhere (Garcia et al. 2007a). The lines were maintained in vials on a 12-hour light/dark cycle at 25°C and 65% humidity and expanded in bottles by synchronized 24 h egg lay. The original reporter lines were outcrossed 5 times with a w1118 line originally obtained from Caltech, followed by two homozygosity crosses. Passage over dextrose containing food supplemented with gentamycin and doxycyclin was performed before expansion to ensure removal of high mucus producing bacterial load present in the original lines.
Media
Fly media were prepared by combining ingredients listed (Table 2) in a final volume of 1 liter dH2O. The fly food was boiled for 10 min under continuous stirring and allowed to cool to ~50°C. After addition of propionic/phosphoric acid, fungicidal mix, food was poured in bottles or vials (80 ml per 8 oz polypropylene fly bottles, 5–10 ml per narrow polystyrene vial; Genesee). For sugar, a concentration of 5% was chosen because at this concentration lifespan and fecundity were optimal for females and lower concentrations negatively affected male lifespan at low yeast concentrations according to our initial diet screen. Agar concentrations were at 4.6 g/l in media containing cornmeal (Brewer’s yeast and yeast extract) and at 10 g/l in whole yeast diets without cornmeal, which optimally prevented dehydration of the food at 29°C. Diet media were prepared freshly every 4 days and stored at RT. Live yeast was sprinkled on regular diet immediately before use.
Paraquat treatment
Food was prepared using our regular dietary composition. Heated food was allowed to cool to ~50°C and paraquat (methyl viologen, Sigma) was added to 1 mM final concentration (≤1:1000) from a stock solution freshly prepared in dH2O.
Life span assays
Young flies (3+/− 1 day old) were placed on regular diet in bottles (~80 ml food) at 25°C for mating and egg lay (24 h). Adults were removed and after 12 to 14 days young flies were transferred to fresh bottles within two days of eclosion to allow for mating. DR was initiated on the 3rd day after eclosion to allow for recovery from CO2 anesthesia during sorting. Flies were harvested by CO2 exposure, sorted by sex and 40 flies each were transferred to vials containing 5 ml of regular diet. After allowing recovery from CO2 anaesthetization for 24 h at 25 °C, flies were placed on specific diets and transferred to 29 °C. Fly death was recorded as flies were transferred to fresh vials every 48 h. The total number recorded disregards rare escape or accidental death of flies. All lifespan determinations were performed independently of the mutation frequency assessments.
Mutation frequency assessment
For mutation frequency assessments separate studies were initiated in parallel with the life span studies using flies obtained from the same large population. Samples of 50 flies were taken after 2, 20 or 30 days on specific diets, or 5, 10 and 15 days on paraquat. Mutation frequencies were measured on samples of 50 whole flies according to the protocol described by Garcia et al. (Garcia et al. 2007b) with the following modifications. Proteinase K digestion in lysis buffer and SDS was extended to 90 min. at 65°C. DNA binding to anti-β-galactosidase/fusion protein coated magnetic beads was supplemented with 1 μg/ml RNAse to ensure complete RNA removal. Transformations yielding fewer than 100,000 or greater than 1,000,000 colonies were excluded from analysis. Positive controls (mouse lacZ DNA with known mutation frequencies) and negative controls (non-lacZ fly DNA) were included in every mutation frequency determination. All data points are averages of at least three independent groups of 50 flies analyzed in the same experiment.
Statistical analyses
Survival analysis (Prism 4) was used to calculate percent survival of paired groups on restricted versus non-restricted diets. Two-tailed p values were <0.0001 for all lifespan comparisons (Logrank test). Very few flies escaped or died accidentally and were not left-censored. Median survival (rather than average) was determined in Prism 4. All flies were always followed to the end; samples for the mutation frequency assessments were taken from separate cohorts. The built-in regular two-way ANOVA of Prism was used to evaluate effects of time, paraquat or diet on lacZ mutation frequencies.
Acknowledgments
We thank Mr. Brent Calder for his help in preparing the manuscript. This work was supported by NIH grants AG18923 (JV) and U54 RR024346 (JV and PK).
References
- Aidoo A, Mittelstaedt RA, Bishop ME, Lyn-Cook LE, Chen YJ, Duffy P, Heflich RH. Effect of caloric restriction on Hprt lymphocyte mutation in aging rats. Mutat Res. 2003;527:57–66. doi: 10.1016/s0027-5107(03)00072-1. [DOI] [PubMed] [Google Scholar]
- Barja G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic Biol Med. 2002;33:1167–1172. doi: 10.1016/s0891-5849(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busuttil RA, Garcia AM, Reddick RL, Dolle ME, Calder RB, Nelson JF, Vijg J. Intra-organ variation in age-related mutation accumulation in the mouse. PLoS ONE. 2007;2:e876. doi: 10.1371/journal.pone.0000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution I. Nutrition and the cost of reproduction. J Evol Biology. 1993;6:171–193. [Google Scholar]
- Dempsey JL, Pfeiffer M, Morley AA. Effect of dietary restriction on in vivo somatic mutation in mice. Mutat Res. 1993;291:141–145. doi: 10.1016/0165-1161(93)90153-q. [DOI] [PubMed] [Google Scholar]
- Dollé ME, Snyder WK, Gossen JA, Lohman PH, Vijg J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc Natl Acad Sci U S A. 2000;97:8403–8408. doi: 10.1073/pnas.97.15.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Calder RB, Dolle ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech Ageing Dev. 2008;129:528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Derventzi A, Busuttil R, Calder RB, Perez E, Jr, Chadwell L, Dolle ME, Lundell M, Vijg J. A model system for analyzing somatic mutations in Drosophila melanogaster. Nat Methods. 2007a;4:401–403. doi: 10.1038/NMETH1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Rodriguez A, Cabrera C, Lundell M, Dolle ME, Vijg J. Detection and analysis of somatic mutations at a lacZ reporter locus in higher organisms: application to Mus musculus and Drosophila melanogaster. Methods Mol Biol. 2007b;371:267–287. doi: 10.1007/978-1-59745-361-5_20. [DOI] [PubMed] [Google Scholar]
- Garinis GA, vander Horst GT, Vijg J, Hoeijmakers JH. DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol. 2008;10:1241–1247. doi: 10.1038/ncb1108-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Partridge L. Role of dFOXO in lifespan extension by dietary restriction in Drosophila melanogaster: not required, but its activity modulates the response. Aging Cell. 2008;7:187–198. doi: 10.1111/j.1474-9726.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Natural selection for extended longevity from food restriction. Growth Dev Aging. 1989;53:3. [PubMed] [Google Scholar]
- Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? Bioessays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- Kabil H, Partridge L, Harshman LG. Superoxide dismutase activities in long-lived Drosophila melanogaster females: chico 1 genotypes and dietary dilution. Biogerontology. 2007;8:201–208. doi: 10.1007/s10522-006-9065-3. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–1733. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Partridge L. Dietary restriction in Drosophila: delayed aging or experimental artefact? PLoS Genet. 2007;3:e57. doi: 10.1371/journal.pgen.0030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher SD, Libert S, Skorupa D. Flies and their golden apples: the effect of dietary restriction on Drosophila aging and age-dependent gene expression. Ageing Res Rev. 2005;4:451–480. doi: 10.1016/j.arr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijg J. Aging of the Genome. New York: Oxford University Press; 2007. [Google Scholar]