Abstract
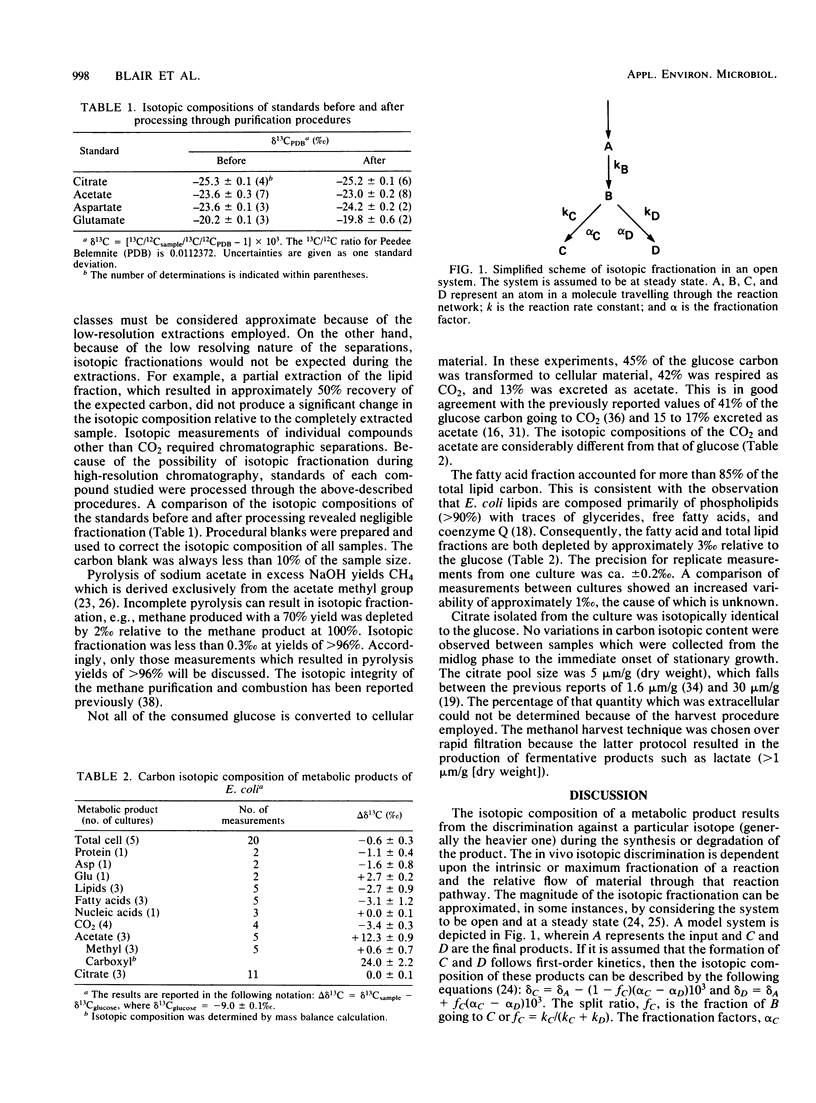
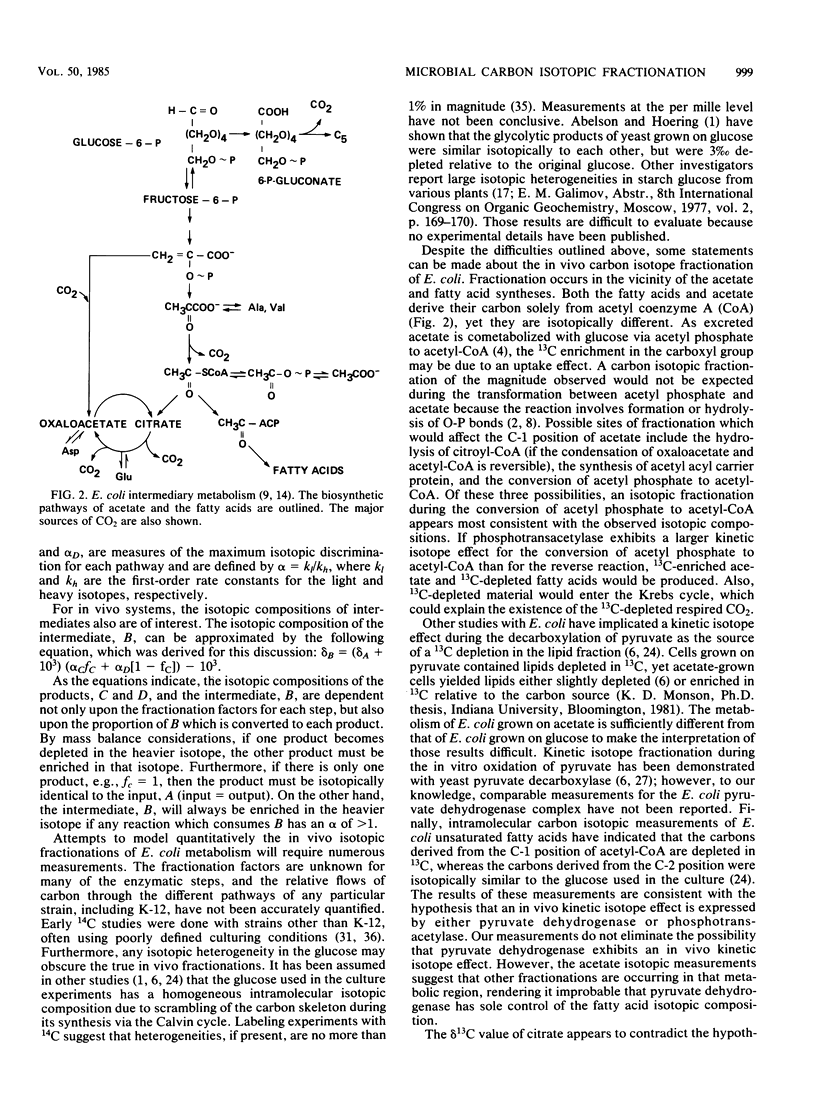
Differences in the natural-abundance carbon stable isotopic compositions between products from aerobic cultures of Escherichia coli K-12 were measured. Respired CO2 was 3.4% depleted in 13C relative to the glucose used as the carbon source, whereas the acetate was 12.3% enriched in 13C. The acetate 13C enrichment was solely in the carboxyl group. Even though the total cellular carbon was only 0.6% depleted in 13C, intracellular components exhibited a significant isotopic heterogeneity. The protein and lipid fractions were -1.1 and -2.7%, respectively. Aspartic and glutamic acids were -1.6 and +2.7%, respectively, yet citrate was isotopically identical to the glucose. Probable sites of carbon isotopic fractionation include the enzyme, phosphotransacetylase, and the Krebs cycle.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELSON P. H., HOERING T. C. Carbon isotope fractionation in formation of amino acids by photosynthetic organisms. Proc Natl Acad Sci U S A. 1961 May 15;47:623–632. doi: 10.1073/pnas.47.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blättler W. A., Knowles J. R. Stereochemical course of phosphokinases. The use of adenosine [gamma-(S)-16O,17O,18O]triphosphate and the mechanistic consequences for the reactions catalyzed by glycerol kinase, hexokinase, pyruvate kinase, and acetate kinase. Biochemistry. 1979 Sep 4;18(18):3927–3933. doi: 10.1021/bi00585a013. [DOI] [PubMed] [Google Scholar]
- Brown T. D., Jones-Mortimer M. C., Kornberg H. L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977 Oct;102(2):327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- DeNiro M. J., Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977 Jul 15;197(4300):261–263. doi: 10.1126/science.327543. [DOI] [PubMed] [Google Scholar]
- Holms W. H., Bennett P. M. Regulation of isocitrate dehydrogenase activity in Escherichia coli on adaptation to acetate. J Gen Microbiol. 1971 Jan;65(1):57–68. doi: 10.1099/00221287-65-1-57. [DOI] [PubMed] [Google Scholar]
- Kates M. Bacterial lipids. Adv Lipid Res. 1964;2:17–90. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Meinschein W. G., Rinaldi G. G., Hayes J. M., Schoeller D. A. Intramolecular isotopic order in biologically produced acetic acid. Biomed Mass Spectrom. 1974 Jun;1(3):172–174. doi: 10.1002/bms.1200010306. [DOI] [PubMed] [Google Scholar]
- Monson K. D., Hayes J. M. Biosynthetic control of the natural abundance of carbon 13 at specific positions within fatty acids in Saccharomyces cerevisiae. Isotopic fractionation in lipid synthesis as evidence for peroxisomal regulation. J Biol Chem. 1982 May 25;257(10):5568–5575. [PubMed] [Google Scholar]
- O'Leary M. H. Carbon isotope effect of the enzymatic decarboxylation of pyruvic acid. Biochem Biophys Res Commun. 1976 Dec 6;73(3):614–618. doi: 10.1016/0006-291x(76)90854-8. [DOI] [PubMed] [Google Scholar]
- Park R., Epstein S. Metabolic fractionation of C & C in plants. Plant Physiol. 1961 Mar;36(2):133–138. doi: 10.1104/pp.36.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster R. Determination of free amino acids by high performance liquid chromatography. Anal Chem. 1980 Apr;52(4):617–620. doi: 10.1021/ac50054a005. [DOI] [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorio P. V., Krotkov G., Reed G. B. Degradation of Radioactive Glucose. Science. 1952 May 23;115(2995):567–568. doi: 10.1126/science.115.2995.567. [DOI] [PubMed] [Google Scholar]
- WANG C. H., STERN I., GILMOUR C. M., KLUNGSOYR S., REED D. J., BIALY J. J., CHRISTENSEN B. E., CHELDELIN V. H. Comparative study of glucose catabolism by the radiorespirometric method. J Bacteriol. 1958 Aug;76(2):207–216. doi: 10.1128/jb.76.2.207-216.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Sackett W. M. Isotope Fractionation in Photosynthetic Bacteria during Carbon Dioxide Assimilation. Plant Physiol. 1975 Mar;55(3):475–479. doi: 10.1104/pp.55.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen G., Blair N., Des Marais D. J., Chang S. Carbon isotope composition of low molecular weight hydrocarbons and monocarboxylic acids from Murchison meteorite. Nature. 1984 Jan 19;307(5948):252–254. doi: 10.1038/307252a0. [DOI] [PubMed] [Google Scholar]


