Abstract
Multiple randomized controlled trials (RCTs) have established the efficacy of statins for prevention of cardiovascular disease. The benefits observed are often framed in terms of percent reductions in low-density lipoprotein cholesterol (LDL-C) from baseline or percent reduction between control and treatment groups, even though epidemiological data suggest that absolute inter-group difference in LDL-C (ΔLDLControl-Rx) is the more informative measure. We conducted a systematic review of large-scale trials of statins versus placebo, usual care or active (lower-dose statin) control to calculate updated summary estimates of risk reduction in coronary heart disease (CHD) and all-cause mortality. Meta-regression analysis was used to ascertain the relations of different LDL-C metrics to outcome. In 20 eligible RCTs, there were significant overall reductions for CHD (OR=0.72, 95% CI=0.67–0.78) and mortality (OR=0.89, 95% CI=0.84–0.94), but with substantial variability in trial results. ΔLDLControl-Rx was the strongest determinant of CHD risk reduction, particularly after excluding active-comparator studies, and was independent of baseline LDL-C. By contrast, baseline LDL-C edged ΔLDLControl-Rx as the strongest determinant of mortality, but neither was significant after exclusion of active-comparator studies. Exclusion of 3 RCTs involving distinct populations, however, rendered ΔLDLControl-Rx the predominant determinant of mortality reduction. In conclusion, these findings underscore the primacy of absolute reductions in LDL-C to the design and interpretation of RCTs of lipid-lowering therapies, and to framing treatment recommendations based on the proven coronary benefits of these drugs.
A multitude of randomized controlled trials (RCTs) have documented the efficacy of statins in reducing coronary events in diverse populations.1 These benefits are well-established in RCTs comparing statins against placebo or usual care,1 with accumulating data suggesting that this is also true for high- versus low-intensity statin treatment.2–4 Demonstration of statin-related benefits versus control has not been uniform, however, even in such mega-trials.3–5 A potential explanation cited for null results is that, despite major percent reductions in LDL-C from baseline in the active group, the percent difference in LDL-C achieved between study arms has been more modest.5–6 Yet assessments of benefit of lipid-lowering agents, whether in RCTs5,7–10 or meta-analyses,11–14 have often been framed in terms of percent cholesterol reductions associated with active treatment. The construct of percent cholesterol lowering from baseline has similarly been used in national treatment guidelines.15 Focus on percent, rather than absolute, reductions is itself problematic, however, because available epidemiological evidence shows that the relation between cholesterol level and cardiovascular risk is curvilinear.15–16 Hence, for any absolute change in serum cholesterol irrespective of its starting clinical value, the corresponding change in the relative risk of CHD is constant. Consequently, percent cholesterol reductions, whether longitudinally in the intervention arm11 or between study arms,17 may not be the most apt metric for describing statin-related benefits. We sought to define in comparative fashion the relation between various measures of LDL-C lowering to outcomes using meta-regression in an updated systematic review of large-scale statin trials.
Methods
We conducted a systematic search of the English-language literature using PubMed, EMBASE, BIOSIS, Web of Science, Cochrane Systematic Reviews, DARE, Central Register of Controlled Trials and clinicaltrials.gov from January 1994 to December 2008 to identify pertinent studies. Nineteen relevant keywords were entered, and clinical trials, qualitative and quantitative reviews, and editorials thus identified were reviewed to select relevant studies. Reference sections of articles served to identify additional studies, as did knowledge of experts in the field (C.J.V., A.M.G., and R.C.P.).
We focused our systematic review on large-scale RCTs18 of statins versus placebo, usual care, or active comparator having clinical cardiovascular events or all-cause mortality as their primary endpoint. Eligible RCTs required a minimum enrollment of 1,000 participants at risk for, or with stable, CHD and follow-up duration >1 year. We excluded a priori clinical trials that evaluated statins in combination with other therapies, that were designed to assess impact on intermediate primary endpoints, or that focused on distinct clinical populations or acute settings (which could introduce additional heterogeneity), namely, patients with acute coronary syndromes or advanced renal disease, or patients undergoing cardiac catheterization.
After selection of RCTs meeting inclusion criteria, a single investigator (J.R.K.) extracted data from individual studies. The 2 endpoints of interest were the primary or secondary outcomes of major CHD events and all-cause mortality. Major CHD events comprised non-fatal myocardial infarction and fatal CHD, as defined in individual RCTs. When resuscitated cardiac arrest formed part of an RCT’s major CHD endpoint, and could not be clearly excluded from the composite CHD outcome based on the information presented, it was included in the analysis. If fatal CHD was not reported, fatal myocardial infarction was used instead. Mean or median LDL-C at baseline and following allocation to treatment or control was abstracted directly or computed from available information. Where the mean in-trial follow-up LDL-C in the placebo group was not provided, it was imputed from the baseline value.
Five measures of LDL-C were examined: (i) baseline LDL-C in the treatment group (baseline LDLRx); (ii) absolute change in LDL-C in the treatment group (ΔLDLBaseline-Final); (iii) percent change in LDL-C in the treatment group (%ΔLDLBaseline-Final); (iv) absolute difference between achieved in-trial LDL-C in the control versus treatment group (ΔLDLControl-Rx); (v) percent difference between achieved LDL-C in the control versus treatment group (%ΔLDLControl-Rx).
STATA, version 10.0 (College Station, Texas), was used in all analyses. Separate analysis of RCTs not having active comparator was planned a priori. For individual trials, the numbers of participants with and without events in the treatment and control arms at the conclusion of the study were recorded. Where ascertainment of follow-up LDL-C was performed prior to conclusion of the trial, events reported as of the time of such measurements were used in the analyses. In view of the presence of significant heterogeneity (Cochran’s Q test), a random-effects approach (DerSimonian and Laird) was used to calculate pooled odds ratios (Ors). The quantity I2, the component of heterogeneity not attributable to chance, was also computed.19 Because only mega-trials were included, an approach that avoids potential biases associated with smaller studies,18,20 assessment for publication bias was not undertaken.
To investigate the observed study heterogeneity, random effects meta-regression of RCT results was performed against different measures of LDL-C or trial duration.21–22 Sensitivity analysis of ORs, I2s and meta-regression coefficients entailed serial exclusion of studies or groups of studies from consideration.
Results
The search yielded 31 candidate RCTs meeting sample size and duration criteria, of which 11 did not meet additional inclusion criteria (4 for acute coronary syndromes or enrollment following cardiac catheterization; 2 for assessing drug-combinations; 2 owing to a focus on end-stage renal disease or renal transplantation; 1 with an intermediate primary endpoint; 1 for failed randomization; and 1 because of incompleteness of endpoint ascertainment). The characteristics of the 20 large-scale trials2–5,7–9,23–35 selected for inclusion are presented in Table 1. There were a total of 155,255 participants, of whom 11,508 experienced major CHD events, and 13,687 had all-cause fatal events. With exclusion of the 3 active-comparator trials 2–4, there were 124,302 individuals, with 8,332 and 10,448 developing major CHD and fatal events, respectively.
Table 1.
Characteristics of Large-scale Randomized Controlled Trials
| Trial | Dates | N | Treatment Arm (mg/d) |
Control Arm (mg/d) |
Double- Blinded |
Baseline LDL-C*† |
ΔLDLBaseline -Final† |
ΔLDLControl- Treatment† |
Cumulative CHD Incidence-Controls |
F/U mo |
|---|---|---|---|---|---|---|---|---|---|---|
| 4S | 1988–1994 | 4,444 | Simvastatin 20‡ | Placebo | + | 188 | 66 (35%) | 68 (36%) | 27.3% (5.1%/y) | 65 |
| WOSCOPS | 1989–1995 | 6,595 | Pravastatin 40 | Placebo | + | 192 | 50 (26% ) | 50 (26%) | 7.9% (1.6%/y) | 59 |
| CARE | 1989–1996 | 4,159 | Pravastatin 40 | Placebo | + | 139 | 42 (30%) | 42 (30%) | 13.2% (2.4%/y) | 60 |
| LIPID | 1990–1997 | 9,014 | Pravastatin 40 | Placebo | + | 150 | 38 (25%) | 38 (25%) | 15.9% (2.6%/y) | 73 |
| AFCAPS/ TexCAPS |
1990–1997 | 6,605 | Lovastatin 20‡ | Placebo | + | 150 | 35 (23%) | 41 (26%) | 3.1% (0.6%/y) | 62 |
| GISSI-P | 1993–1996 | 4,271 | Pravastatin 20‡ | Usual Care | 0 | 152 | 23 (15%) | 18 (12%) | 3.9% (2.0%/y) | 23 |
| HPS | 1994–2001 | 20,536 | Simvastatin 40 | Placebo | + | 131 | 54 (41%) | 38 (32%) | 11.8% (2.4%/y) | 60 |
| ALLHAT- LLT |
1994–2002 | 10,355 | Pravastatin 40 | Usual Care | 0 | 146 | 42 (29%) | 17 (14%) | 10.4% (2.2%/y) | 58 |
| PROSPER | 1997–2001 | 5,804 | Pravastatin 40 | Placebo | + | 147 | 50 (34%) | 50 (34%) | 12.2% (3.8%/y) | 38 |
| ASCOT- LLA |
1998–2002 | 10,305 | Atorvastatin 10 | Placebo | + | 132 | 42 (32%) | 37 (29%) | 3.0% (0.9%/y) | 40 |
| GREACE | 1998–2001 | 1,600 | Atorvastatin10‡ | Usual Care | 0 | 179 | 83 (46%) | 72 (43%) | 11.2% (3.7%/y) | 36 |
| CARDS | 1997–2003 | 2,838 | Atorvastatin10 | Placebo | + | 117 | 36 (31%) | 39 (32%) | 5.5% (1.4%/y) | 47 |
| TNT | 1998–2005 | 10,001 | Atorvastatin 80 | Atorvastatin10 | + | 152 | 75 (49%) | 24 (24%) | 8.3% (1.7%/y) | 59 |
| IDEAL | 1999–2005 | 8,888 | Atorvastatin 80 | Simvastatin 20–40 |
0 | 122 | 42 (34%) | 20 (20%) | 23.8% (5.0%/y) | 58 |
| SPARCL | 1998–2005 | 4,731 | Atorvastatin 80 | Placebo | + | 133 | 60 (45%) | 56 (43%) | 5.1% (1.0%/y) | 59 |
| MEGA | 1994–2004 | 7,832 | Pravastatin 10‡ + Diet |
Diet/Usual Care |
0 | 157 | 29 (18%) | 23 (15%) | 1.1% (0.2%/y) | 64 |
| ASPEN | 1996–2003 | 2,410 | Atorvastatin 10 | Placebo | + | 114 | 34 (30%) | 34 (30%) | 4.0% (1.0%/y) | 48 |
| CORONA | 2003–2007 | 5,001 | Rosuvastatin 10 | Placebo | + | 137 | 61 (45%) | 62 (45%) | 6.0% (2.2%/y) | 33 |
| JUPITER | 2003–2008 | 17,802 | Rosuvastatin 20 | Placebo | + | 108 | 53 (49%) | 54 (50%) | 0.8% (0.4%/y) | 23 |
| SEARCH | NA | 12,064 | Simvastatin 80 | Simvastatin 20 | + | 97 | 11 (11%) | 11 (11%) | 13.4% (2.0%/y) | 80 |
All means except for LIPID, where median is reported.
in mg/dL (percent reduction, i.e., %ΔLDLBaseline-Final and %ΔLDLControl-Rx).
With upward titration of dose as indicated.
AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT-LLT = Antihypertensive and Lipid Lowering Treatment to prevent Heart Attack Trial – Lipid Lowering Trial; ASCOT-LLA = Anglo-Scandinavian Cardiac Outcomes Trial – Lipid Lowering Arm (ASCOT-LLA); ASPEN = Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS = Collaborative AtoRvastatin Diabetes Study; CARE = Cholesterol and Recurrent Events; CHD = Coronary Heart Disease; CORONA = Controlled Rosuvastatin Multinational Trial in Heart Failure; F/U = Follow Up; GISSI-P = Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico – Prevenzione; GREACE = GREek Atorvastatin and Coronary-heart-disease Evaluation; HPS = Heart Protection Study; IDEAL = Incremental Decrease in End Points through Aggressive Lipid Lowering; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL-C = Low-density Lipoprotein Cholesterol; LIPID = Long-term Intervention with Pravastatin in Ischaemic Disease; MEGA = Management of Elevated cholesterol in the primary prevention Group of Adult Japanese; mg/d = milligrams per day; mo = months; NA = Not available; PROSPER = PROspective Study of Pravastatin in the Elderly at Risk; 4S = Scandinavian Simvastatin Survival Study; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SPARCL = Stroke Prevention by Aggressive Reduction in Cholesterol Levels; TNT = Treating to New Targets; WOSCOPS = West Of SCOtland COronary Prevention Study
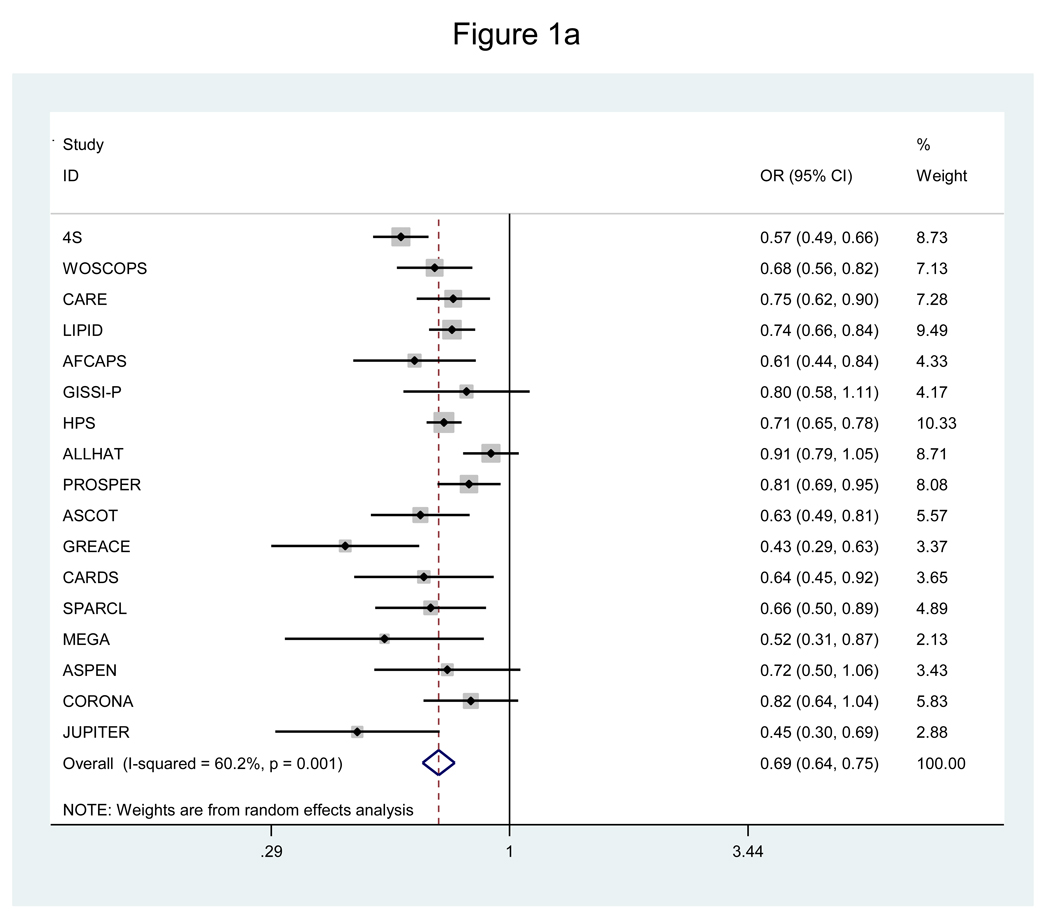
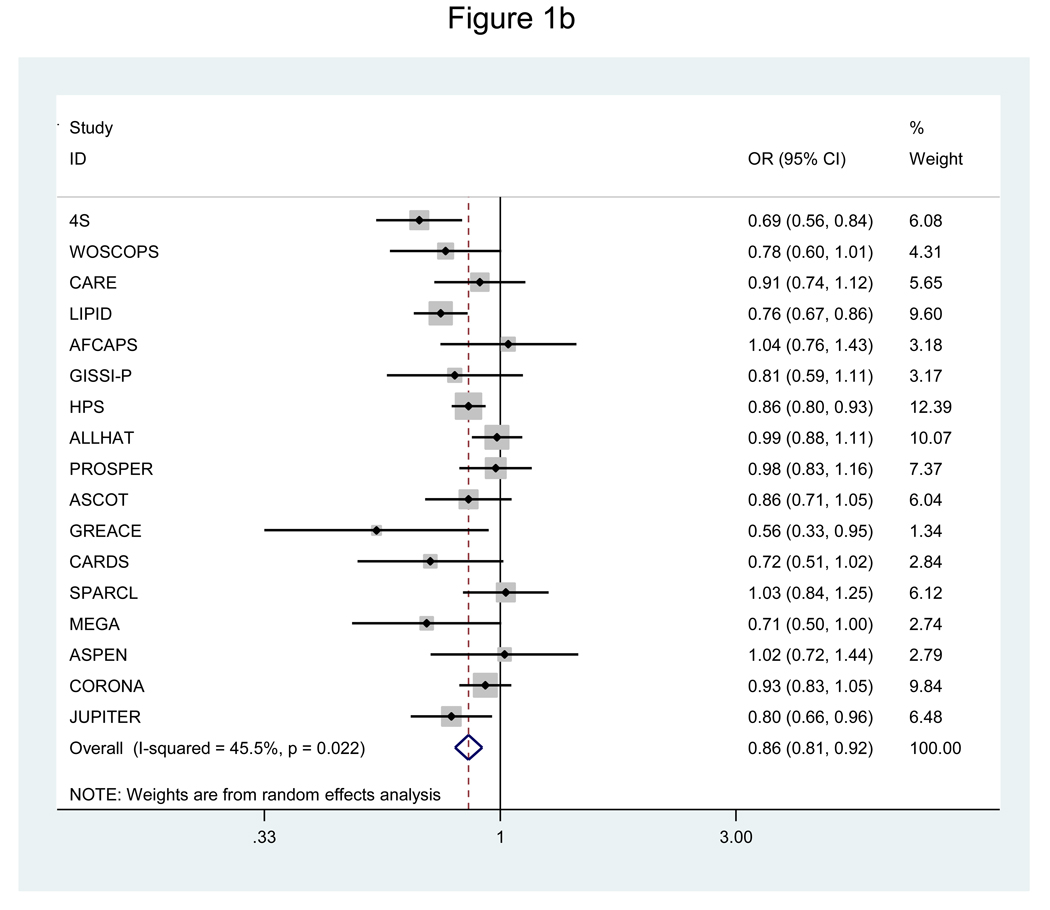
Analysis of all 20 RCTs showed significant overall reductions in risk of CHD (OR=0.72, 95% CI=0.67–0.78) and mortality (OR=0.89, 95% CI=0.84–0.94), but this was associated with significant underlying heterogeneity for both outcomes (P≤0.005). The lack of consistency in study findings was not attributable to chance, with moderate-to-high (I2=69.7%) or moderate (I2=51.1%) variability in effect estimates across RCTs. Figure 1 shows the corresponding findings for the analysis focused on the 17 RCTs of statins versus placebo or usual care. Significant risk reductions were again achieved for CHD and mortality, with less, though still significant, heterogeneity for both outcomes.
Figure 1.
a. Pooled odds ratio of coronary heart disease for statins versus placebo or usual care.
b. Pooled odds ratio of all-cause mortality for statins versus placebo, usual care.
The results of meta-regression modeling of risk reduction as a function of various measures of LDL-C or trial duration are shown in Table 2. Taking all trials into account, ΔLDLControl-Rx exhibited the strongest inverse association (highest negative standardized regression coefficient, β) with relative reduction in CHD events, while that for the percent difference (%ΔLDLControl-Rx) was minimally weaker. According to the random-effects model, every 39 mg/dL (1mmol/L) inter-group difference in achieved LDL-C was associated with a 25% relative reduction in CHD risk (OR=0.75, 95% CI=0.68–0.82). There were significant, but weaker associations for baseline LDLRx, and absolute – though not percent – reduction in LDL-C in the treatment arm (ΔLDLBaseline-Final), whereas trial duration bore no significant relation to CHD risk reduction.
Table 2.
Meta-Regression* for Coronary Heart Disease or Death as a Function of Lipid Measures or Trial Duration
| RCTs w/o Active Comparator | All RCTs | |||
|---|---|---|---|---|
| β per SD† Increase | P | β per SD‡ Increase | P | |
| Coronary Heart Disease | ||||
| LnORCoronary Heart Disease | ||||
| Baseline LDLRx | −0.045 | 0.320 | −0.083 | 0.014 |
| ΔLDLBaseline-Final | −0.091 | 0.052 | −0.082 | 0.029 |
| %ΔLDLBaseline-Final | −0.063 | 0.221 | −0.062 | 0.143 |
| ΔLDLControl-Rx | −0.108 | <0.001 | −0.129 | <0.001 |
| %ΔLDLControl-Rx | −0.095 | 0.030 | −0.127 | <0.001 |
| Trial Duration | 0.011 | 0.813 | 0.052 | 0.237 |
| All-cause Mortality | ||||
| LnORMortality | ||||
| Baseline LDLRx | −0.065 | 0.081 | −0.067 | 0.022 |
| ΔLDLBaseline-Final | −0.018 | 0.686 | −0.021 | 0.511 |
| %ΔLDLBaseline-Final | 0.020 | 0.581 | 0.002 | 0.941 |
| ΔLDLControl-Rx | −0.030 | 0.397 | −0.055 | 0.041 |
| %ΔLDLControl-Rx | 0 | 0.993 | −0.030 | 0.297 |
| Trial Duration | −0.018 | 0.585 | 0.012 | 0.702 |
Models are univariable, i.e., the β coefficients refer to the relationship with each measure as a single independent variable.
SD (RCTs w/o Active Comparator): Baseline LDLRx = 24 mg/dL; ΔLDLBaseline-Final = 15 mg/dL ; %ΔLDLBaseline-Final = 10%; = ΔLDLControl-Rx = 16 mg/dL ; %ΔLDLControl-Rx = 11%; Trial Duration = 15 months.
SD (All RCTs): Baseline LDLRx = 25 mg/dL; ΔLDLBaseline-Final = 17 mg/dL; %ΔLDLBaseline-Final = 11%; ΔLDLControl-Rx = 17 mg/dL; %ΔLDLControl-Rx = 11%; Trial Duration = 23 months.
Ln = Natural logarithm. OR = Odds Ratio. RCT = Randomized Controlled Trial. SD = Standard Deviation.
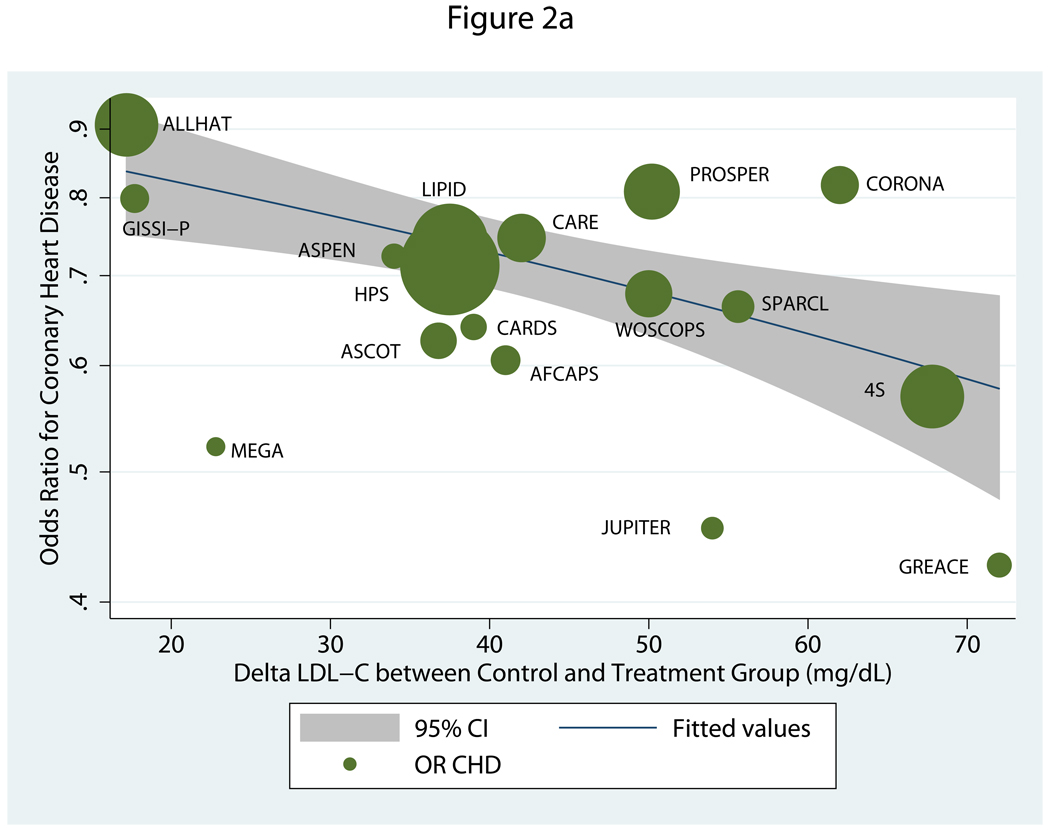
When active-comparator trials were excluded, the absolute inter-group difference in LDL-C (ΔLDLControl-Rx) again showed the strongest association to CHD risk reduction, followed by %ΔLDLControl-Rx (Table 2). The associations between these inter-group differences, whether in absolute or percent terms, and CHD risk decreased with exclusion of active-comparator trials. Figure 2a shows that, based on the model, there was a 23% relative reduction in CHD risk for every 39 mg/dL absolute inter-group decrease in LDL-C (OR=0.77, 95% CI=0.66–0.89). As compared with ΔLDLControl-Rx, ΔLDLBaseline-Final was more modest and fell just short of significance. No other variables were significantly related to CHD risk reduction.
Figure 2.
a. Meta-regression of odds ratio for coronary heart disease relative to absolute difference in post-treatment LDL-C between treatment arms in trials of statins versus placebo or usual care.
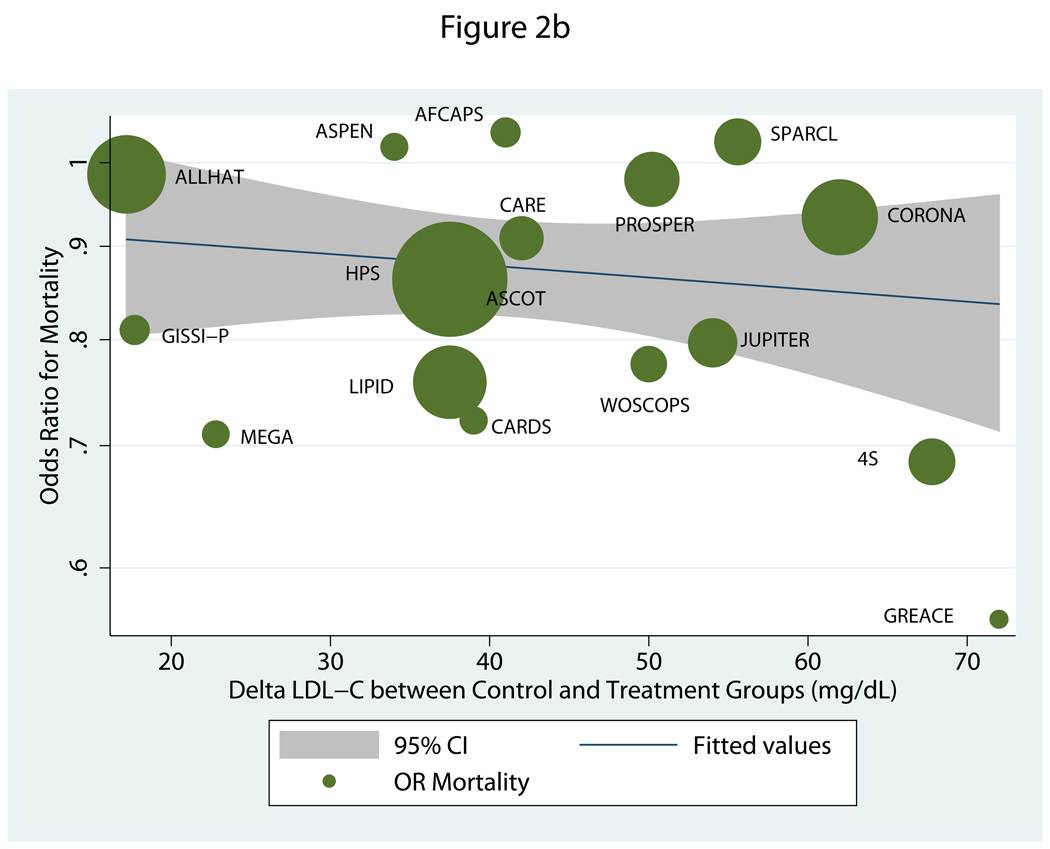
b. Meta-regression of odds ratio for all-cause mortality relative to absolute difference in post-treatment LDL-C between treatment arms in trials of statins versus placebo or usual care.
Regarding all-cause mortality, analysis of all 20 RCTs showed significant relations only for ΔLDLControl-Rx and baseline LDLRx, but it was baseline LDLRx that had the higher negative regression coefficient. Accordingly, for every 39 mg/dL higher value for baseline LDL-C there was an associated 10% reduction in the relative risk of death (OR=0.90, 95% CI=0.83–0.99). The corresponding reduction for every 39 mg/dL of ΔLDLControl-Rx was 12% (OR=0.88, 95% CI=0.78–0.99). Notably, analyses of the 17-RCT subset revealed no significant associations for any of the independent measures (Table 2 and Figure 2b).
The association between ΔLDLControl-Rx and relative risk of CHD was not meaningfully altered by adjustment for baseline LDLRx, whether or not active-comparator trials were taken into account (data not shown). With respect to mortality, inclusion of both ΔLDLControl-Rx and baseline LDLRx in the full meta-regression model rendered both variables non-significant (P≥0.116).
Since exclusion of active-comparator RCTs was found to reduce heterogeneity, sensitivity analyses focused on the 17 trials of statin versus placebo or usual care. Exclusion of each of these RCTs individually did not materially influence estimates of risk reduction for CHD or mortality (data not shown). Selected individual and multiple exclusions based in part on outliers in meta-regression analysis (Figure 2) are shown in Table 3.
Table 3.
Sensitivity Analysis of Trials of Statins versus Placebo or Usual Care – Effect Estimate and Correlations to Lowering or Baseline Value of Low-Density Lipoprotein Cholesterol
| RCTs | Summary OR 95% CI |
I2(%) | Standardized β ΔLDLControl-Rx |
P | Standardized β ΔLDLBaseline-Final |
P |
|---|---|---|---|---|---|---|
| Coronary Heart Disease | ||||||
| 17 RCT Meta-Analysis | 0.69 (0.64–0.75) | 60 | −0.108 | <0.001 | −0.091 | 0.052 |
| Excluding MEGA | 0.70 (0.64–0.76) | 61 | −0.116 | <0.001 | −0.106 | 0.023 |
| Excluding PROSPER | 0.68 (0.63–0.75) | 60 | −0.121 | <0.001 | −0.094 | 0.042 |
| Excluding CORONA | 0.68 (0.63–0.75) | 62 | −0.128 | <0.001 | −0.110 | 0.019 |
| Excluding PROSPER & CORONA |
0.67 (0.62–0.74) | 61 | −0.142 | <0.001 | −0.116 | 0.012 |
| Excluding MEGA & CORONA |
0.69 (0.63–0.75) | 63 | −0.134 | <0.001 | −0.126 | 0.007 |
| Excluding MEGA, PROSPER & CORONA |
0.68 (0.62–0.74) | 63 | −0.148 | <0.001 | −0.131 | 0.004 |
| Excluding JUPITER | 0.70 (0.65–0.76) | 58 | −0.104 | <0.001 | −0.087 | 0.054 |
| Excluding GREACE | 0.71 (0.65–0.76) | 55 | −0.094 | 0.003 | −0.052 | 0.325 |
| Excluding ALLHAT | 0.68 (0.63–0.73) | 46 | −0.081 | 0.041 | −0.078 | 0.056 |
| Excluding GISSI-P | 0.69 (0.63–0.75) | 62 | −0.110 | 0.001 | −0.091 | 0.081 |
| Excluding 4S | 0.71 (0.66–0.77) | 50 | −0.092 | 0.019 | −0.070 | 0.180 |
| Excluding SPARCL | 0.69 (0.64–0.76) | 63 | −0.108 | 0.001 | −0.091 | 0.063 |
| Excluding High-Risk CHD* | 0.65 (0.59–0.72) | 0 | −0.057 | 0.437 | −0.046 | 0.522 |
| Excluding Low-Risk CHD† | 0.72 (0.66–0.78) | 63 | −0.115 | <0.001 | −0.125 | 0.004 |
|
Standardized β ΔLDLControl-Rx |
P |
Standardized β Baseline LDLRx |
P | |||
| All-cause Mortality | ||||||
| 17 RCT Meta-Analysis | 0.86 (0.81–0.92) | 46 | −0.030 | 0.397 | −0.065 | 0.081 |
| Excluding MEGA | 0.87 (0.81–0.93) | 47 | −0.041 | 0.265 | −0.061 | 0.101 |
| Excluding PROSPER | 0.86 (0.80–0.91) | 45 | −0.036 | 0.311 | −0.067 | 0.067 |
| Excluding CORONA | 0.86 (0.80–0.92) | 46 | −0.051 | 0.178 | −0.063 | 0.101 |
| Excluding PROSPER & CORONA |
0.85 (0.79–0.91) | 45 | −0.064 | 0.072 | −0.064 | 0.086 |
| Excluding MEGA and CORONA |
0.86 (0.80–0.92) | 48 | −0.063 | 0.104 | −0.059 | 0.123 |
| Excluding MEGA, PROSPER & CORONA |
0.85 (0.79–0.92) | 47 | −0.074 | 0.038 | −0.061 | 0.105 |
| Excluding JUPITER | 0.87 (0.81–0.93) | 47 | −0.027 | 0.469 | −0.095 | 0.017 |
| Excluding GREACE | 0.87 (0.82–0.92) | 44 | −0.019 | 0.610 | −0.055 | 0.144 |
| Excluding ALLHAT | 0.85 (0.80–0.91) | 39 | −0.003 | 0.951 | −0.067 | 0.051 |
| Excluding GISSI-P | 0.87 (0.81–0.92) | 49 | −0.038 | 0.317 | −0.064 | 0.088 |
| Excluding 4S | 0.88 (0.83–0.93) | 37 | −0.003 | 0.924 | −0.039 | 0.404 |
| Excluding SPARCL | 0.85 (0.80–0.91) | 44 | −0.042 | 0.230 | −0.059 | 0.112 |
| Excluding High-Risk CHD* | 0.86 (0.79–0.95) | 10 | 0.048 | 0.452 | −0.021 | 0.657 |
| Excluding Low-Risk CHD† | 0.87 (0.81–0.94) | 51 | −0.037 | 0.337 | −0.114 | 0.023 |
For both outcomes, exclusion of ALLHAT-LLT 5 and the Scandinavian Simvastatin Survival Study (4S)23 individually led to a decrease in variability across studies. Omission of each also led to appreciable weakening in the relationship between ΔLDLControl-Rx and either outcome. So too did exclusion of the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE),31 particularly for CHD, but this was accompanied by an even more marked decrease in effect estimate for ΔLDLBaseline-Final. The same effect was evident with exclusion of high-risk CHD trials, of which ALLHAT-LLT, 4S, and GREACE are part. By contrast, exclusion of the Management of Elevated cholesterol in the primary prevention Group of Adult Japanese (MEGA) study,34 the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER)29, and the Controlled Rosuvastatin in Multinational Trial in Heart Failure (CORONA),8 both individually and collectively, resulted in strengthening of the associations between ΔLDLControl-Rx and both outcomes, as well as ΔLDLBaseline-Final with regard to CHD events. In fact, exclusion of all three RCTs uncovered a significant association between ΔLDLControl-Rx and mortality. Last, exclusion of low-risk CHD trials, led to a stronger relation for ΔLDLBaseline-Final with respect to CHD events, but this depended entirely on exclusion of the MEGA study (data not shown). It also resulted in a stronger and significant association of baseline LDLRx with mortality, driven by exclusion of the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) study.7
Discussion
The present study provides updated summary estimates regarding the impact of statins on major CHD and all-cause mortality, accounting for several important mega-trials published since completion of a prospective systematic review by the Cholesterol Treatment Trialists’ Collaboration (CTTC), based on patient-level data.1 Strikingly, after incorporating an additional 34,246 participants (65,199 including active-comparator trials), and despite the use of study-level data and somewhat divergent inclusion criteria, the resulting effect estimates were nearly identical to those reported previously by the CTTC,1 including in a recent update.4 These findings were robust to single trial exclusions, yet even though removal of active-comparator trials and of either ALLHAT-LLT or 4S dampened heterogeneity, moderate variability remained across studies.
Beyond confirming these results, the significance of the present work lies in its focus on an issue that has heretofore received insufficient attention, namely, understanding which measure of LDL-C concentration or lowering best relates to the benefits conferred by statins – and, by extension, other lipid-lowering therapies. To our knowledge, the current investigation is the first to undertake a direct comparison of such different LDL-C metrics in relation to the magnitude of risk reductions achieved by statin medications in RCTs.
Our meta-regression analyses show that different measures of LDL-C help to explain the heterogeneity observed in the effect estimates from different studies. In these analyses, ΔLDLControl-Rx emerged as the strongest determinant of CHD risk reduction in the full range of trials considered, as well as in the narrower set excluding trials with active-comparator arms. Importantly, these relationships were not influenced by baseline LDL-C level. The same was not true for total mortality, however, where ΔLDLControl-Rx was less strongly associated with outcome than baseline LDLRx in the broader meta-analysis, and none of these measures was significant in the more restrictive meta-analysis.
The observation regarding all-cause mortality runs contrary to expectation. Yet, to the extent that a higher baseline LDL-C is strongly linked to risk of coronary mortality, the component of all-cause mortality most directly influenced by statin-associated LDL-C lowering, this finding reflects a greater contribution of coronary death reduction to the overall mortality risk. That the relationship between baseline LDLRx and all-cause mortality was bolstered by exclusion of low-risk CHD trials, and particularly JUPITER, is in turn consistent with the removal of other influences on mortality risk, such as low-grade inflammation.7
Moreover, it is notable that exclusion of PROSPER, MEGA and, especially, CORONA heightened the relationship between ΔLDLControl-Rx and death (as well as CHD), with the association becoming both significant and stronger than the baseline LDL-C level when all 3 trials were omitted. These studies included special populations – older adults with8 or without 29 symptomatic heart failure, Japanese subjects34 – apt to have different relations of LDL-C to disease outcomes, as compared with participants in the majority of statin trials. In the case of PROSPER and CORONA, competing, non-coronary risks for mortality would alter the expected influence of LDL-C lowering on the outcomes considered here. Indeed, neither showed a significant reduction in all-cause mortality. By contrast, in MEGA, disproportionate reductions in CHD and total mortality in the face of modest LDL-C lowering would have the opposite distorting effect on the relation between ΔLDLControl-Rx and outcomes.
Another important message from these findings is that measures of absolute reduction generally have stronger relations with outcome than measures expressing these reductions as percents. This was the case for ΔLDLBaseline-Final in the 2 analyses of major CHD, as well as for ΔLDLControl-Rx (even if minimally) in all relevant instances but one.
Apart from the influence of the various LDL-C measures, key differences in the design features among the trials included are likely to account in substantial measure for the moderate degree of heterogeneity observed. These include the types and doses of statins evaluated; the approach to, and extent of, in-trial ascertainment of LDL-C (including in non-random subsets); duration of follow-up period; definitions and ascertainment of CHD outcomes; and conduct of the trials over the span of nearly 2 decades, with the associated influence of secular trends. In the context of such disparate trial-specific features, the pre-eminence of ΔLDLControl-Rx as a determinant of major CHD events and its robustness to sensitivity analysis are all the more notable, even if this measure proved to be of less consequence to all-cause mortality.
The finding that ΔLDLControl-Rx outperforms ΔLDLBaseline-Final, even in trials of statins versus non-active control, is expected because the former better reflects adherence to study assignment and extent of cross-over between study arms. In turn, the superiority of ΔLDLControl-Rx is consistent with observations from cohort studies and RCTs wherein the relation between cholesterol and risk of CHD is curvilinear.15–16 The implication of this log-linear relation is that the same absolute change at a different starting level of LDL-C will translate into an identical reduction in the relative risk of CHD. Using the percent change in lieu of the absolute change violates this relation.
The superior validity of ΔLDLControl-Rx is of special relevance because clinical trials,5,7–10 meta-analyses11 and even professional guidelines15 have often used the percent LDL-C reduction from baseline as the reference in discussing the magnitude of expected benefit. The present findings emphasize the importance of incorporating absolute changes in LDL-C and an assessment of baseline coronary risk in discussing the degree of risk reduction anticipated with statin use. They also underscore the need to consider post-treatment differences between comparison arms in designing future clinical studies of emerging therapies, whether for lipid lowering or otherwise.
A limitation of the present work is our lack of patient-level data, which would have permitted more thorough evaluation of lipid measures and other covariates, including sociodemographic and clinical subgroups. Individual-participant data also would have allowed assessment of the impact of estimated cardiovascular risk, because relating the observed risk in control groups to risk reduction is fraught with the potential for regression to the mean.36 More granular data could be used to probe further the questions addressed here.
Acknowledgments
Dr. Kizer was supported in part by Grant K23 HL070854 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Dr. Kizer has received honoraria from Merck & Co., Inc., and research support from diaDexus, Inc. Dr. Gotto is a current consultant for KOWA Pharmaceuticals, Merck, and Roche Pharmaceuticals, and he is on advisory boards for DuPont and Novartis. Dr. Gotto serves on corporate boards for Aegerion Pharmaceuticals, Arisaph Pharmaceuticals, and Vatera Capital. Dr. Pasternak is an employee of Merck & Co., Inc. None of the other authors have potential conflicts of interest to report.
References
- 1.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen TR, Faergeman O, Kastelein JJ, Olsson AG, Tikkanen MJ, Holme I, Larsen ML, Bendiksen FS, Lindahl C, Szarek M, Tsai J. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. doi: 10.1001/jama.294.19.2437. [DOI] [PubMed] [Google Scholar]
- 4.University of Oxford CTSU. American Heart Association Scientific Sessions Presentation Slides: Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Results. 2008 November 9; www.ctsu.ox.ac.uk/search/results/results.htm.
- 5.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 6.Pasternak RC. The ALLHAT Lipid Lowering Trial - Less Is Less. JAMA. 2002;288:3042–3044. doi: 10.1001/jama.288.23.3042. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 8.Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec J, Janosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 9.GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico) Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J. 2000;1:810–820. [PubMed] [Google Scholar]
- 10.Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 11.Gould AL, Rossouw JE, Santanello NC, Heyse JF, Furberg CD. Cholesterol reduction yields clinical benefit. Impact of statin trials. Circulation. 1998;97:946–952. doi: 10.1161/01.cir.97.10.946. [DOI] [PubMed] [Google Scholar]
- 12.Amarenco P, Labreuche J, Lavallee P, Touboul PJ. Statins in stroke prevention and carotid atherosclerosis: systematic review and up-to-date meta-analysis. Stroke. 2004;35:2902–2909. doi: 10.1161/01.STR.0000147965.52712.fa. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JG, Smith B, Maheshwari N, Schrott H. Pleiotropic effects of statins: benefit beyond cholesterol reduction? A meta-regression analysis. J Am Coll Cardiol. 2005;46:1855–1862. doi: 10.1016/j.jacc.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 14.Briel M, Schwartz GG, Thompson PL, de Lemos JA, Blazing MA, van Es GA, Kayikcioglu M, Arntz HR, den Hartog FR, Veeger NJ, Colivicchi F, Dupuis J, Okazaki S, Wright RS, Bucher HC, Nordmann AJ. Effects of early treatment with statins on short-term clinical outcomes in acute coronary syndromes: a meta-analysis of randomized controlled trials. JAMA. 2006;295:2046–2056. doi: 10.1001/jama.295.17.2046. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Merz CNB, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 16.Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–1576. doi: 10.1136/bmj.324.7353.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease. A meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 18.Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analysis. Ann Intern Med. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson SG, Higgins JPT. How should meta-regression analyses be undertaken and interpreted? Statist Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 22.Sharp SJ. Meta-analysis regression. Stata Tech Bull. 1998;42:16–22. [Google Scholar]
- 23.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 24.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;1995:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 25.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JMO, Wun CC, Davis BR, Braunwald E. The effects of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 26.The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 27.Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM., Jr Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279:1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 28.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd J, Blauw GJ, Murphy MB, Bollen ELEM, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlance PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RGJ. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 30.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collings R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O'Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 31.Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus 'usual' care in secondary coronary heart disease prevention. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 32.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomized placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 33.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 35.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 36.Sharp SJ, Thompson SG, Altman DG. The relation between treatment benefit and underlying risk in meta-analysis. BMJ. 1996;313:735–738. doi: 10.1136/bmj.313.7059.735. [DOI] [PMC free article] [PubMed] [Google Scholar]