Abstract
Regulators of G Protein Signaling (RGS) accelerate GTP hydrolysis by Gα subunits and profoundly inhibit signaling by G protein-coupled receptors. The distinct expression patterns and pathophysiologic regulation of RGS proteins suggest that inhibitors may have therapeutic potential. We previously reported the design, mechanistic evaluation and structure-activity relationships (SAR) of a disulfide-containing cyclic peptide inhibitor of RGS4, YJ34 (Ac-Val-Lys-c[Cys-Thr-Gly-Ile-Cys]-Glu-NH2, S-S) (Roof, et al. Chem Biol Drug Des 2006; 67:266-274). Using a focused one-bead, one-compound (OBOC) peptide library that contains features known to be necessary for the activity of YJ34, we now identify peptides that bind to RGS4. Six peptides showed confirmed binding to RGS4 by flow cytometry. Two analogs of peptide 2, (Gly-Thr-c[Cys-Phe-Gly-Thr-Cys]-Trp-NH2, S-S with a free or acetylated N-terminus) inhibited RGS4-stimulated Gαo GTPase activity at 25–50 μM. They selectively inhibit RGS4 but not RGS7, RGS16 and RGS19. Their inhibition of RGS4 does not depend on cysteine-modification of RGS4, as they do not lose activity when all cysteines are removed from RGS4. Peptide 2 has been modeled to fit in the same binding pocket predicted for YJ34 but in the reverse orientation.
Keywords: One-Bead, One-Compound Library (OBOC), Focused library, Regulators of G-Protein Signaling (RGS), Protein-protein interaction (PPI) inhibitors, Structure-activity relationship (SAR)
Introduction
Regulators of G Protein Signaling (RGS) proteins are GTPase accelerating proteins (GAPs) for Gα subunits (1). They diminish G protein-coupled receptor (GPCR) signaling by binding to the activated Gα protein and stabilizing the transition state for GTP hydrolysis (2). Because RGS proteins have Gα specificity (1), receptor specificity (3–6), and unique expression patterns (7), it has been suggested that RGS inhibitors could selectively potentiate GPCR signaling in specific tissues or brain regions (8–10). Others have suggested that RGS inhibitors would be useful treatments for hypertension (11), Parkinson’s Disease (12), pain (13), cocaine reward (14), asthma (15), diabetes (16,17) and cancer (18,19).
There have been over 30 RGS proteins identified (20), which are divided into 8 families (RZ, R4, R7, R12, RA, GEF, GRK and SNX families) based on the homology of the 120 amino acid RGS domain as well as the presence or absence of other domains (1). The R4 family contains the RGS domain as well as an amphipathic N-terminus (21,22) that plays a role in membrane targeting and/or receptor specificity (21–23). RGS4 is the prototypical member of the R4 family. It has roles in cardiovascular and central nervous system signaling and was the starting point for our RGS inhibition efforts.
We previously reported the first RGS inhibitor, YJ34 (Ac-Val-Lys-c[Cys-Thr-Gly-Ile-Cys]-Glu-NH2, S-S) (24). This peptide was designed to mimic the switch 1 region of the Gα subunit and was modeled from the RGS4-Gαi1 crystal structure (25). It has a sequence similar to the switch 1 region with 2 amino acid substitutions (Thr181 to Cys and Val185 to Cys) to incorporate a disulfide bridge, which constrains the peptide in the correct conformation (24,26). This peptide has an IC50 of 26 μM in a membrane based steady state GTPase assay (24), a 9 μM IC50 in a purified protein single turnover GTPase assay (26), inhibits RGS4 81% at 40 μM in a capillary electrophoresis assay (27), and an analog inhibits RGS modulation of G-protein inwardly rectifying potassium current kinetics in atrial myocytes (26). It has been found that BR2 (Ac-Val-Lys-c[Cys-Thr-Ser-Ile-Cys]-Glu-NH2, S-S), a peptide that mimics the RGS-insensitive G184S mutation in the Gα protein is inactive, suggesting that YJ34 binds to RGS4 as designed (i.e. in the same way that the Gα switch 1 binds). SAR studies on this peptide showed that the following features were necessary for peptide activity: the N-terminal acetyl group, C-terminal amide, the Gly at position 5, and Cys residues at positions 3 and 7 linked by a disulfide bridge (26).
Peptide YJ34 is a useful tool for studying RGS function but we wanted to find other, hopefully more potent, RGS inhibitors. We report here the use of a focused one-bead, one-compound (OBOC) (28) peptide library to screen for RGS4 inhibitors. The library was restricted to contain peptides with the features mentioned above that are required for the function of YJ34. The other 5 amino acids were randomized among the 19 natural amino acids (except Cys) to afford 2.5 million possible peptide sequences. The library was synthesized and screened for RGS4 binding using beads containing only one peptide sequence each. Half of the peptides on each bead had an acetyl group, because it is important for peptide YJ34 function, while half had a free N-terminus, to allow sequencing by Edman degradation. From this screen we identified 7 peptide sequences that bound RGS4. Two analogs of peptide 2, 2ad (Ac-Gly-Thr-c[Cys-Phe-Gly-Thr-Cys]-Trp-NH2, S-S) and 2nd (Gly-Thr-c[Cys-Phe-Gly-Thr-Cys]-Trp-NH2, S-S) are novel inhibitors of RGS4.
Materials and Methods
Materials
Fmoc-protected amino acids and Rink amide resin were purchased from Advanced ChemTech (Louisville, KY). Fmoc protected and acetylated amino acid purity was verified by high performance liquid chromatography (HPLC). Tentagel Resin was purchased from ChemImpex (Wood Dale, Il) or Rapp-Polymer (Tubingen, Germany), and Alexa Fluor label from Invitrogen (Carlsbad, California). Peptide synthesis grade chemicals were purchased from either Applied Biosystems (Foster City, CA) or Sigma-Aldrich (St. Louis, MO). γ[32P]GTP (10 mCi/ml) was purchased from Perkin Elmer (Boston, MA) and diluted with unlabeled GTP to the desired level of radioactivity. Amylose resin was purchased from New England Biolabs and the Ni-NTA resin from Qiagen.
Protein Expression, Purification and Labeling
His6-Gαo (rat), and RGS4Δ18N (rat), were expressed and purified according to previous protocols (29–32). Mbp-His6-RGS4Δ51N (human), Mbp-His6-RGS16 (human) Mbp-His6-RGS19Δ11C (human) Mbp-His6-RGS7 RGS domain (human, nucleotides 915–1359), and the mutant Mbp-His6-RGS4Δ51N lacking all seven cysteines (called “−7C”) were in Gateway pMAL vectors and were expressed using a similar protocol as the RGS4Δ18 construct and purified over an amylose column followed by a Ni-NTA column according to the manufacturer’s protocol. In some cases this was followed by a size exclusion column as necessary. The mutagenesis was done using the “QuickChange Multi Site-Directed Mutagenesis Kit” from Stratagene according to the manufacturer’s protocol. Labeling of RGS4 with succinimide ester fluorophores (Alexa Fluor 568 and Alexa Fluor 532) was done according to the manufacturer’s protocol using approximately 2- to 3-fold excess fluorophore.
OBOC Peptide Library Synthesis
The protocol was based on previous reports (28,33) using a manual “mix and split” synthesis. TentaGel amide resin (10 g of 130 μm sized beads with a substitution level of 300 pmole) was swelled in N-methylpyrrolidone (NMP) and divided by volume into 19, 8.0 ml polypropylene filter columns (Alltech). To each column a solution of a different Fmoc-protected amino acid in NMP was added; all natural amino acids were used except cysteine. This was followed by a solution containing a 3-fold excess 2-(1H-Benzotriazole-1-yl)-1,1,3,3,-tetramethylaminium hexafluorophosphate/1-hydroxybenzotria-zole (HBTU/HOBt) and 2M N,N′-diisopropylethylamine (DIEA) in NMP and the mixture was shaken for 1 hour at room temperature. Then the beads were rinsed thoroughly with NMP. Unreacted amino groups on the resin were acetylated with a 20-fold excess of acetic anhydride with DIEA and HOBt in NMP. After a negative bromophenol blue test for free amines, the beads were pooled and treated with 50% piperidine in NMP. The splitting, coupling, pooling, and deprotection (but not the acetylation) steps were repeated for the randomized positions. For the non-random positions, the resin was not split and Fmoc-Cys(trt)-OH (positions 3 or 7) or Fmoc-Gly-OH (position 5) was coupled to the whole batch of beads. In the final coupling step, a 1:1 mixture of acetylated and Fmoc-protected amino acids was used. To remove the side chain protecting groups without cleaving the peptide from the resin (33), all the beads were pooled and treated with 50 ml ice-cold 88:5:5:2 trifluoroacetic acid (TFA):phenol:water:triisopropylsilane (TIPS) and shaken for 3 hours at room temperature. Treatment of test peptides with deprotection reagents resulted in no detectable cleaved peptide as checked by HPLC. After thorough washing (including a wash in 10% DIEA in order to neutralize obtained TFA salts), the resin was added to 7.8 L oxidation solution (20% DMSO (Dimethylsulfoxide), 5% Acetic acid, in water pH 6.0 purged of air with N2) and shaken for 48 hours.
Library Screening
Beads were washed and plated in a single layer (on average about 1,500 beads per well) in 96 well plates in buffer (1% BSA in 20 mM Hepes pH 8.0) and incubated with 25 nM RGS4Δ18N-Alexa Fluor 568. Wells containing beads with YJ34 were included in every scan as a control. Fluorescence was imaged on a Typhoon 9200 Gel Imager using an excitation of 532 nm and an emission of 610 nm and the fluorescence was quantified with the accompanying ImageQuant software. To do this, a 12×8 grid was placed over the image such that each grid block contained only one well. Grid blocks that contained an object with intensity more than 5–10 fold over the most intense object in the wells containing YJ34 were collected and pooled. The image was visually inspected and any objects that were clearly not beads, such as dust or other debris, were excluded. Pooled beads were washed with buffer, diluted and re-screened against 10 nM RGS4Δ18N-Alexa Fluor 568. The process was repeated with lower RGS4 concentrations until a single bead per well was obtained. The top 20 hits were then isolated with a needle under a dissecting microscope and sent for sequencing by Edman degradation at the Biomedical Research Core Facilities at the University of Michigan.
Synthesis of individual Peptides
Soluble (not bead-bound) peptides (for GAP assays) were synthesized on Rink resin, cleaved from the resin and cyclized as described previously (24,26). Peptide purity (at least 95%) and solubility were verified by HPLC and correct mass was verified by mass spectrometry analysis (26). YJ34 and BR2 bead bound controls were synthesized on 130 μm Tentagel resin using the same protocol as the library except no “mix and split” steps were incorporated. Hit peptides on beads (for the FACScan assays) were synthesized on 20 μm Tentagel resin using a LabMate apparatus (Advanced Chem Tech) and cyclized manually using the similar chemistries as for the library with no “mix and split” randomization. The deprotection mixture was 83:5:5:5:2 TFA:thioanisole:phenol:TIPS and an Ellman test was performed to ensure complete oxidation.
Binding of test peptides
Unreacted 130 μm TentaGel amide resin, or resin containing YJ34 or BR2 was put into wells of a 96 well plate with 5 mg/well in 0.5 ml buffer (1% BSA in 20 mM Hepes pH 8.0) for 1 hour. The supernatant was removed and various concentrations of Alexa Fluor 568 labeled RGS4Δ18N were added in 200 μl followed by incubation for 15 min. After a wash with 500 μl buffer, fluorescence was measured in black Costar 96 well plates in a Victor2 fluorescence plate reader with an excitation filter at 560 and an emissions filter at 595. Samples were measured in duplicate.
FACScan Flow Cytometry
Peptide beads (about 5 × 103 per sample for a maximum of 5 nmoles peptide) were washed then incubated with 25 nM RGS4Δ51N-Alexa Fluor 532 in 300 μl for at least 15 min at room temperature under foil. The Becton Dickinson FACScan was gated and the laser intensity set in CellQuest such that control beads (acetylated TentaGel resin) were gated and had low but measurable fluorescence. No compensation was set. Fluorescence of RGS4Δ51N-Alexa Fluor 532 bound to acetylated beads (about 60 % of YJ34 beads) was considered background and subtracted from all samples. Samples were read in duplicate.
RGS-Stimulated GTPase
Single turnover GTP hydrolysis measurements with and without RGS were performed as described previously (26).
Modeling
Peptides were modeled using Quanta (Accelrys Inc) by modifying the residues from the Gαi1 switch 1 in the RGS4-Gαi1 (PDB entry: 1agr) crystal structure (25). Upon residue substitution and formation of the disulfide bond, energy minimization of each peptide with all hydrogen atoms added was performed using the Quanta/CHARMm simulation package with dielectric constant (ε)=10 and the Adopted-Basis Newton Raphson method (50 steps). To model the peptide-RGS complex, each minimized peptide with the hydrogens removed was substituted for the corresponding Gαi fragment from the Gαi-RGS4 complex. Images were prepared using PyMol for OS X (http://www.pymol.org).
Statistical Analysis
Data are expressed as mean ± S.E.M (or ± S.D for n=2) and analyzed by either a t-test (GAP data) or a one-way ANOVA (Victor based test binding and FACScan data). A Bonferroni post-test was done on the FACScan data. Significance is indicated as follows: *p<0.05, **p<0.01, ***p<0.001.
Results and Discussion
OBOC Library Design and Screening
It has been found previously that several structural features were necessary for activity of YJ34: the N-terminal acetyl group, the C-terminal amide, Gly at position 5, and Cys at positions 3 and 7 with their side chains linked via a disulfide bridge. However, the other 5 positions can be varied (26). As seen in Table 1, the features necessary for YJ34 function were constrained in the library, while the other positions were randomized. All natural amino acids except Cys were used in the other 5 positions to give 2.5 million possible sequences. Using 10 g of 130 μm TentaGel beads provides approximately 7.9 million beads, thus each sequence would have been present on 3 beads in the library on average. In an OBOC library, each bead has only one amino acid sequence. However, for this library, half of the peptides on each bead were acetylated, since this is necessary for the function of YJ34, and half had a free N-terminus, to allow sequencing by Edman degradation. The beads had a substitution level of 300 pmoles/bead, resulting in about 150 pmoles of free-amine-containing peptides for sequencing. This was found to be sufficient for sequencing of test peptides (data not shown). Also, as a control, we ensured that YJ34 on beads bound to RGS4 (Figure 1). Although TentaGel resin is known not to be cleavable with TFA treatment (33), it was verified that deprotection conditions did not remove test peptides from resin (see methods). Tentagel resin was chosen because it has been used extensively and has good mechanical stability and has a uniform size. Unfortunately, it has green autofluorescence (34), so a red dye (Alexa Fluor 568) was chosen for RGS4 labeling for the library screen. Others have found non-specific binding to be problematic with this resin (Anna Mapp, personal communication). We did find high non-specific binding to unreacted resin (data not shown), but this was not observed with acetylated resin or with resin containing BR2 (Ac-Val-Lys-c[Cys-Thr-Ser-Ile-Cys]-Glu-NH2, S-S, which is an inactive peptide that mimics the RGS insensitive mutation in the Gα protein (26)).
Table 1.
OBOC library design. The key features of YJ34 were constrained in the library. The library had the Gly at position 5, the Cys at positions 3 and 7 with a disulfide bonds, and a C-terminal amide. The other 5 amino acid positions were randomized such that each bead had only one sequence. Half of the peptides on each bead had an acetyl group and the other half had a free N-terminus. There were 300 pmoles peptide on each bead, 2.5 million sequences and 7.9 million beads in the library.
| Name | Sequence | Bridge |
|---|---|---|
| Gαi switch I | …Val-Lys-Thr-Thr-Gly-Ile-Val-Glu… | none |
| YJ34 | Ac-Val-Lys-[Cys-Thr-Gly-Ile-Cys]-Glu-NH2 | (S-S) |
| Library | (S-S) |
Fig. 1.
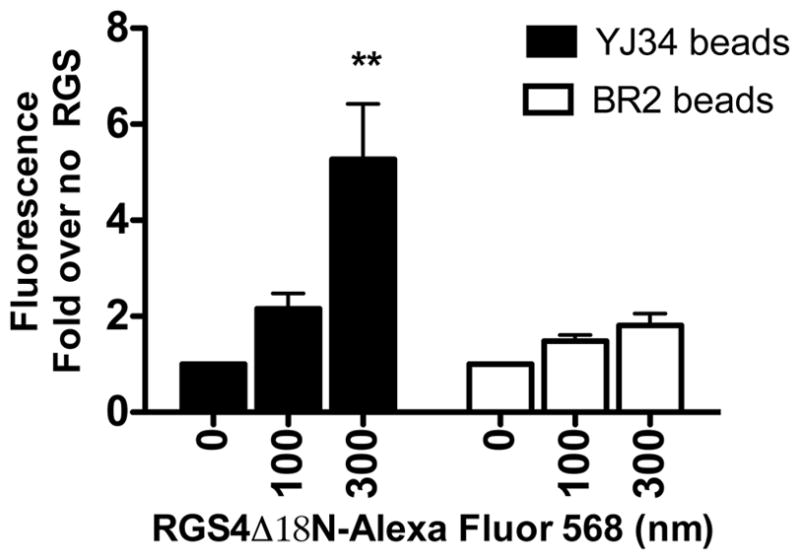
Alexa Fluor 568 labeled RGS4Δ18N binds peptide YJ34 (filled bars) on beads but not peptide BR2 (open bars) on beads. Binding of RGS4 to beads was measured as described. The experiment was done in duplicate. (mean ± S.D., n =2). *p<0.05, **p<0.01, ***p<0.001 compared to no RGS4Δ18N-Alexa Fluor 568.
To screen the library, the beads were incubated with Alexa Fluor 568 labeled RGS4Δ18N and imaged in a Typhoon Gel Imager as shown in Figure 2. The top twenty most fluorescent beads were isolated and sequenced by Edman degradation. Unfortunately, six of the hits yielded only partial sequences, leaving 14 fully sequenced hits for further evaluation.
Fig. 2.
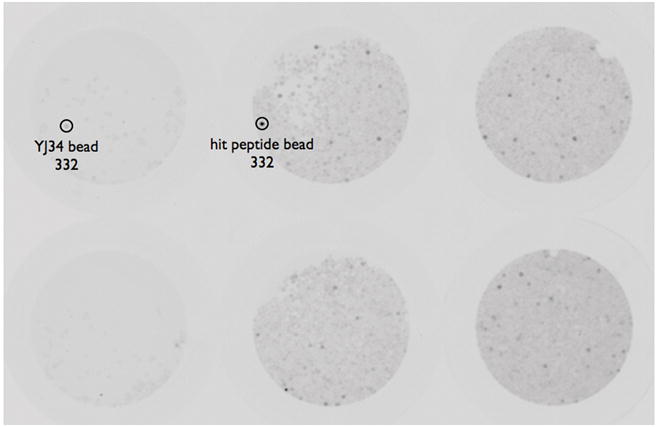
Screening of the library. The library was screened as described. The left 2 wells contain peptide YJ34 on beads while the right 4 wells contain library beads. Fluorescence was quantified and represented by the number below the circled beads.
Hit verification
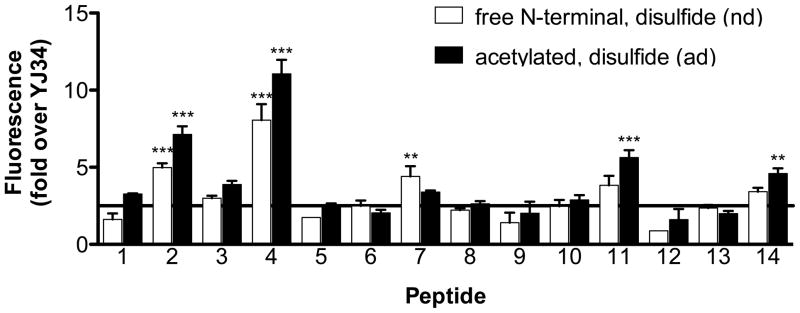
Each of the 14 sequenced hit peptides was resynthesized on 20 μm TentaGel beads. Since both were present in the library, free N-terminal, disulfide bridged (nd) and acetylated, disulfide bridged (ad) versions of each sequence were synthesized. Interaction with Alexa Fluor 532 labeled RGS4Δ51N was detected in a FACScan Flow Cytometer (Figure 3). The RGS4Δ51N protein lacking the amphipathic helix in the N-terminus was used to ensure that the verified hits bound to the RGS domain of RGS4. Using 2.5-fold increased binding of RGS4Δ51N-Alexa Fluor 532 compared to YJ34 on beads as a cut-off, it was found that some of the initial hits were false positives, but peptides 1, 2, 3, 4, 7, 11, and 14 (Figure 3, Table 2) were verified and were chosen for further evaluation. The hits were structurally divergent from our lead compound. Both the free N-terminal and the acetylated versions of the verified hits bound RGS4Δ51N. This is in contrast to YJ34, where only the acetylated version has activity in the GAP assay (26).
Fig. 3.
Hit verification. The 14 hits for which complete sequences were obtained were resynthesized on beads and tested for binding to RGS4Δ15N-Alexa Fluor 532 as described (mean ± S.EM. (or S.D. where n=2), n≥2). Acetylated peptides are the filled bars and the free N-terminal peptides are in the open bars. *p<0.05, **p<0.01, ***p<0.001 compared to YJ34 on beads.
Table 2.
The verified hits. Peptides from Figure 3 that bind RGS4Δ51N-Alexa Fluor 532 at least 2.5-fold more than YJ34 are shown. Note that both acetylated, disulfide bridged (ad) and free N-terminal, disulfide bridged (nd) peptides were chosen for further evaluation.
| YJ34 | Ac-VKc[CTGIC]E-NH2, S-S |
|---|---|
| 1 | YNc[CQGEC]E-NH2, S-S |
| 2 | GTc[CFGTC]W-NH2, S-S |
| 3 | LVc[CKGYC]Q-NH2, S-S |
| 4 | KVc[CMGGC]T-NH2, S-S |
| 7 | YWc[CKGLC]K-NH2, S-S |
| 11 | KLc[CHGYC]H-NH2, S-S |
| 14 | KHc[CYGFC]K-NH2, S-S |
Peptide activity in GAP assays
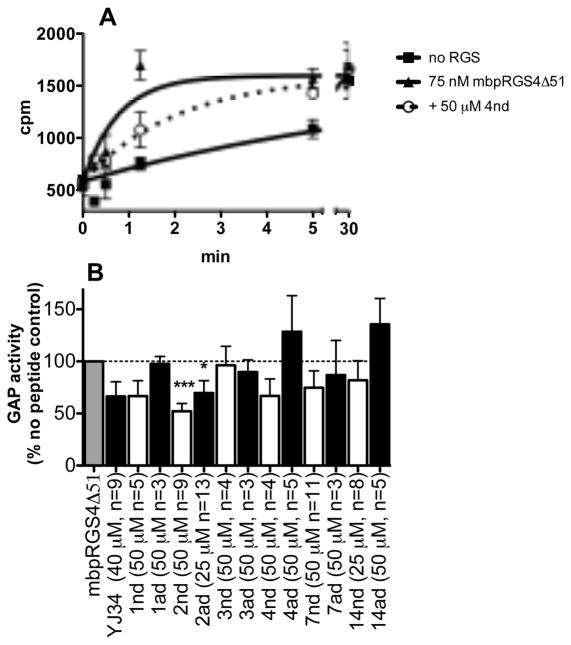
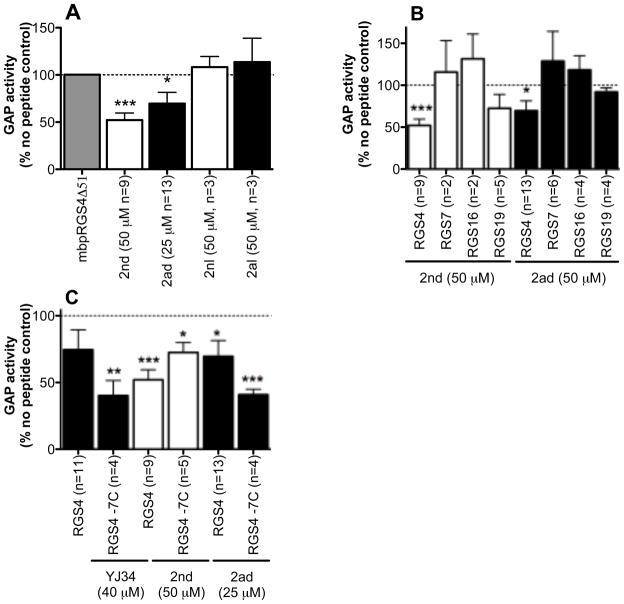
Peptides were tested at 50 μM except for YJ34, 2ad, and 14nd, which where limited by solubility. Effects on the rate of RGS stimulated Gαo GTPase activity were measured in a single turnover assay with purified proteins and peptides in solution (Figure 4). Hit 11 was not evaluated because of signs of aggregation. Only peptides 2nd (the free N-terminal, disulfide bridged hit 2) and 2ad (the acetylated, disulfide bridged peptide 2) had statistically significant inhibition of RGS4 activity (48 ± 7 percent inhibition at 50 μM and 30 ± 12 percent inhibition at 25 μM respectively). Based on these values, both peptides have an estimated IC50 of about 50 μM. Other peptides that had some activity included 1nd (33 ± 15 percent inhibition at 50 μM) and 4nd (33 ± 16 percent inhibition at 50 μM, Figure 4). The activity of hit 2 was investigated further in Figure 5. Linear as well as disulfide bridged versions of peptide 2 (2nl: free N-terminal linear and 2al: acetylated linear) were tested in the GAP assay since cyclization yields may have been less than 100% in the synthesis of the library leaving some linear peptides present on each bead, which may have contributed to the observed fluorescence of the bead (Figure 5A). Consistent with previous results for YJ34, the linear peptides were inactive. RGS selectivity was also investigated. Interestingly, peptides 2ad and 2nd were inactive against RGS7, RGS16 and RGS19 (Figure 5B). RGS16, like RGS4, is in the R4 family, while RGS7 and RGS19 are in different families (R7 and RZ respectively) and have lower homology with RGS4.
Fig. 4.
Activity of hit peptides in a single turnover GAP assay. A) Representative time course. B) Rates were calculated based on the time courses, and percent decrease in the rate of RGS stimulated GTP hydrolysis by peptides was measured (mean ± S.E.M., n ≥3). *p<0.05, **p<0.01, ***p<0.001 compared to no peptide.
Fig. 5.
Functional activity of peptide 2. A) Disulfide-bridged (2nd and 2ad), but not linear (2nl and 2al) peptides inhibit RGS4Δ51N GAP activity. B) Peptides 2nd and 2ad are selective for RGS4Δ51N (75–125 nM) over RGS7box (500 nM), RGS16 (1.5 μM) or RGS19 (200 nM). C) Activity of 2nd and 2ad on wild type and −7C mutant (200 nM) RGS4 (mean ± S.E.M., (or S.D. where n=2), n ≥2). *p<0.05, **p<0.01, ***p<0.001 compared to no peptide.
Recently, CCG-4986, a small molecule inhibitor of RGS4 was identified and found to interact with RGS4 through cysteine modification ((32,35), David Roman, unpublished observation). For this reason, the activity of our original peptide, YJ34, as well as that of hit 2 was evaluated with an RGS4Δ51N mutant lacking all 7 cysteines (−7C) (Figure 5C). The −7C mutant was inhibited by 2ad, 2nd and YJ34. There was no statistically significant difference between the activity on wild type and the −7C RGS4Δ51N for 2ad, 2nd or YJ34 (59 ± 4, 27 ± 7, 60 ± 11 percent inhibition for 2ad, 2nd and YJ34 respectively). This indicates that hit 2 does not inhibit RGS4 through cysteine modification.
Conclusions
Inhibition of protein-protein interactions (PPIs) are particularly challenging. Unlike enzymes and receptors that have well defined pockets, protein-protein interfaces tend to be large and flat (36). Because of this, many PPIs have been declared “non-amenable” to inhibition by drug-like molecules based on computational measurements (37). In fact, the RGS4-Gαo interaction falls in this category (David Fry, personal communication). However, some protein-protein interfaces have been found to have “hot spots”, which are a limited number of residues that are responsible for the majority of the interaction energy. Others have taken advantage of this to successfully create peptide and small molecule inhibitors of PPIs (38–40). Also, computational approaches such as used in Fry and Vassilev (37) are based on rigid crystal structures of proteins. Others have shown that protein interfaces are adaptable and in multiple cases small molecules bind in induced pockets that are not observed in the crystal structures of the protein alone or in complex with its natural protein binding partner ((41) and references therein). Thus these interactions could not be rationally predicted in spite of the existence of a structure of the target protein and could only come from experimental screening. Hence, although this was an ambitious project, there are ample precedents for success. In fact, the above mentioned small molecule inhibitor of RGS4, CCG-4986, was found in a screen to block the RGS4-Gαo interaction (32). Inhibitors of RGS4 were also found in a yeast based screen, but unfortunately, no structures were reported (42).
Of the seven RGS4-binding peptides found in our OBOC peptide library screen, one peptide sequence (in both free amide and acetylated form) was found to inhibit RGS4 GAP activity in the single turnover GAP assay. Although the potency of the new compounds is no greater then the starting peptide, we have identified new peptides that we believe have the desired mode of binding to RGS4, and the desired RGS4 inhibitor activity.
Based on the crystal structure of RGS4 bound to Gαi (25), Thr182 of Gαi forms hydrogen-bonding and hydrophobic interactions with several residues in RGS4 including Asn88, Asp163, Ser164 and Leu159. The corresponding residue in YJ34, Thr4, may similarly interact with RGS4 residues. However, there is a Phe at this position in peptide 2 and it is unlikely that this bulkier, non-polar side chain interacts with RGS in the same manner as does the Thr4 side chain of YJ34 (Figure 6A). However, if the peptide were rotated around the central Gly (i.e. oriented as a retrobinder in the YJ34 binding site), then the Thr in position 6 would be placed into the pocket where Thr182 of switch 1 binds and the Thr4 of YJ34 is predicted to bind. This would also position the Phe4 of hit 2 above Tyr84 in RGS4 where Ile185 from switch 1 is located. These two amino acid interactions are reminiscent of a previously identified YJ34 analog, (Ac-Val-Lys-[Cys-Thr-Gly-Phe-Cys]-Glu-NH2, S-S, peptide 20 in Roof et al (26)), which has a Thr at position 4 and a Phe at position 6. This peptide inhibited RGS4 GAP activity by nearly 40% at 100 μM (26). Thus we propose that hit 2 interacts with RGS4 in the switch 1 binding site in an inverse orientation (Figure 6). Based on our model, the Trp8 residue of 2 also makes favorable hydrophobic interactions with Leu159. On the other hand, due to the inverse position of the peptide 2 in the binding site, its N-terminus likely occupies a position of YJ34 C-terminus, while C-terminal amide of peptide 2 is positioned similarly to YJ34 N-terminus. The presence of positively charged Lys155 and Lys162 of RGS4 near N-terminus of YJ34 may explain the poor activity of its non-acetylated version. However, the proposed different location of the peptide 2 N-terminus would not require its acetylation.
Fig. 6.
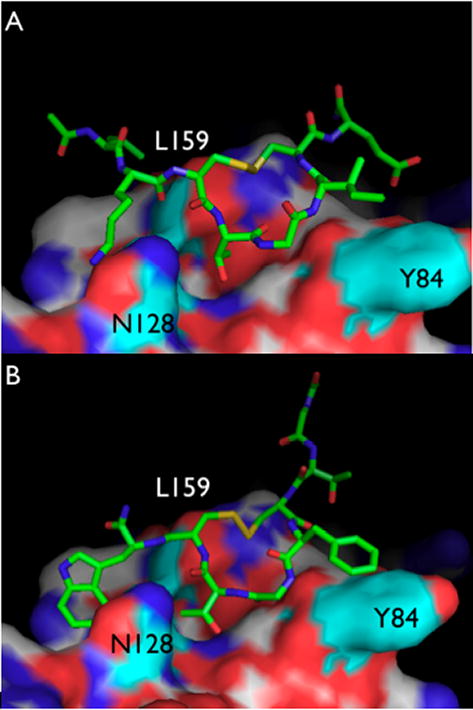
Model of YJ34 and 2ad in the switch 1 binding site of RGS4. Amino acids in RGS4 mentioned in the text are in cyan. A) YJ34 is modeled to bind RGS4 the same way the switch 1 region of Gαi binds. B) 2ad is modeled in the same pocket but in the reverse orientation.
Although it is believed that hit 2 binds the YJ34 binding site, it is possible that some of the other peptides do not. In fact, the observation that many of them do not have an effect on GAP activity would suggest that this may be the case and that these analogs interact with RGS in alternate ways. This may explain why peptide 4, for example, bound RGS4 so well in the FACScan assay (Figure 3) while having little effect on RGS4 GAP activity (Figure 4). RGS4 has been shown to have an allosteric site where phosphatidylinositol-3,4,5,-trisphosphate (PIP3) and calmodulin are known to bind (43). Calmodulin binding alone does not affect GAP activity, but blocks the inhibition by PIP3 (43). It is therefore possible that some of the peptides isolated in the library bind RGS4 at this site without affecting RGS4 GAP activity. Alternatively, these peptides may bind other, perhaps as of yet unidentified, protein-protein interaction sites. The fact that the sequences obtained in the library are so divergent from the sequence of YJ34 also supports the possibility that many of the hit peptides do not bind at the YJ34 binding site. In fact, it is hard to find any clear structural clusters based on the homology of the varied positions. It is therefore speculated that RGS4 may have several peptide (and therefore endogenous protein or lipid) binding sites.
As in the YJ34 series, linear versions of hit 2 are inactive. Also, as in the YJ34 series, hit 2 is most active on RGS4 compared to RGS7. It is thought that CCG-4986 inhibits RGS4 through cysteine modification ((32,35), David Roman, personal communication). Because of the apparent sensitivity of RGS4 to cysteine modifications, and because of cysteines in the peptides, it was examined whether a similar mechanism might exist here. Hence peptides 2ad, 2nd and YJ34 were tested against the −7Cys RGS4Δ51N mutant. The observation that none of the peptides differentiated between wild type and the −7C mutant supports a mechanism of action like that of YJ34, rather than via a covalent interaction with RGS4 cysteines.
The side chain order and ring size of 2ad and 2nd are the same as in peptide 20 in Roof et al (26) within the cycle, however, the backbone is different. The fact that both have activity opens up the possibility for non-peptide analogs such as β-amino acid and peptoid analogs. Peptidomimetics have the advantage of not being protease substrates and this increased stability makes them more useful in cells and in vivo (44), making them an interesting avenue for future investigations.
Acknowledgments
The authors would like to acknowledge David Fry from Roche Reseach Center, Nutley, NJ for his analysis of the RGS4-Gαo interaction pocket, as well as Roger Sunahara, John Tesmer and Anna Mapp at the University of Michigan for helpful discussions. We would also like to thank Roger Sunahara, Kathleen Collins and Gus Rosania for sharing equipment (Top Count plate reader, FACScan flow cytometer, and Typhoon gel imager respectively). We would like to acknowledge the support of the Michigan Chemistry-Biology Interface Training Program, which is funded through the National Institutes of Health under grant number T32 GM00008597. This work was also supported by DA003910 (HIM) and GM39561 (RRN).
Abbreviations
- ANOVA
analysis of variance
- DIEA
N,N′-diisopropylethylamine
- DMSO
dimethylsulfoxide
- GAP
GTPase accelerating protein
- GPCR
G protein-coupled receptor
- HBTU
2-(1H-Benzotriazole-1-yl)-1,1,3,3,-tetramethylaminium hexafluorophosphate
- HPLC
high performance liquid chromatography
- HOBT
1-hydroxybenzotriazole
- NMP
N-methylpyrrolidone
- OBOC
one-bead, one-compound
- PPI
protein-protein interaction
- RGS
regulator of G protein signaling
- SAR
structure activity relationship
- TFA
trifluoroacetic acid
- TIPS
triisopropylsilane
References
- 1.Hepler JR. Emerging roles for RGS proteins in cell signalling. Trends Pharmacol Sci. 1999;9:376–382. doi: 10.1016/s0165-6147(99)01369-3. [DOI] [PubMed] [Google Scholar]
- 2.Berman DM, Kozasa T, Gilman AG. The GTPase-activating protein RGS4 stabilizes the transition state for nucleotide hydrolysis. J Biol Chem. 1996;44:27209–27212. doi: 10.1074/jbc.271.44.27209. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Liu M, Mullah B, Siderovski DP, Neubig RR. Receptor-selective effects of endogenous RGS3 and RGS5 to regulate mitogen-activated protein kinase activation in rat vascular smooth muscle cells. J Biol Chem. 2002;28:24949–24958. doi: 10.1074/jbc.M203802200. [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Zeng W, Popov S, Berman DM, Davignon I, Yu K, Yowe D, Offermanns S, Muallem S, Wilkie TM. RGS proteins determine signaling specificity of Gq-coupled receptors. J Biol Chem. 1999;6:3549–3556. doi: 10.1074/jbc.274.6.3549. [DOI] [PubMed] [Google Scholar]
- 5.Hague C, Bernstein LS, Ramineni S, Chen Z, Minneman KP, Hepler JR. Selective inhibition of alpha1A-adrenergic receptor signaling by RGS2 association with the receptor third intracellular loop. J Biol Chem. 2005;29:27289–27295. doi: 10.1074/jbc.M502365200. [DOI] [PubMed] [Google Scholar]
- 6.Krumins AM, Barker SA, Huang C, Sunahara RK, Yu K, Wilkie TM, Gold SJ, Mumby SM. Differentially regulated expression of endogenous RGS4 and RGS7. J Biol Chem. 2004;4:2593–2599. doi: 10.1074/jbc.M311600200. [DOI] [PubMed] [Google Scholar]
- 7.Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;20:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Traynor JR, Neubig RR. Regulators of G protein signaling & drugs of abuse. Mol Interv. 2005;1:30–41. doi: 10.1124/mi.5.1.7. [DOI] [PubMed] [Google Scholar]
- 9.Neubig RR. Regulators of G protein signaling (RGS proteins): novel central nervous system drug targets. J Pept Res. 2002;6:312–316. doi: 10.1034/j.1399-3011.2002.21064.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhong H, Neubig RR. Regulator of G protein signaling proteins: novel multifunctional drug targets. J Pharmacol Exp Ther. 2001;3:837–845. [PubMed] [Google Scholar]
- 11.Cho H, Park C, Hwang IY, Han SB, Schimel D, Despres D, Kehrl JH. Rgs5 Targeting Leads to Chronic Low Blood Pressure and a Lean Body Habitus. Mol Cell Biol. 2008 doi: 10.1128/MCB.01889-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding J, Guzman JN, Tkatch T, Chen S, Goldberg JA, Ebert PJ, Levitt P, Wilson CJ, Hamm HE, Surmeier DJ. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;6:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Munoz M, de la Torre-Madrid E, Gaitan G, Sanchez-Blazquez P, Garzon J. RGS14 prevents morphine from internalizing Mu-opioid receptors in periaqueductal gray neurons. Cell Signal. 2007;12:2558–2571. doi: 10.1016/j.cellsig.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, et al. RGS9 modulates dopamine signaling in the basal ganglia. Neuron. 2003;6:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- 15.Druey KM. Regulators of G protein signalling: potential targets for treatment of allergic inflammatory diseases such as asthma. Expert Opin Ther Targets. 2003;4:475–484. doi: 10.1517/14728222.7.4.475. [DOI] [PubMed] [Google Scholar]
- 16.Usui I, Imamura T, Satoh H, Huang J, Babendure JL, Hupfeld CJ, Olefsky JM. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004;14:2821–2829. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X, Charbeneau RA, Fu Y, Kaur K, Gerin I, MacDougald OA, Neubig RR. Resistance to diet-induced obesity and improved insulin sensitivity in mice with a regulator of G protein signaling-insensitive G184S Gnai2 allele. Diabetes. 2008;1:77–85. doi: 10.2337/db07-0599. [DOI] [PubMed] [Google Scholar]
- 18.Heo K, Ha SH, Chae YC, Lee S, Oh YS, Kim YH, Kim SH, Kim JH, Mizoguchi A, Itoh TJ, Kwon HM, Ryu SH, Suh PG. RGS2 promotes formation of neurites by stimulating microtubule polymerization. Cell Signal. 2006;12:2182–2192. doi: 10.1016/j.cellsig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Boss CN, Grunebach F, Brauer K, Hantschel M, Mirakaj V, Weinschenk T, Stevanovic S, Rammensee HG, Brossart P. Identification and characterization of T-cell epitopes deduced from RGS5, a novel broadly expressed tumor antigen. Clin Cancer Res. 2007;11:3347–3355. doi: 10.1158/1078-0432.CCR-06-2156. [DOI] [PubMed] [Google Scholar]
- 20.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;5:579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein LS, Grillo AA, Loranger SS, Linder ME. RGS4 binds to membranes through an amphipathic alpha-helix. J Biol Chem. 2000;24:18520–18526. doi: 10.1074/jbc.M000618200. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein LS, Ramineni S, Hague C, Cladman W, Chidiac P, Levey AI, Hepler JR. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11alpha signaling. J Biol Chem. 2004;20:21248–21256. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- 23.Gu S, He J, Ho WT, Ramineni S, Thal DM, Natesh R, Tesmer JJ, Hepler JR, Heximer SP. Unique hydrophobic extension of the RGS2 amphipathic helix domain imparts increased plasma membrane binding and function relative to other RGS R4/B subfamily members. J Biol Chem. 2007;45:33064–33075. doi: 10.1074/jbc.M702685200. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Zhong H, Omnaas JR, Neubig RR, Mosberg HI. Structure-based design, synthesis, and pharmacologic evaluation tf peptide RGS4 inhibitors. J Pept Res. 2004;2:141–146. doi: 10.1111/j.1399-3011.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 25.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4--activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997;2:251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 26.Roof RA, Jin Y, Roman DL, Sunahara RK, Ishii M, Mosberg HI, Neubig RR. Mechanism of action and structural requirements of constrained peptide inhibitors of RGS proteins. Chem Biol Drug Des. 2006;4:266–274. doi: 10.1111/j.1747-0285.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 27.Jameson EE, Roof RA, Whorton MR, Mosberg HI, Sunahara RK, Neubig RR, Kennedy RT. Real-time detection of basal and stimulated G protein GTPase activity using fluorescent GTP analogues. J Biol Chem. 2005;9:7712–7719. doi: 10.1074/jbc.M413810200. [DOI] [PubMed] [Google Scholar]
- 28.Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. A new type of synthetic peptide library for identifying ligand-binding activity. Nature. 1991;6348:82–84. doi: 10.1038/354082a0. [DOI] [PubMed] [Google Scholar]
- 29.Lan KL, Sarvazyan NA, Taussig R, Mackenzie RG, DiBello PR, Dohlman HG, Neubig RR. A point mutation in Galphao and Galphai1 blocks interaction with regulator of G protein signaling proteins. J Biol Chem. 1998;21:12794–12797. doi: 10.1074/jbc.273.21.12794. [DOI] [PubMed] [Google Scholar]
- 30.Lan KL, Zhong H, Nanamori M, Neubig RR. Rapid kinetics of regulator of G-protein signaling (RGS)-mediated Galphai and Galphao deactivation. Galpha specificity of RGS4 AND RGS7. J Biol Chem. 2000;43:33497–33503. doi: 10.1074/jbc.M005785200. [DOI] [PubMed] [Google Scholar]
- 31.Lee E, Linder ME, Gilman AG. Expression of G-protein alpha subunits in Escherichia coli. Methods Enzymol. 1994:146–164. doi: 10.1016/s0076-6879(94)37059-1. [DOI] [PubMed] [Google Scholar]
- 32.Roman DL, Talbot JN, Roof RA, Sunahara RK, Traynor JR, Neubig RR. Identification of small-molecule inhibitors of RGS4 using a high-throughput flow cytometry protein interaction assay. Mol Pharmacol. 2007;1:169–175. doi: 10.1124/mol.106.028670. [DOI] [PubMed] [Google Scholar]
- 33.Cabilly S. Combinatorial peptide library protocols. Totowa, N.J.: Humana Press; 1998. [Google Scholar]
- 34.Olivos HJ, Bachhawat-Sikder K, Kodadek T. Quantum dots as a visual aid for screening bead-bound combinatorial libraries. Chembiochem. 2003;11:1242–1245. doi: 10.1002/cbic.200300712. [DOI] [PubMed] [Google Scholar]
- 35.Kimple AJ, Willard FS, Giguere PM, Johnston CA, Mocanu V, Siderovski DP. The RGS protein inhibitor CCG-4986 is a covalent modifier of the RGS4 Galpha-interaction face. Biochim Biophys Acta. 2007;9:1213–1220. doi: 10.1016/j.bbapap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitty A, Kumaravel G. Between a rock and a hard place? Nat Chem Biol. 2006;3:112–118. doi: 10.1038/nchembio0306-112. [DOI] [PubMed] [Google Scholar]
- 37.Fry DC, Vassilev LT. Targeting protein-protein interactions for cancer therapy. J Mol Med. 2005;12:955–963. doi: 10.1007/s00109-005-0705-x. [DOI] [PubMed] [Google Scholar]
- 38.Scott JK, Huang SF, Gangadhar BP, Samoriski GM, Clapp P, Gross RA, Taussig R, Smrcka AV. Evidence that a protein-protein interaction ‘hot spot’ on heterotrimeric G protein betagamma subunits is used for recognition of a subclass of effectors. EMBO J. 2001;4:767–776. doi: 10.1093/emboj/20.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trosset JY, Dalvit C, Knapp S, Fasolini M, Veronesi M, Mantegani S, Gianellini LM, Catana C, Sundstrom M, Stouten PF, Moll JK. Inhibition of protein-protein interactions: the discovery of druglike beta-catenin inhibitors by combining virtual and biophysical screening. Proteins. 2006;1:60–67. doi: 10.1002/prot.20955. [DOI] [PubMed] [Google Scholar]
- 40.Thanos CD, DeLano WL, Wells JA. Hot-spot mimicry of a cytokine receptor by a small molecule. Proc Natl Acad Sci USA. 2006;42:15422–15427. doi: 10.1073/pnas.0607058103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;7172:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 42.Young KH, Wang Y, Bender C, Ajit S, Ramirez F, Gilbert A, Nieuwenhuijsen BW. Yeast-based screening for inhibitors of RGS proteins. Methods Enzymol. 2004:277–301. doi: 10.1016/S0076-6879(04)89017-7. [DOI] [PubMed] [Google Scholar]
- 43.Ishii M, Fujita S, Yamada M, Hosaka Y, Kurachi Y. Phosphatidylinositol 3,4,5-trisphosphate and Ca2+/calmodulin competitively bind to the regulators of G-protein-signalling (RGS) domain of RGS4 and reciprocally regulate its action. Biochem J Pt. 2005;1:65–73. doi: 10.1042/BJ20040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fear G, Komarnytsky S, Raskin I. Protease inhibitors and their peptidomimetic derivatives as potential drugs. Pharmacol Ther. 2007;2:354–368. doi: 10.1016/j.pharmthera.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]