Abstract
A two-step solution phase synthesis employing a double UDC (Ugi/Deprotect/Cyclize) strategy has been utilized to obtain fused 6,7,6,6-quinoxalinone-benzodiazepines and 6,7,7,6-bis-benzodiazepines. Optimization of the methodology to produce these tetracyclic scaffolds was enabled by microwave irradiation, incorporation of trifluoroethanol as solvent, and the use of the convertible isocyanide, 4-tert-butyl cyclohexen-1-yl isocyanide.
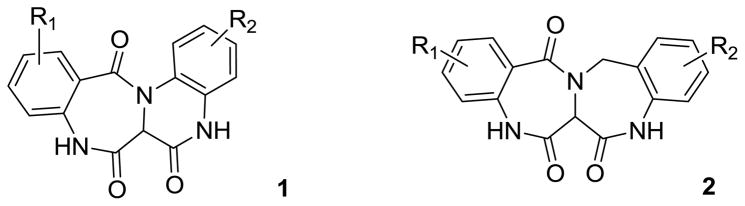
Application of the multi-component reaction (MCR) is a well-known synthetic strategy in which three or more starting materials react in a convergent manner to obtain a single highly functionalized product.1 In drug discovery, MCRs are of interest as an enabling technology to efficiently probe new dimensions of chemical space in the production of small molecule libraries that contain high iterative efficiency potential.2 Of particular note is the Ugi reaction, an isonitrile based MCR in which an amine, carbonyl compound, carboxylic acid, and isonitrile undergo a condensation reaction to afford the corresponding functionalized α–acylamino amide.2b,3 UDC (Ugi/de-protect/cyclize) methodologies employ the Ugi MCR as the initial diversity generating event and subsequently, strategically positioned internal masked nucleophiles are deprotected, promoting a series of ring closing events.4 This methodology has been utilized in the production of a plethora of pharmacologically relevant templates some of which have ultimately led to clinical evaluation.4f, 5 In this report, we describe a 2 step UDC based methodology to obtain fused 6,7,6,6-quinoxalinone-benzodiazepines 1 and 6,7,7,6-bis-benzodiazepines 2 Figure 1 that relies on the incorporation of two masked amine nucleophiles, ethyl glyoxalate as the carbonyl component, and the convertible isocyanide, 4-tert-butyl cyclohexen-1-yl isocyanide. One example of scaffold 1 (R1=R2=H) has been previously reported in 1980 by Massa et al. prepared conventionally in 5 steps, whereas scaffold 2 is completely unreported.6
Figure 1.
Targeted fused tetracyclic quinoxalinone-benzodiazepines 1, and bis-benzodiazepines 2.
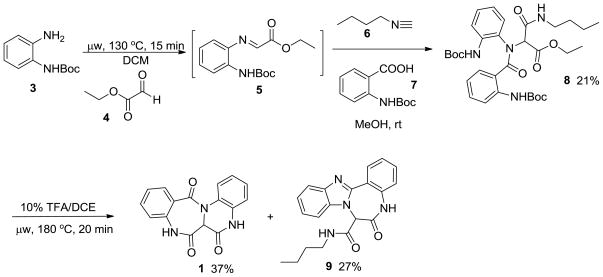
As indicated in Scheme 1, compound 1 was initially prepared by a four-component Ugi reaction involving N-Boc-1,2-phenylenediamine 3, ethyl glyoxylate 4, n-butylisonitrile 6, and Boc-2-aminobenzoic acid 7. However, attempts to form the desired Ugi product by mixing the four components all at once were unsuccessful and resulted in a significant amount of Passerini product and un-reacted amine.7 Accordingly, a microwave procedure was incorporated to pre-form the Schiff base 5 by reacting N-Boc-1,2-phenylenediamine 3 with ethyl glyoxylate 4 in DCM at 130°C for 15 minutes. Lower temperatures required longer times for complete disappearance of starting material and conversion to Schiff base and they were not compatible with succinct, short microwave reaction times. Solvent was then evaporated and the Schiff base, n-butyl isonitrile 6, and Boc-2-aminobenzoic acid 7 were reacted in methanol at room temperature. The Ugi product 8 was successfully obtained albeit in low yield (21%) with the majority of mass balance accounted for by the highly conjugated Schiff base starting material (tlc). The Ugi product 8 was further reacted in the presence of 10% TFA in DCE under microwave irradiation at 180 °C for 20 min to give the target product 1 in low yield (37%). The major side product observed was tetra-cyclic triazadibenzoazulenone 9 (27% yield).
Scheme 1.
Initial synthetic route to scaffold 1.
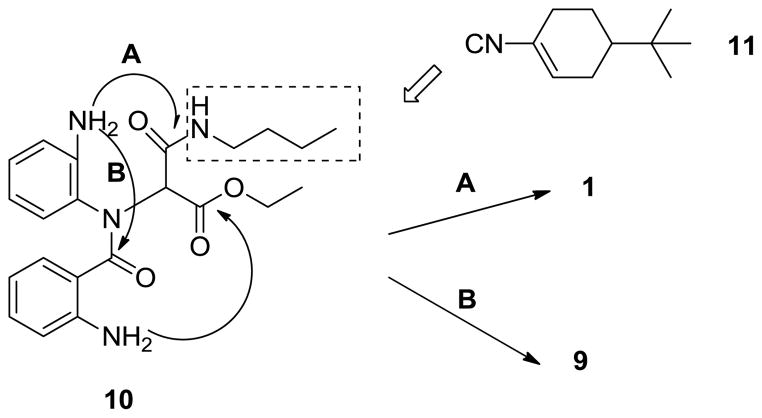
This finding suggested that two electrophilic carbonyls (one derived from the carboxylic acid input, the other emanating from the isonitrile) were competing for the same internal amine. As shown in Scheme 2, upon removal of the N-Boc protecting group on the 1,2-phenylenediamine buried in the Ugi scaffold, this amino group can follow one of two ring closing routes - nucleophilic displacement of the n-butyl group which was derived from the n-butyl isonitrile (path A) or alternatively, a cyclodehydration reaction with the amide group derived from the acid input in the Ugi reaction (path B). The other amine derived from the protected anthranilic acid input however, exclusively reacts with the electrophilic carbonyl from the ester group, which is expected to be rapid.4e In order to redirect the reaction to proceed solely through path A, we incorporated the convertible isonitrile, 4-tert-butyl cyclohexen-1-yl isocyanide 11 in the initial Ugi reaction to increase the electophilic nature of the corresponding amide (this occurs via formation of an activated N-acyliminium species under acidic conditions). Alternatively, we also hypothesized that introduction of a bulkier isonitrile such as tert-butyl isonitrile would favor formation of the corresponding novel triazadibenzoazulenone 9, another potential route to a new generic library in just 2 steps. Thus, subsequently the yield of the Ugi reaction was optimized by the use of co-solvents trifluoroethanol and dichloroethane. As before, the
Scheme 2.
Proposed mechanism of synthesizing benzodiazepines.
Schiff based was formed in DCM via microwave irradiation and then trifluoroethanol was added (1:1, v/v, trifluoroethanol:DCM) along with the acid and isonitrile components. When using this alternative procedure, the Ugi yield increased from 21% to 46%, Scheme 3. Addition of trifluoroethanol has been shown to produce a similar increase in yields for other Ugi reactions.8
Scheme 3.
New process of synthesizing compound 1.
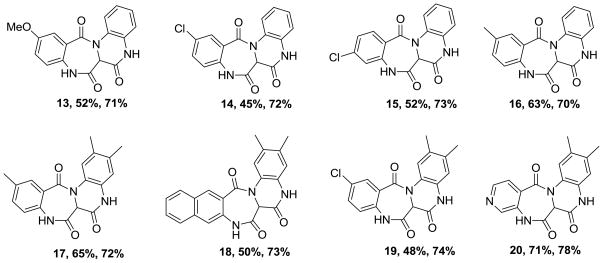
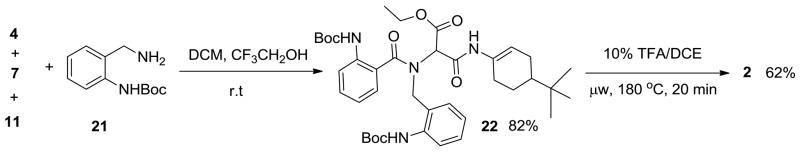
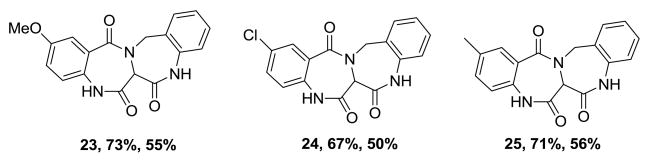
With the Ugi product 12 in-hand, cyclization under microwave irradiation at 180°C for 20 min afforded compound 1 good yield (67%, 31% overall, two steps).9 With satisfactory conditions in place, a small array of fused 6,7,6,6-quinoxalinone-benzodiazepine derivatives was thus synthesized with diversity being generated through the utilization of various benzoic acids and N-Boc diamines to afford the Ugi and final cyclization products with yields of 45–71% and 67–78%, respectively (Figure 2). In synthesizing the small array of molecules, we observed a slight increase in the yield of the Ugi product when using mono-N-Boc-3,4-dimethylphenylenediamine. This may be attributed to the increased basicity of the highly conjugated Schiff base derived from this input. Consequently, a methylene linker was inserted to break conjugation of the Schiff base intermediate by use of tert-butyl 2-(aminomethyl)phenylcarbamate 21 as the amine source, Scheme 4. Schiff base formation was accomplished in just 15 minutes at room temperature and addition of acid and isonitrile gave the Ugi product 22 in high yield (82%). However, the increased flexibility associated with an additional methylene linker and increased free energy associated with forming a 7-member ring in lieu of a 6-member ring, had a negative effect on the second cyclization to form 2 in lower observed yields (62%).10 Three additional tetracyclic bis-benzodiazepines 23, 24 and 25 were also synthesized and yields are shown, Figure 3.
Figure 2.
Structures of bicyclic quinoxalinone-benzodiazepines (Ugi % yield, Cyclization % yield).
Scheme 4.
Route to fused bis-benzodiazepine 2.
Figure 3.
Structures of fused bis-benzodiazepines (Ugi % yield, Cyclization % yield).
In summary, two series of tetracyclic quinoxalinone-benzodiazepines and novel fused bis-benzodiazepine scaffolds were synthesized in two steps employing a UDC strategy. Ugi reactions were optimized by addition of trifluoroethanol and control over cyclization modes was attained by employing 4-tert-butyl cyclohexen-1-yl isocyanide, respectively. Based on the uniqueness of these scaffolds, the desirable drug-like properties of the molecules (compound 1, MW=293, cLogP=1.19, tPSA=78.51), and ease of synthesis, these scaffolds have the potential to be of interest in future library enrichment strategies.
Acknowledgments
We would like to thank the Office of the Director, NIH and the National Institute of Mental Health for funding (1RC2MH090878-01). Abbott Laboratories are also thanked for a New Faculty Award to CH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Bienayme H, Hulme C, Oddon G, Schmitt P. Chem Eur J. 2000;6(10):3321. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; (b) Larsen SD, Grieco PA. J Am Chem Soc. 1985;107:1768. [Google Scholar]; (c) Frantz DE, Morency L, Soheili A, Murry JE, Grabowski EJJ, Tillyer RD. Org Lett. 2004;6:843. doi: 10.1021/ol0498803. [DOI] [PubMed] [Google Scholar]; (d) Dhawan R, Dghaym RD, Arndtsen BA. J Am Chem Soc. 2003;125:1474. doi: 10.1021/ja027474d. [DOI] [PubMed] [Google Scholar]; (e) Rossen K, Pye PJ, Di Michele LM, Volante K, Reider PJ. Tetrahedron Lett. 1998;39:6823. [Google Scholar]
- 2.(a) Dolle RE, La Bourdonnec B, Goodman AJ, Morales GA, Thomas CJ, Zhang W. J Comb Chem. 2009;11:739–790. doi: 10.1021/cc9000828. [DOI] [PubMed] [Google Scholar]; (b) Dõmling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]; (c) Hulme C, Gore V. Curr Med Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]; (d) Hulme C, Lee YS. Mol Div. 2008;12:1–15. doi: 10.1007/s11030-008-9072-1. [DOI] [PubMed] [Google Scholar]; (e) Hulme C, Nixey T. Curr Opin Drug Discovery Dev. 2003;6:921–929. [PubMed] [Google Scholar]
- 3.(a) Ugi I. Angew Chem. 1962;74:9–22. [Google Scholar]; (b) Dõmling A, Ugi I. Angew Chem, Int Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]; (c) Hulme C, Peng J, Louridas B, Menard P, Krolikowski P, Kumar NV. Tetrahedron Lett. 1998;39:8047. [Google Scholar]; (d) Hulme C, Cherrier MP. Tetrahedron Lett. 1999;40:5295. [Google Scholar]; (e) Hulme C, Ma L, Labaudiniere R. Tetrahedron Lett. 2000;41:1509. [Google Scholar]; (f) Hulme C, Ma L, Romano J, Morton G, Tang SY, Cherrier MP, Choi S, Labaudiniere R. Tetrahedron Lett. 2000;41:1883. [Google Scholar]
- 4.(a) Nixey T, Tempest P, Hulme C. Tetrahedron Lett. 2002;43:1637. [Google Scholar]; (b) Hulme C, Ma L, Romano J, Cherrier MP, Salvino J, Labaudiniere R. Tetrahedron Lett. 2000;41:1889. [Google Scholar]; (c) Hulme C, Cherrier MP. Tetrahedron Lett. 1999;40:5295. [Google Scholar]; (d) Hulme C, Ma L, Labaudiniere R. Tetrahedron Lett. 2000;41:1509. [Google Scholar]; (e) Hulme C, Chappeta S, Griffith C, Lee YS, Dietrich J. Tetrahedron Lett. 2009;50:1939–1942. [Google Scholar]
- 5.(a) Hulme C, Peng J, Tang SY, Burns CJ, Morize I, Labaudiniere R. J Org Chem. 1998;63:8021–8023. [Google Scholar]; (b) Nixey T, Hulme C. Tetrahedron Lett. 2002;43:6833–6835. [Google Scholar]; (c) Habashita H, Kokubo M, Hamano SI, Hamanaka N, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. J Med Chem. 2006;49:4140–4152. doi: 10.1021/jm060051s. [DOI] [PubMed] [Google Scholar]; (d) Nishizawa R, Nishiyama T, Hisaichi K, Matsunaga N, Minamoto C, Habashita H, Takaoka Y, Toda M, Shibayama S, Tada H, Sagawa K, Fukushima D, Maeda K, Mitsuya H. Bioorg Med Chem Lett. 2007;17:727–731. doi: 10.1016/j.bmcl.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 6.Massa S, Corelli F, Stefancich G, De Martino G. J Heterocyclic Chem. 1980;17:1781–1782. [Google Scholar]
- 7.(a) Ugi I, Steinbrucker C. Chem Ber. 1961;94:734–742. [Google Scholar]; (b) Ugi I. Angew Chem, Int Ed Engl. 1962;1:8–20. [Google Scholar]; (c) Ugi I, Dõmling A, Horl W. Endeavor. 1994;18:115. [Google Scholar]
- 8.(a) Marcaccini S, Torroba T. Nature Protocols. 2007;2:632–639. doi: 10.1038/nprot.2007.71. [DOI] [PubMed] [Google Scholar]; (b) Mossetti R, Pirali T, Tron GC. J Org Chem. 2009;74:4890–4892. doi: 10.1021/jo9005969. [DOI] [PubMed] [Google Scholar]; (c) Zhdanko AG, Gulevich AV, Nenajdenko VG. Tetrahedron Lett. 2009;65:4692–4702. [Google Scholar]
- 9.For the preparation of 12 and general library protocol: A solution of N-BOC-1,2-phenylenediamine (0.21 g, 1.00 mmol) and ethyl glyoxalate (50%, 0.20 ml, 1.00 mmol) in DCM (1.00 ml) was reacted in microwave at 130°C for 15 min. After the reaction mixture was cooled down to room temperature, appropriate benzoic acid (1.00 mmol), trifluoroethanol (1.0 ml) and 4-tert-butyl cyclohexen-1-yl isocyanide (0.16 g, 1.00 mmol) were added. The resulting solution was stirred overnight, and the solvent was removed in vacuo. The product was purified by column chromatography (hexanes: ethyl acetate, 1:4) to afford a white solid 12 (320mg, 0.46mmol).
- 10.For the preparation of 22 and general library protocol: A solution of tert-Butyl 2-(aminomethyl)phenylcarbamate (0.22 g, 1.00 mmol) and ethyl glyoxalate (50%, 0.20 ml, 1.00 mmol) in DCM (1.00 ml) was reacted at room temperature for 15 min. Then, appropriate benzoic acid (1.00 mmol), trifluoroethanol (1.0 ml) and 4-tert-butyl cyclohexen-1-yl isocyanide were added with stirring and followed by TLC (reactions completed between 20 min and 60 min). When there was no SM left, the solvent was removed in vacuo. The product was purified by column chromatography (hexane: ethyl acetate, 1:4) to afford a white solid (580mg, 0.82 mmol). For the preparation of 1, 2 and general library protocol: The appropriate Ugi product (0.20 mmol) in 10% TFA/DCE (5.0 ml) was reacted in microwave at 180°C for 20 min. The solvent was removed under reduced pressure and the residue was purified by column chromatography (hexanes: ethyl acetate, 2:3) to afford the pure solid. Compound 1: 1H NMR (300 MHz, DMSO) 11.08 (s, 1H), 10.92 (s, 1H), 8.06 (d, J = 8.2 Hz, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.62 (t, J = 7.7 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.10 (d, J = 7.1 Hz, 1H), 7.04–6.95 (m, 2H), 5.12 (s, 1H); 13C NMR (75MHz, DMSO) 166.73, 165.84, 161.10, 136.89, 133.21, 131.81, 128.37, 126.55, 125.38, 124.62, 122.85, 122.60, 121.78, 120.90, 116.39, 57.74. Compound 2: 1H NMR (300 MHz, DMSO) 10.52 (s, 2H), 7.78 (d, J = 7.8 Hz, 1H), 7.52 (t, J = 7.7 Hz, 1H), 7.37–7.16 (m, 3H), 7.08 (t, J = 7.4 Hz, 3H), 5.38 (d, J = 13.8 Hz, 1H), 4.88 (s, 1H), 4.35 (s, 1H); 13C NMR (75 MHz, DMSO) 168.27, 166.12, 137.26, 133.58, 132.30, 130.14, 127.34, 125.15, 121.71, 121.62, 100.67, 79.37, 45.81.