Synopsis
In 1923, Friedrich Wohlwill described two patients with a “microscopic form of periarteritis nodosa”, which was distinct from classical polyarteritis nodosa. This disease, now known as microscopic polyangiitis (MPA), is a primary systemic vasculitis characterized by inflammation of the small-caliber blood vessels and the presence of circulating antineutrophil cytoplasmic antibodies (ANCA). Typically, microscopic polyangiitis presents with glomerulonephritis and pulmonary capillaritis, although involvement of the skin, nerves, and gastrointestinal tract is not uncommon. Treatment of MPA generally requires use of a cytotoxic agent (such as cyclophosphamide) in addition to high-dose glucocorticoids. Recent research has focused on identifying alternate treatment strategies that minimize or eliminate exposure to cytotoxic agents. This article will review the history, pathogenesis, clinical manifestations, and treatment of MPA.
Microscopic polyangiitis is an idiopathic autoimmune disease characterized by a systemic vasculitis that predominantly affects the small- caliber blood vessels, and is associated with the presence of antineutrophil cytoplasmic autoantibodies (ANCA). Because of its relationship to ANCA, it is often classified as a form of ANCA-associated vasculitis, an important subset of the primary systemic vasculitides that includes Wegener’s granulomatosis (WG), the Churg-Strauss syndrome (CSS), and renal-limited vasculitis. Because it can lead to both pulmonary capillaritis and glomerulonephritis, MPA is also a prime cause of the pulmonary-renal syndrome, a group of disorders that includes Goodpasture’s syndrome (which is associated with anti-glomerular basement membrane [GBM] antibodies), systemic lupus erythematosus, and WG. In this review, we will discuss the history, pathogenesis, clinical manifestations, and treatment of MPA.
Historical Overview and Epidemiology
Although syphilitic aneurysms had been recognized since the 1500s, the first complete description of a primary systemic vasculitis came in 1866, when Kussmaul and Maier described the plight of Carl Seufarth, a 27 year old journeyman tailor who had rapidly become incapacitated by fevers, myalgias, renal insufficiency, neuropathy, and abdominal pain. At autopsy, they described “[p]eculiar mostly nodular thickening … of countless arteries of and below the caliber of the liver artery and the major branches of the coronary arteries of the heart, principally in the bowel, stomach, kidneys, spleen, heart, and voluntary muscles, and to a lesser extent also in the liver, subcutaneous cell tissue and the bronchial and phrenic arteries.”[1] Although the significance of these findings, which they dubbed “periarteritis nodosa”, was not immediately clear, this is now widely recognized as the archetypal description of polyarteritis nodosa.[2]
For years after this description, all patients with a non-infectious arteritis were classified as having polyarteritis nodosa. In 1923, Friedrich Wohlwill described two patients who appeared to have a novel form of this disease, characterized by the presence of glomerulonephritis and non-granulomatous inflammation of the small-caliber blood vessels.[3] This “microscopic form of periarteritis nodosa” was gradually recognized as a new entity, distinct from classic polyarteritis nodosa. In 1953, Pearl Zeek noted that this disease was pathologically similar to hypersensitivity vasculitis, preferentially involving the arterioles and venules of the visceral organs (including the lung) but often sparing the medium-caliber blood vessels.[4] In 1950, Wainwright and Davson used the phrase “microscopic polyarteritis” to describe this phenotype.[5]
In 1985, Caroline Savage et al. defined “microscopic polyarteritis” as a small vessel vasculitis associated with focal segmental glomerulonephritis and hemoptysis.[6] In 1994, the Chapel Hill Consensus Conference proposed the term “microscopic polyangiitis” to describe patients with a small-vessel vasculitis characterized by the absence of immune complex deposition on immunofluorescence, and the presence of pulmonary capillaritis and glomerulonephritis.[7] The new name emphasized the differences between this phenomenon and “classic” polyarteritis nodosa, which was defined as a medium-vessel vasculitis that spared the arterioles and venules. Despite this clear distinction, distinguishing these two phenomena clinically is not always straightforward; the classic description of polyarteritis nodosa by Kussmaul and Maier, for example, includes evidence of a small vessel vasculitis.[8] Moreover, the Chapel Hill Consensus Conference criteria do not always clearly distinguish MPA from other forms of vasculitis, such as Wegener’s granulomatosis.[9] Regardless, the introduction of this nomenclature resulted in a rapid reduction in the prevalence of polyarteritis nodosa, due to the reclassification of many of these patients as having MPA.[10]
In 1954, Godman and Churg noted that the “microscopic form of periarteritis” was closely related to WG and CSS.[11] In the ensuing years, it gradually became clear that these three forms of systemic vasculitis were also linked by the presence of anticytoplasmic antibodies directed against neutrophils. Antineutrophil cytoplasmic antibodies (ANCA) were first reported in association with focal segmental glomerulonephritis in the 1980s.[12] Subsequent work demonstrated that these antibodies were associated with distinct staining patterns when alcohol-fixed neutrophils were used as a substrate. In 1988, Jennette and Falk reported that serum from patients with WG, renal-limited vasculitis, and MPA was associated with antibodies that created a perinuclear staining pattern.[13] This p-ANCA pattern is caused by antibodies against myeloperoxidase. Some authors have suggested that MPO-ANCA be used to distinguish MPA from polyarteritis nodosa, [14] although these antibodies are also found in other forms of vasculitis, including drug-induced ANCA-associated vasculitis, CSS, and WG.
Regardless, ANCA has become a useful tool for the diagnosis of vasculitis, and may be partially responsible for the perceived increase in prevalence of the primary systemic vasculitides.[15] Southern Sweden has the highest reported prevalence of MPA, with 94 cases per million.[16] Overall, however, the incidence of MPA is higher in southern Europe than in northern Europe; for example, the incidence of MPA in Norway is 2.7 per million,[17] but 11.6 per million in Spain (see Table 1).[18] The incidence and prevalence of MPA in other parts of the world is less clear, but the prevalence seems to be higher in European populations.[19]
Table 1. Incidence of microscopic polyangiitis in Europe.
Data from reference [84]
| Cases per million population | |
|---|---|
| Norway | 2.7 |
| United Kingdom | 5.8 |
| Germany | 2.6 |
| Spain | 11.6 |
Pathogenesis
Growing evidence indicates that ANCA play a role in the pathogenesis of MPA. In theory, this might occur in two steps. In the first step, neutrophils are primed by exposure to low-levels of proinflammatory cytokines, such as interleukin-1 or tumor necrosis factor-α.[20] This process leads to surface expression of myeloperoxidase, followed by adherence of neutrophils to the endothelial surface of blood vessels or glomeruli. In the second step, neutrophils are activated by interaction with MPO-ANCA, either through binding of its substrate[21] or interaction with neutrophil Fc receptors.[22]
Two animal models support a potential role for MPO-ANCA in the pathogenesis of MPA,[23, 24] demonstrating that MPO-ANCA are sufficient to induce pulmonary capillaritis and glomerulonephritis given the correct biologic milieu. Also in support of this role is a case report describing pulmonary hemorrhage and renal insufficiency newborn infant, presumably mediated by passage of MPO-ANCA from mother to fetus.[25] A subsequent case report, however, documents that placental transmission of MPO-ANCA is not sufficient to induce disease.[26] The development of vasculitis likely requires the presence of several co-factors, including genetic predisposition, in order for ANCA to be pathogenic.
This model fails to address the substantial number of patients who are ANCA-negative at the time of diagnosis.[27] It is interesting to note that not all patients with active vasculitis are ANCA positive, and MPO-ANCA titers themselves correlate poorly with disease activity in MPA. These observations imply that ANCA are not essential to pathogenesis in all patients with MPA, or that more than one mechanism can lead to the same clinical diagnosis. For example, recent work indicates that ANCA directed against lysosomal membrane protein-2 (LAMP-2), possibly induced by exposure to FimH-expressing gram negative bacteria, may play a key role in the development of vasculitis in some patients[28] (although this work has not yet been widely replicated).
Clinical Features
MPA has a slight male predominance (male:female ratio of 1.8:1) [14, 29, 30], with an average age of onset between 50–60 years [14, 19, 29]. As expected for an illness that affects multiple organ systems, patients with MPA can present with a myriad of different symptoms. However, over 70% of patients have constitutional symptoms such as fever or weight loss at diagnosis.[6, 29] Patients can present acutely (i.e., having symptoms from days to weeks) or have an indolent course before diagnosis. For example, nonspecific symptoms such as a flu-like illness[6] or arthralgias can be present for months to years prior to diagnosis.[30] In this section, we will discuss the major clinical manifestations of MPA, presented by organ system.
Renal manifestations
Renal involvement, characterized by rapidly progressive glomerulonephritis (RPGN), is the major clinical feature of MPA. Previous studies report that 80–100% of patients with MPA experience renal manifestations[6, 14], which can range from an asymptomatic urinary sediment to end-stage renal disease requiring dialysis.[30] Consistent with glomerulonephritis, the most common clinical manifestations of renal involvement are proteinuria (in the nephrotic range in up to 50% of patients), microscopic hematuria, and urinary granular or red blood cell casts.[6]
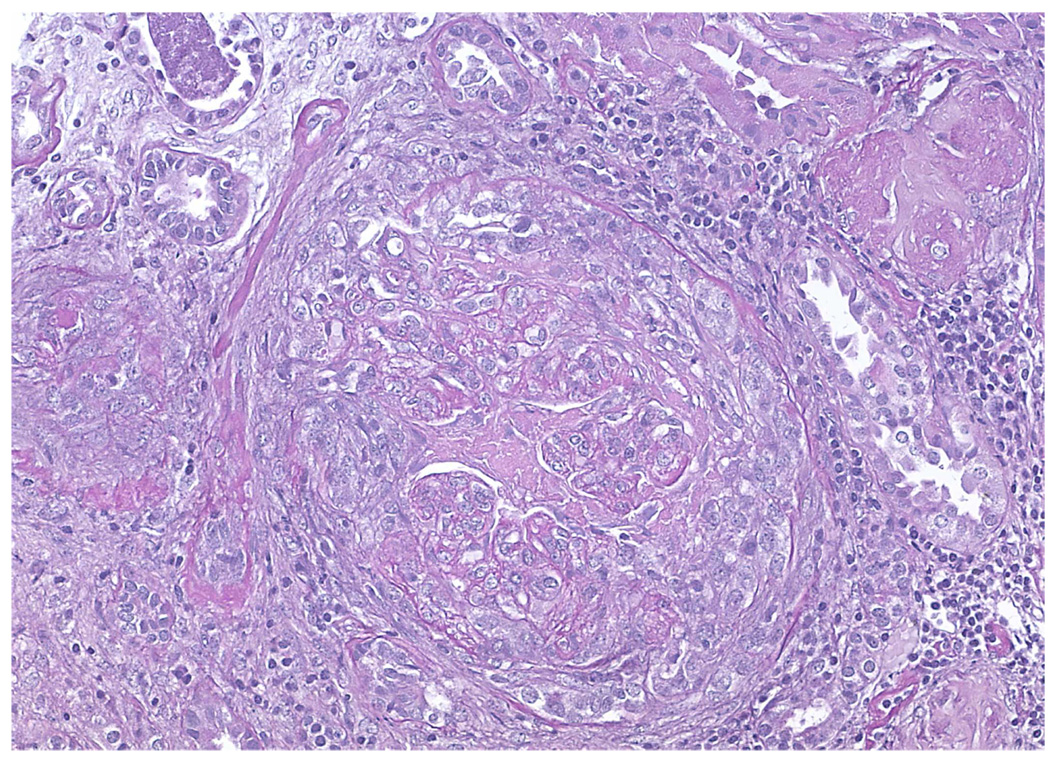
The hallmark finding on renal biopsy is focal segmental necrotizing glomerulonephritis, which is seen in up to 100% of patients (Figure 1).[6] Glomerular crescents are also common, and can be present in approximately 90% of patients.[6] Frank vasculitis and fibrinoid necrosis are seen less frequently, and observed in less than 20% of patients.[6] Areas away from the glomeruli can be affected as well; for example, interstitial nephritis and tubular atrophy are seen in approximately half of MPA patients.[6] Immunofluorescence demonstrates minimal deposition of immunoglobulins or complement in the glomeruli and renal vessels (hence the descriptive term “pauci-immune”), unlike other small vessel vasculitis diseases such as Henoch-Schönlein purpura, cryoglobulinemic vasculitis, or anti-GBM disease.[31] The changes seen on renal biopsy are similar for the three ANCA-associated vasculitidies (MPA, WG, and CSS), and thus cannot be used to distinguish between these entities.
Figure 1.
Crescentic glomerulonephritis in a patient with microscopic polyangiitis (hematoxylin and eosin stain).
The mainstay of treatment for renal involvement in MPA is glucocorticoids and cyclophosphamide. With such therapy, approximately 90% of patients enter a complete or partial remission.[32] However, despite immunosuppression, approximately 20% of patients in one series progressed to end stage renal disease and required renal replacement therapy, either in the form of kidney transplant or dialysis. Not surprisingly, a low serum creatinine at time of diagnosis in this group predicted a better renal survival rate.[32]
Of note, ANCA and anti-GBM antibodies can co-exist. Approximately 30% of patients with anti-GBM antibodies have circulating ANCA.[33, 34] Conversely, a lower percentage (5–14%) of patients with a positive ANCA have evidence of anti-GBM antibodies.[33, 35] In patients with concurrent ANCA and anti-GBM antibodies, the majority of the ANCA are directed against MPO (66–100%).[33–35] In a series of 22 patients with both antibodies who underwent renal biopsy, all biopsies showed linear deposition of IgG and C3 on immunofluorescence. Four of the 22 biopsies (18.2%) also showed granular deposition of IgG, IgM, and C3. Patients with both ANCA and anti-GBM antibodies are sometimes treated with plasma exchange in addition to conventional immunouppression.[33] Studies of whether these “double positive” patients have worse renal outcomes compared to those with only ANCA or anti-GBM antibodies have conflicting results.[33] Some suggest that these patients are more likely to relapse than those patients with anti-GBM antibodies alone.[34, 36] Regardless, lower serum creatinine at diagnosis is associated with an increased renal survival rate.[33]
Pulmonary manifestations
Pulmonary involvement can be seen in 25–55% of patients. Manifestations include hemoptysis and alveolar hemorrhage, infiltrates, pleural effusion, pulmonary edema, pleuritis, and interstitial fibrosis.[6, 14, 30] The classic pulmonary manifestation of MPA is diffuse alveolar hemorrhage caused by pulmonary capillaritis, which has been reported in 12–55% of patients.[37–39] Common presenting symptoms of alveolar hemorrhage include dyspnea, cough, hemoptysis, and pleuritic chest pain.[40]
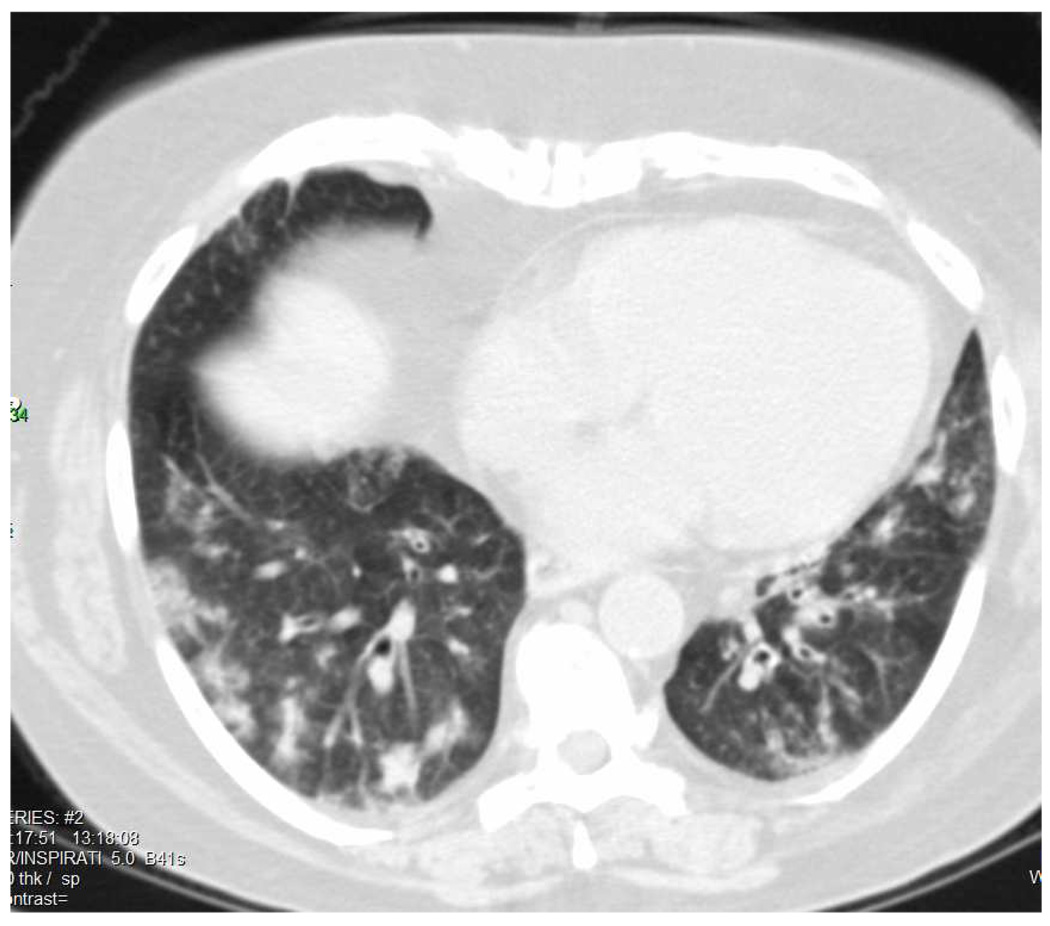
In patients with alveolar hemorrhage, chest radiographs show patchy, bilateral airspace opacities, usually involving both the upper and lower lung fields.[37, 39] The most common finding on computed tomography (CT) is ground-glass attenuation (seen in >90% of patients), which corresponds to alveolar hemorrhage, interstitial chronic inflammation of the alveolar septa, and capillaritis (see Figure 2). Consolidation is seen in ~80% of patients. Thickening of the bronchovascular bundles and honeycombing is also observed.[37, 39]
Figure 2.
Computed tomography scan demonstrating evidence of pulmonary hemorrhage in a patient with microscopic polyangiitis.
Patients often undergo bronchoscopy to evaluate the cause of bleeding. For patients with alveolar hemorrhage, bronchoalveolar lavage (BAL) fluid is usually grossly hemorrhagic, and on sequential lavage, the fluid remains bloody.[37, 39, 41] Perls Prussian blue staining of the BAL fluid shows elevated numbers of hemosiderin-laden macrophages (which are present in over 30% of patients with MPA).[37, 39]
Lung biopsy of alveolar hemorrhage can show intra-alveolar and interstitial red blood cells, pauci-immune, hemorrhagic necrotizing alveolar capillaritis[38], neutrophilic infiltration resulting in fibrinoid necrosis and dissolution of the arterial and venular walls [41], and intra-alveolar hemosideroisis.[37] Of note, granulomatous inflammation is usually not observed in MPA and its presence suggests an alternative diagnosis, such as WG.
Pulmonary function testing can show either restrictive or obstructive patterns. The most frequent abnormality is reduced carbon monoxide diffusing capacity, which can increase dramatically during active alveolar hemorrhage.[38]
Treatment options for patients with alveolar hemorrhage due to MPA include aggressive immunosuppression and plasma exchange.[39] In severe cases, mechanical ventilation can be required to maintain oxygenation. A few case reports demonstrate successful use of extracorporeal membrane oxygenation.[39] However, alveolar hemorrhage is associated with a worse prognosis in patients with MPA. Patients with pulmonary hemorrhage are nine times more likely to die and have higher rates of relapse.[39]
Given the predominance of pulmonary and renal manifestations in MPA, MPA is a well-recognized cause of pulmonary-renal syndromes, along with other autoimmune diseases such as WG, anti-GBM disease, and systemic lupus erythematosus. One study suggests that MPA is the most common cause of pulmonary-renal syndromes.[42]
Pulmonary fibrosis is a less well-recognized pulmonary manifestation of MPA.[43–45] Fibrosis can present months to years prior to, at time of, or years after the diagnosis of MPA.[45, 46] While the etiology of the fibrosis is unclear, chronic subclinical alveolar hemorrhage has been presented as a possible cause.[44] The prognosis for patients with pulmonary fibrosis is poor[45], but may be improved by the institution of immunosuppressive agents.[38, 44]
More unusual pulmonary manifestations attributed to MPA include pulmonary artery aneurysms[47] and panbronchiolitis.[48]
Skin manifestations
Skin lesions are found in 30–60% of patients [6, 14, 30, 49], and are the initial presenting sign in 15–30% of patients.[49] Palpable purpura is the most common manifestation, and occurs in 30–40% of patients.[49, 50] Other manifestations include livedo reticularis, nodules, urticaria, and skin ulcers with necrosis.[49] Dermatologic manifestations have been associated with arthralgias in patients with MPA.[49]
Biopsies of palpable purpura often show leukocytoclastic vasculitis, with neutrophilic infiltration of the small-caliber vessels in the superficial dermis, fibrinoid necrosis, and nuclear dust.[50] However, a nonspecific perivascular lymphocytic infiltration can also be seen.[49, 51] Biopsies of cutaneous nodules generally show vasculitis involving vessels of the deep dermis or subcutis.[49] Immunofluorescence studies are generally negative or show few deposits of immunoglobulins and complement.[49, 51] While nodules are seen more frequently in polyarteritis nodosa, and palpable purpura is more frequent in MPA, both dermatologic manifestations occur in both diseases. Thus, these manifestations and their histologic findings cannot be used to differentiate between polyarteritis nodosa and MPA.
Gastrointestinal manifestations
The most frequently reported gastrointestinal symptom in MPA is abdominal pain[52], which can occur in 30–58% of patients.[14, 30] While gastrointestinal bleeding occurs in up to 21–29% of patients[52, 53], massive hemorrhage is rare.[54] Angiographic studies, although not routinely performed, have shown arterial aneursyms[54, 55] as a potential source of bleeding. Other gastrointestinal manifestations such as colonic ulcerations[56], intestinal ischemia[52, 57], and bowel perforation[52] have been reported. However, they are likely less frequent in MPA compared to polyarteritis nodosa, since there are fewer reports of these manifestations in MPA patients in the published literature.
Involvement of the liver occurs rarely in MPA. Liver dysfunction in MPA may present as elevated liver enzymes, with alkaline phosphatase and γ-glutamyl transferase more affected than aspartate or alanine transaminase. These abnormal findings can precede the development of glomerulonephritis or pulmonary hemorrhage.[58–61] Histologic findings from liver biopsies performed from these cases have shown fibrinoid degeneration of an interlobular arteriole[60] as well as necrotizing arteritis and lymphocytic infiltration of portal tracts.[61] In addition, primary biliary cirrhosis has been reported in patients with MPA[62, 63], but whether the association is causal is unknown.
Neurologic manifestations
Neurologic involvement in MPA is common, and affects between 37–72% of patients.[14, 29, 64] Peripheral neuropathy occurs more frequently than central nervous system involvement, with mononeuritis multiplex and distal symmetrical polyneuropathy as the predominant peripheral nervous system manifestations. Necrotizing vasculitis can be seen on sural nerve biopsy in up to 80% of affected patients, and nerve conduction studies typically show acute axonopathy.[65] Some studies suggest that relapse rates of peripheral neuropathy are low [65, 66], but this area warrants further investigation.
Central nervous system manifestations account for 17–30% of the neurologic involvement seen in MPA.[14, 64] Manifestations are quite varied, and can include cerebral hemorrhage[29], pachymeningitis[67], and non-hemorrhagic cerebral infarctions.[68]
Laboratory testing
Currently, there is no laboratory test that has diagnostic specificity for MPA. Since ANCA are detected in only 50–75% of MPA patients[14, 30], the absence of circulating ANCA does not exclude this diagnosis. ANCA associated with MPA generally has a perinuclear staining pattern (P-ANCA) caused by antibodies against myeloperoxidase (MPO-ANCA), which can be detected using enzyme-linked immunoassays (ELISA). Immunofluorescence has greater sensitivity, but the ELISA has greater specificity for the diagnosis of MPA. Unfortunately, neither test is specific for MPA, since these antibodies can be found in patients with the other ANCA-associated vasculitides in addition to other inflammatory diseases[69], such as drug-induced ANCA-associated vasculitis[70], cystic fibrosis[71], and various infections[72, 73].
Nonspecific markers of inflammation are also observed in patients with MPA. The most common findings are an elevated erythrocyte sedimentation rate and C-reactive protein. Other findings include elevated white blood cell and platelet counts, and a normochromic, normocytic anemia.[53]
Treatment
Given that most patients will present with glomerulonephritis, initial therapy of MPA generally entails the use of glucocorticoids and a cytotoxic agent such as cyclophosphamide. Using this regimen, remission can be achieved in 90% of patients. The regimen, first described by Anthony Fauci and Sheldon Wolfe for the treatment of WG, used daily oral cyclophosphamide (2 mg/kg/day) for years, both to induce and maintain remission.[74] Although effective, this regimen is associated with substantial toxicity, including infertility, malignancy, and hemorrhagic cystitis.[75] For this reason, substantial effort has been expended to find ways to minimize cyclophosphamide exposure, either by developing alternate dosing regimens or identifying subsets of patients who can be treated without resorting to cytotoxic agents.
Remission maintenance strategies
The Cyclophosphamide versus Azathioprine for early Remission phase of vasculitis trial (CYCAZAREM) treated 60 subjects with MPA and 95 subjects with WG who had involvement of the kidneys or another vital organ.[76] All subjects were treated with a remission induction regimen of daily oral cyclophosphamide (2 mg/kg/day) and prednisolone for 3 to 6 months, after which subjects were randomized to receive either continued therapy with cyclophosphamide (1.5 mg/kg/day) or azathioprine (2 mg/kg/day). Patients who had been randomized to receive cyclophosphamide were transitioned to azathioprine after having received 1 year of therapy. The primary endpoint of the trial was relapse rate, which was demonstrated to be equivalent among both groups (15% versus 10%, P=0.94).
CYCAZAREM effectively demonstrated that induction of remission with cyclophosphamide, followed by remission maintenance with azathioprine, was as effective at preventing disease flare as a prolonged course of cyclophosphamide. However, it was less clear if other remission maintenance agents, such as methotrexate, might be equally as effective. This question was addressed by the French Vasculitis Study Group, which treated 30 subjects with MPA and 96 subjects with WGs with intravenous cyclophosphamide (0.6 mg/m2 every 2 weeks for 3 doses, then 0.6 mg/m2 every 3 weeks until remission was achieved, then an additional 0.7 mg/m2 every 3 weeks for 3 additional doses).[77] These subjects were subsequently randomized to receive remission maintenance therapy with either oral methotrexate (titrated to 25 mg/week) or azathioprine (2 mg/kg/day). The relapse rate was again determined to be equivalent in both groups (33% versus 36%, P=0.71).
Taken together, these trials indicate that for the treatment of MPA, remission induction with a limited course of cyclophosphamide, followed by remission maintenance with an antimetabolite such as methotrexate or azathioprine, is an appropriate treatment strategy for patients with severe disease. This leaves open the question, however, of whether adjunctive therapies, such as plasma exchange, might further augment the response to immunosuppression. This possibility was examined by the Methylprednisolone versus Plasma Exchange as additional therapy for severe ANCA associated glomerulonephritis trial (MEPEX) which enrolled 95 patients with MPA and 24 patients with WG, all of whom had biopsy-proven glomerulonephritis and a serum creatinine ≥5.8 mg/dL.[78] All patients received treatment with oral glucocorticoids and cyclophosphamide (2.5 mg/kg/day for 3 months, then 1.5 mg/kg/day for an additional 3 months) followed by azathioprine (2 mg/kg/day). In addition, patients were randomized to receive adjunctive therapy with either intravenous methylprednisolone (1 g daily for 3 days) or a series of 7 plasma exchange procedures over 14 days. The primary endpoint of renal recovery at 3 months occurred more frequently among patients who had received plasma exchange (49% versus 69%, P=0.02). Randomization to plasma exchange resulted in a 24% risk reduction of end-stage renal disease at 12 months (43% versus 19%). However, mortality was equivalent in both groups, and long-term follow-up studies demonstrate no difference in mortality or renal survival.
Remission induction strategies
Because of the toxicities inherent to the use of cyclophosphamide, investigators have focused on developing strategies to treat MPA that avoid using cytotoxic agents altogether. One such strategy involves identifying patients with milder disease, who may not require aggressive treatment to achieve remission. Silva et al. recruited 17 patients with MPA with mild to moderate renal involvement (defined as a serum creatinine ≤3 mg/dL) for treatment with glucocorticoids and mycophenolate mofetil (2–3 g total dose daily).[79] Thirteen subjects (76%) met the primary endpoint, and 12 remained in remission until month 18. Regimens that avoid the use of cyclophosphamide for disease activity that does not affect life or the function of a vital organ may be a valuable strategy to help some patients avoid cytotoxic agents; research in analogous patients with WG indicates that methotrexate[80] and leflunomide[81] may also be effective for such patients.
Rituximab may represent an important alternative to cyclophosphamide for patients with higher levels of disease activity, which may not respond adequately to antimetabolite therapies. The Rituximab versus cyclophosphamide for induction of remission in ANCA-associated Vasculitis (RAVE) is a multi-center, double-blinded trial that randomized 48 subjects with MPA and 147 subjects with WG to receive either standard therapy or treatment with rituximab.[82] Standard therapy included a remission induction regimen of daily oral cyclophosphamide (2 mg/kg/day) for 3–6 months, followed by a remission maintenance regimen of azathioprine (2 mg/kg/day). Rituximab was administered using a standard lymphoma protocol (375 mg/m2 IV weekly for four weeks). This trial demonstrated that rituximab is non-inferior to cyclophosphamide for the induction of remission at 6 months (63.6% versus 53.1%, P=0.089). Remission rates at 6 months increase if subjects who were allowed to remain on low-dose glucocorticoids are included in the calculations (70.7% versus 62.2%, P=0.103). Post hoc analysis indicates that rituximab may be especially effective for patients with relapsing disease (66.7% versus 42.0%, P=0.013). Although the long-term consequences associated with rituximab treatment are not entirely clear for this patient population,[83] this trial strongly supports rituximab as an alternative to cyclophosphamide for the treatment of MPA.
Summary
MPA is a systemic necrotizing vasculitis with significant renal and pulmonary manifestations. The pathogenesis of MPA has not been clearly defined, although current evidence supports a role for ANCA. Diagnosis can be challenging, and relies on the physician drawing together elements of the patient’s clinical history and symptoms with diagnostic tests such as tissue biopsy and autoantibody testing. Prognosis for MPA has greatly improved with the use of cyclophosphamide and glucocorticoids. The future of MPA treatment appears bright, as newer medications like rituximab show great promise as effective alternative therapeutic agents with potentially less toxicity.
Acknowledgement
Dr. Seo is a Lowe Family Scholar in the Johns Hopkins University Center for Innovative Medicine
Funding
The National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (1K23AR052820-01)
American College of Rheumatology Physician Scientist Development Award National Institutes of Health/National Center for Research Resources (5 KL2 RR024130-04)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sharon A. Chung, Assistant Professor of Medicine, Rosalind Russell Medical Research Center for Arthritis, Division of Rheumatology, University of California, San Francisco, CA, Sharon.Chung@ucsf.edu.
Philip Seo, Co-Director, the Johns Hopkins Vasculitis Center, Assistant Professor of Medicine, Johns Hopkins University Division of Rheumatology, seo@jhmi.edu.
References
- 1.Matteson E. Commemorative translation of the 130-year anniversary of the original article by Adolf Kussmaul and Rudolf Maier, ed. Anonymous. Rochester: Mayo Foundation; 1996. [Google Scholar]
- 2.Matteson EL. Historical perspective on the classification of vasculitis. Arthritis Care Res. 2000;13(2):122–127. doi: 10.1002/1529-0131(200004)13:2<122::aid-anr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Wohlwill F. Über die nur mikroskopisch erkennbare Form der Periarteritis nodosa. Virchows Arch Pathol Anat Physiol. 1923;246:36. [Google Scholar]
- 4.Zeek PM. Periarteritis nodosa and other forms of necrotizing angiitis. N Engl J Med. 1953;248(18):764–772. doi: 10.1056/NEJM195304302481805. [DOI] [PubMed] [Google Scholar]
- 5.Wainwright J, Davson J. The renal appearances in the microscopic form of periarteritis nodosa. J Pathol Bacteriol. 1950;62(2):189–196. doi: 10.1002/path.1700620206. [DOI] [PubMed] [Google Scholar]
- 6.Savage CO, et al. Microscopic polyarteritis: presentation, pathology and prognosis. Q J Med. 1985;56(220):467–483. [PubMed] [Google Scholar]
- 7.Jennette J, et al. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 8.Kussmaul A, Maier R. Ueber eine bisher nicht beschriebenen Eigenthumliche arterienerkrankung (periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner muskellahmung Einhergeht. Dtsch Arch Klin Med. 1866;1:484–518. [Google Scholar]
- 9.Watts R, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis. 2007;66(2):222–227. doi: 10.1136/ard.2006.054593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watts RA, et al. Effect of classification on the incidence of polyarteritis nodosa and microscopic polyangiitis. Arthritis Rheum. 1996;39(7):1208–1212. doi: 10.1002/art.1780390720. [DOI] [PubMed] [Google Scholar]
- 11.Godman G, Churg J. Wegener's granulomatosis: Pathology and review of the literature. Arch Pathol Lab Med. 1954;58:533–553. [PubMed] [Google Scholar]
- 12.Davies D, et al. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology. BMJ. 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Falk R, Jennette J. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Eng J Med. 1988;318:1651. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 14.Guillevin L, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–430. doi: 10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Lane SE, et al. Primary renal vasculitis in Norfolk--increasing incidence or increasing recognition? Nephrol Dial Transplant. 2000;15(1):23–27. doi: 10.1093/ndt/15.1.23. [DOI] [PubMed] [Google Scholar]
- 16.Mohammad AJ, et al. Prevalence of Wegener's granulomatosis, microscopic polyangiitis, polyarteritis nodosa and Churg Strauss syndrome within a defined population in southern Sweden. Rheumatology (Oxford, England) 2007;46(8):1329–1337. doi: 10.1093/rheumatology/kem107. [DOI] [PubMed] [Google Scholar]
- 17.Koldingsnes W, Nossent H. Epidemiology of Wegener's granulomatosis in northern Norway. Arthritis Rheum. 2000;43(11):2481–2487. doi: 10.1002/1529-0131(200011)43:11<2481::AID-ANR15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Gay MA, et al. The epidemiology of the primary systemic vasculitides in northwest Spain: implications of the Chapel Hill Consensus Conference definitions. Arthritis Rheum. 2003;49(3):388–393. doi: 10.1002/art.11115. [DOI] [PubMed] [Google Scholar]
- 19.Mahr A, et al. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51(1):92–99. doi: 10.1002/art.20077. [DOI] [PubMed] [Google Scholar]
- 20.Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of Disease: pathogenesis and treatment of ANCA-associated vasculitides. Nature clinical practice.Rheumatology. 2006;2(12):661–670. doi: 10.1038/ncprheum0355. [DOI] [PubMed] [Google Scholar]
- 21.Guilpain P, et al. Pathogenic effects of antimyeloperoxidase antibodies in patients with microscopic polyangiitis. Arthritis Rheum. 2007;56(7):2455–2463. doi: 10.1002/art.22741. [DOI] [PubMed] [Google Scholar]
- 22.Mulder A, et al. Activation of granulocytes by anti-neutrophil cytoplasmic antibodies (ANCA): a FcgRII-dependent process. Clin Exp Immunol. 1994;98:270. doi: 10.1111/j.1365-2249.1994.tb06137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little MA, et al. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106(6):2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110(7):955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlieben DJ, et al. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 2005;45(4):758–761. doi: 10.1053/j.ajkd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Silva F, et al. Successful pregnancy and delivery of a healthy newborn despite transplacental transfer of antimyeloperoxidase antibodies from a mother with microscopic polyangiitis. Am J Kidney Dis. 2009;54(3):542–545. doi: 10.1053/j.ajkd.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Falk RJ, Hoffman GS. Controversies in small vessel vasculitis--comparing the rheumatology and nephrology views. Current opinion in rheumatology. 2007;19(1):1–9. doi: 10.1097/BOR.0b013e328011cb80. [DOI] [PubMed] [Google Scholar]
- 28.Kain R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14(10):1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agard C, et al. Microscopic polyangiitis and polyarteritis nodosa: how and when do they start? Arthritis Rheum. 2003;49(5):709–715. doi: 10.1002/art.11387. [DOI] [PubMed] [Google Scholar]
- 30.Lhote F, Cohen P, Guillevin L. Polyarteritis nodosa, microscopic polyangiitis and Churg-Strauss syndrome. Lupus. 1998;7(4):238–258. doi: 10.1191/096120398678920055. [DOI] [PubMed] [Google Scholar]
- 31.Jennette JC, Thomas DBS, Falk RJ. Microscopic polyangiitis (microscopic polyarteritis) Semin Diagn Pathol. 2001;18(1):3–13. [PubMed] [Google Scholar]
- 32.Westman KW, et al. Relapse rate, renal survival, and cancer morbidity in patients with Wegener's granulomatosis or microscopic polyangiitis with renal involvement. J Am Soc Nephrol. 1998;9(5):842–852. doi: 10.1681/ASN.V95842. [DOI] [PubMed] [Google Scholar]
- 33.Levy JB, et al. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66(4):1535–1540. doi: 10.1111/j.1523-1755.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 34.Lindic J, et al. Clinical outcome of patients with coexistent antineutrophil cytoplasmic antibodies and antibodies against glomerular basement membrane. Ther Apher Dial. 2009;13(4):278–281. doi: 10.1111/j.1744-9987.2009.00724.x. [DOI] [PubMed] [Google Scholar]
- 35.Hellmark T, et al. Comparison of anti-GBM antibodies in sera with or without ANCA. J Am Soc Nephrol. 1997;8(3):376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]
- 36.Lionaki S, Jennette JC, Falk RJ. Anti-neutrophil cytoplasmic (ANCA) and anti-glomerular basement membrane (GBM) autoantibodies in necrotizing and crescentic glomerulonephritis. Semin Immunopathol. 2007;29(4):459–474. doi: 10.1007/s00281-007-0093-0. [DOI] [PubMed] [Google Scholar]
- 37.Lauque D, et al. Microscopic polyangiitis with alveolar hemorrhage. A study of 29 cases and review of the literature. Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires (GERM"O"P) Medicine (Baltimore) 2000;79(4):222–233. doi: 10.1097/00005792-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Puerta JA, et al. Interstitial lung disease as a presenting manifestation of microscopic polyangiitis successfully treated with mycophenolate mofetil. Clin Exp Rheumatol. 2009;27(1):166–167. [PubMed] [Google Scholar]
- 39.Collins CE, Quismorio FP., Jr Pulmonary involvement in microscopic polyangiitis. Curr Opin Pulm Med. 2005;11(5):447–451. doi: 10.1097/01.mcp.0000170520.63874.fb. [DOI] [PubMed] [Google Scholar]
- 40.Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med. 2000;6(5):430–435. doi: 10.1097/00063198-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Schwarz MI, Brown KK. Small vessel vasculitis of the lung. Thorax. 2000;55(6):502–510. doi: 10.1136/thorax.55.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niles JL, et al. The syndrome of lung hemorrhage and nephritis is usually an ANCA-associated condition. Arch Intern Med. 1996;156(4):440–445. [PubMed] [Google Scholar]
- 43.Eschun GM, Mink SN, Sharma S. Pulmonary interstitial fibrosis as a presenting manifestation in perinuclear antineutrophilic cytoplasmic antibody microscopic polyangiitis. Chest. 2003;123(1):297–301. doi: 10.1378/chest.123.1.297. [DOI] [PubMed] [Google Scholar]
- 44.Birnbaum J, et al. Microscopic polyangiitis presenting as a "pulmonary-muscle" syndrome: is subclinical alveolar hemorrhage the mechanism of pulmonary fibrosis? Arthritis Rheum. 2007;56(6):2065–2071. doi: 10.1002/art.22633. [DOI] [PubMed] [Google Scholar]
- 45.Tzelepis GE, et al. Prevalence and outcome of pulmonary fibrosis in microscopic polyangiitis. Eur Respir J. 2009 doi: 10.1183/09031936.00110109. [DOI] [PubMed] [Google Scholar]
- 46.Foulon G, et al. ANCA-associated lung fibrosis: analysis of 17 patients. Respir Med. 2008;102(10):1392–1398. doi: 10.1016/j.rmed.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 47.Ortiz-Santamaria V, et al. Pulmonary aneurysms in microscopic polyangiitis. Clin Rheumatol. 2003;22(6):498–499. doi: 10.1007/s10067-003-0765-7. [DOI] [PubMed] [Google Scholar]
- 48.Park J, et al. Microscopic polyangiitis associated with diffuse panbronchiolitis. Intern Med. 2004;43(4):331–335. doi: 10.2169/internalmedicine.43.331. [DOI] [PubMed] [Google Scholar]
- 49.Kluger N, et al. Comparison of cutaneous manifestations in systemic polyarteritis nodosa and microscopic polyangiitis. Br J Dermatol. 2008;159(3):615–620. doi: 10.1111/j.1365-2133.2008.08725.x. [DOI] [PubMed] [Google Scholar]
- 50.Kawakami T, et al. Cutaneous manifestations in patients with microscopic polyangiitis: two case reports and a minireview. Acta Derm Venereol. 2006;86(2):144–147. doi: 10.2340/00015555-0034. [DOI] [PubMed] [Google Scholar]
- 51.Seishima M, Oyama Z, Oda M. Skin eruptions associated with microscopic polyangiitis. Eur J Dermatol. 2004;14(4):255–258. [PubMed] [Google Scholar]
- 52.Pagnoux C, et al. Presentation and outcome of gastrointestinal involvement in systemic necrotizing vasculitides: analysis of 62 patients with polyarteritis nodosa, microscopic polyangiitis, Wegener granulomatosis, Churg-Strauss syndrome, or rheumatoid arthritis-associated vasculitis. Medicine (Baltimore) 2005;84(2):115–128. doi: 10.1097/01.md.0000158825.87055.0b. [DOI] [PubMed] [Google Scholar]
- 53.Guillevin L, Pagnoux C, Teixeira L. In: Microscopic polyangiitis, in Vasculitis. Ball G, Bridges S Jr, editors. Oxford: Oxford University Press; 2008. pp. 355–364. [Google Scholar]
- 54.Ueda S, et al. Microscopic polyangiitis complicated with massive intestinal bleeding. J Gastroenterol. 2001;36(4):264–270. doi: 10.1007/s005350170114. [DOI] [PubMed] [Google Scholar]
- 55.Spahn TW, et al. Gastrointestinal bleeding secondary to hepatic artery involvement of microscopic polyangiitis: case report and review of the literature. Dig Dis Sci. 2007;52(6):1558–1561. doi: 10.1007/s10620-006-9267-1. [DOI] [PubMed] [Google Scholar]
- 56.Tsai CN, et al. Extended colonic ulcerations in a patient with microscopic polyangiitis. Ann Rheum Dis. 2004;63(11):1521–1522. doi: 10.1136/ard.2003.018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Passam FH, et al. Intestinal ischemia as the first manifestation of vasculitis. Semin Arthritis Rheum. 2004;34(1):431–441. doi: 10.1016/j.semarthrit.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Ohnuma K, et al. Microscopic polyangiitis initiated with liver dysfunction, calf pain and fever of unknown origin. Rheumatol Int. 2009 doi: 10.1007/s00296-009-1121-2. [DOI] [PubMed] [Google Scholar]
- 59.Takebayashi K, et al. Microscopic polyangiitis presenting with liver dysfunction preceding rapidly progressive necrotizing glomerulonephritis. South Med J. 2004;97(9):911–914. doi: 10.1097/01.SMJ.0000105082.08745.67. [DOI] [PubMed] [Google Scholar]
- 60.Nakamoto T, et al. Microscopic polyangiitis that presented liver dysfunction prior to noted renal manifestations. Intern Med. 2000;39(6):517–521. doi: 10.2169/internalmedicine.39.517. [DOI] [PubMed] [Google Scholar]
- 61.Goritsas CP, et al. Intrahepatic bile duct injury and nodular regenerative hyperplasia of the liver in a patient with polyarteritis nodosa. J Hepatol. 1997;26(3):727–730. doi: 10.1016/s0168-8278(97)80441-2. [DOI] [PubMed] [Google Scholar]
- 62.Iannone F, et al. Microscopic polyangiitis associated with primary biliary cirrhosis. J Rheumatol. 2003;30(12):2710–2722. [PubMed] [Google Scholar]
- 63.Amezcua-Guerra LM, et al. Microscopic polyangiitis associated with primary biliary cirrhosis: a causal or casual association? J Rheumatol. 2006;33(11):2351–2353. [PubMed] [Google Scholar]
- 64.Zhang W, et al. Clinical analysis of nervous system involvement in ANCA-associated systemic vasculitides. Clin Exp Rheumatol. 2009;27(1 Suppl 52):S65–S69. [PubMed] [Google Scholar]
- 65.Hattori N, et al. Mortality and morbidity in peripheral neuropathy associated Churg-Strauss syndrome and microscopic polyangiitis. J Rheumatol. 2002;29(7):1408–1414. [PubMed] [Google Scholar]
- 66.Cattaneo L, et al. Peripheral neuropathy in Wegener's granulomatosis, Churg-Strauss syndrome and microscopic polyangiitis. J Neurol Neurosurg Psychiatry. 2007;78(10):1119–1123. doi: 10.1136/jnnp.2006.111013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furukawa Y, Matsumoto Y, Yamada M. Hypertrophic pachymeningitis as an initial and cardinal manifestation of microscopic polyangiitis. Neurology. 2004;63(9):1722–1724. doi: 10.1212/01.wnl.0000143063.12569.fc. [DOI] [PubMed] [Google Scholar]
- 68.Ku BD, Shin HY. Multiple bilateral non-hemorrhagic cerebral infarctions associated with microscopic polyangiitis. Clin Neurol Neurosurg. 2009;111(10):904–906. doi: 10.1016/j.clineuro.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Stone J, et al. Test characteristics of Immunofluorescence and ELISA tests in 856 consecutive patients with possible ANCA-associated conditions. Arthritis Care and Research. 2000;13(6):424–434. doi: 10.1002/1529-0131(200012)13:6<424::aid-art14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 70.Choi HK, et al. Drug-associated antineutrophil cytoplasmic antibody-positive vasculitis: prevalence among patients with high titers of antimyeloperoxidase antibodies. Arthritis Rheum. 2000;43(2):405–413. doi: 10.1002/1529-0131(200002)43:2<405::AID-ANR22>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 71.Zhao MH, et al. Autoantibodies against bactericidal/permeability-increasing protein in patients with cystic fibrosis. Qjm. 1996;89(4):259–265. doi: 10.1093/qjmed/89.4.259. [DOI] [PubMed] [Google Scholar]
- 72.Bauer A, et al. Vasculitic purpura with antineutrophil cytoplasmic antibody-positive acute renal failure in a patient with Streptococcus bovis case and Neisseria subflava bacteremia and subacute endocarditis. Clin Nephrol. 2004;62(2):144–148. doi: 10.5414/cnp62144. [DOI] [PubMed] [Google Scholar]
- 73.Hermann J, et al. Clinical interpretation of antineutrophil cytoplasmic antibodies: parvovirus B19 infection as a pitfall. Ann Rheum Dis. 2005;64(4):641–643. doi: 10.1136/ard.2004.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fauci A, Wolff S. Wegener's granulomatosis: studies in eighteen patients and a review of the literature. Medicine. 1973;52:53–61. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 75.Hoffman GS, et al. Wegener's Granulomatosis: An Analysis of 158 Patients. Ann Intern Med. 1992;116:488–498. doi: 10.7326/0003-4819-116-6-488. [DOI] [PubMed] [Google Scholar]
- 76.Jayne D. Randomised trial of cyclophosphamide versus azathioprine during remission in ANCA-associated systemic vasculitis (CYCAZAREM) J Am Soc Nephrol. 1999;10:105A. [Google Scholar]
- 77.Pagnoux C, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359(26):2790–2803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 78.Jayne DR, et al. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18(7):2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 79.Silva F, et al. Mycophenolate Mofetil for Induction and Maintenance of Remission in Microscopic Polyangiitis with Mild to Moderate Renal Involvement--A Prospective, Open-Label Pilot Trial. Clin J Am Soc Nephrol. doi: 10.2215/CJN.06010809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Groot K, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis and Rheumatism. 2005;52(8):2461–2469. doi: 10.1002/art.21142. [DOI] [PubMed] [Google Scholar]
- 81.Metzler C, et al. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener's granulomatosis. Rheumatology (Oxford, England) 2007;46(7):1087–1091. doi: 10.1093/rheumatology/kem029. [DOI] [PubMed] [Google Scholar]
- 82.J.H. Stone PAM, Seo P, Spiera R, Langford CA, Gary SHoffman, Kallenberg CGM, William St. Clair E, Fessler BJ, Tchao N, Ding L, Webber LV, Ikle D, Weitzenkamp D, Wu W, Brunetta P, Seismundo L, Fervenza FC, Keogh KA, Kissin EY, Mieras KS, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U The RAVE-ITN Research Group. Rituximab Versus Cyclophosphamide for Induction of Remission in ANCA-Associated Vasculitis: A Randomized Controlled Trial (RAVE) Arthritis Rheum. 2009;60 Supplement [Google Scholar]
- 83.Calabrese LH, Molloy ES. Therapy: rituximab and PML risk-informed decisions needed! Nat Rev Rheumatol. 2009;5(10):528–529. doi: 10.1038/nrrheum.2009.193. [DOI] [PubMed] [Google Scholar]
- 84.Lane SE, Watts R, Scott DG. Epidemiology of systemic vasculitis. Curr Rheumatol Rep. 2005;7(4):270–275. doi: 10.1007/s11926-005-0036-5. [DOI] [PubMed] [Google Scholar]