Abstract
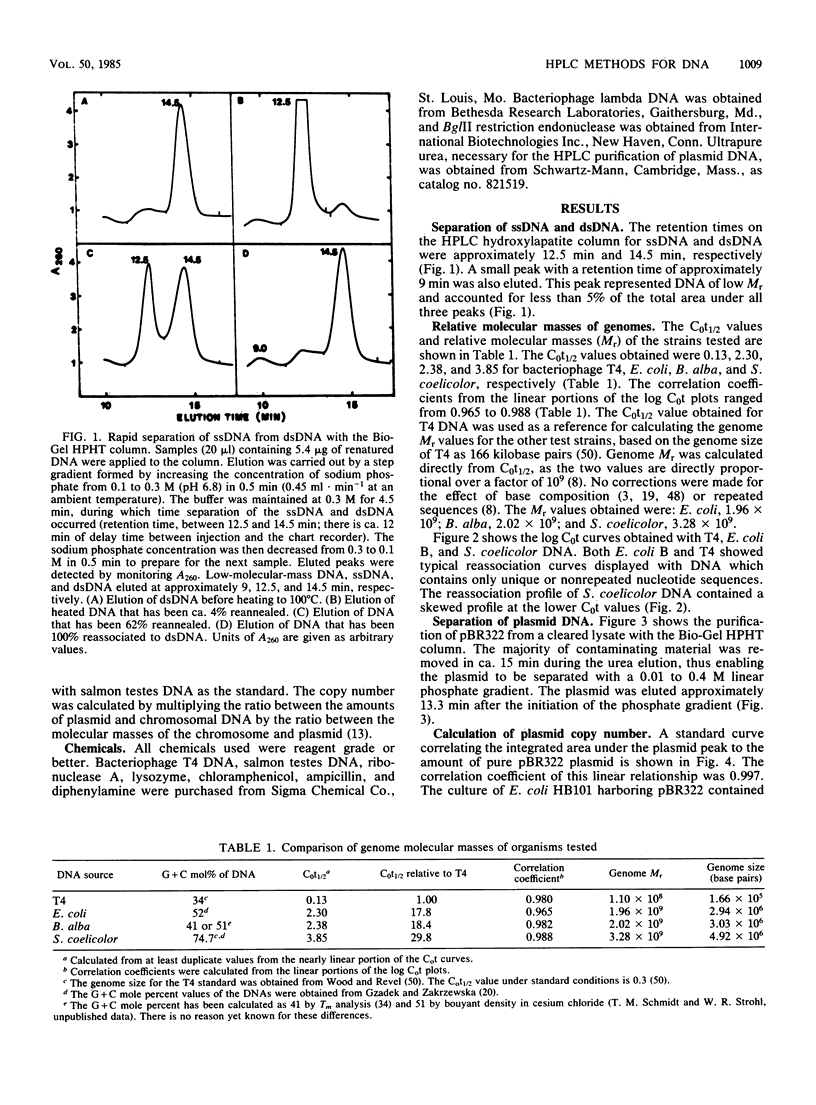
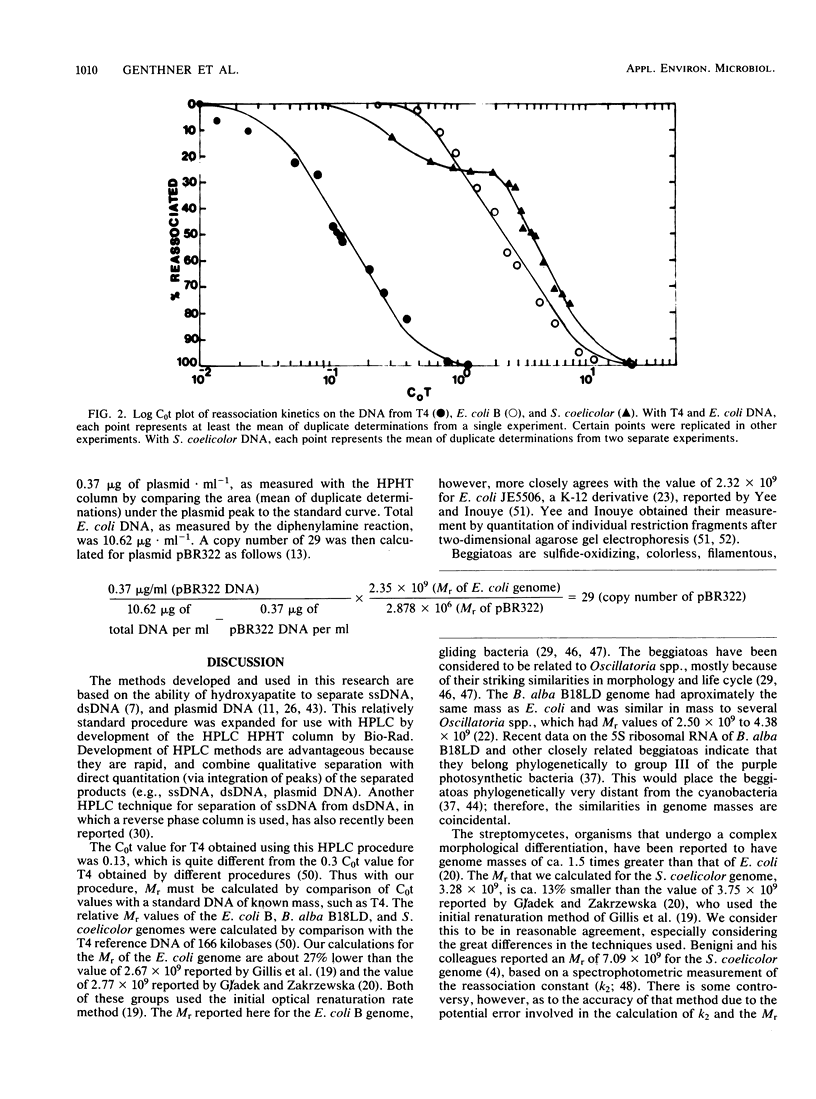
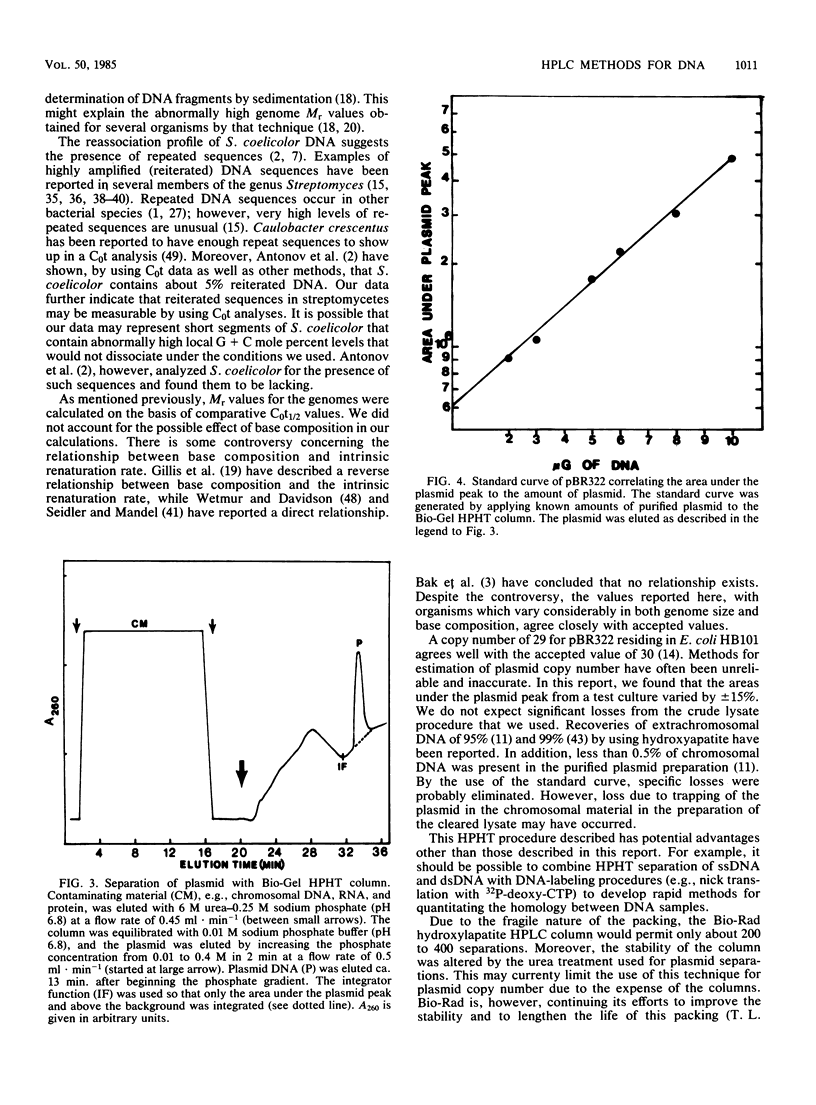
Relatively rapid methods for the determination of relative genome molecular mass (Mr) and the estimation of plasmid copy number have been developed. These methods are based on the ability of the Bio-Rad high-pressure liquid chromatography hydroxylapatite column to separate and quantify single-stranded DNA, double-stranded DNA, and plasmid DNA. Genome Mr values were calculated from reassociation kinetics of single-stranded DNA as measured with the hydroxylapatite column. Bacteriophage T4 DNA was used to establish a C0t (moles of nucleotides times seconds per liter), or standard reassociation value. From this C0t value, C0t values for Escherichia coli B, Beggiatoa alba B18LD, and Streptomyces coelicolor were determined by comparative calculations. From those calculated C0t values, the Mr values of 1.96 X 10(9) for E. coli, 2.02 X 10(9) for B. alba, and 3.28 X 10(9) for S. coelicolor were estimated. Plasmid concentration was determined from cleared lysates by comparing the integrated area under the phosphate buffer-eluted plasmid peak to values obtained with known amounts of plasmid. The plasmid copy number was estimated by multiplying the ratio between the amounts of plasmid and chromosomal DNA by the ratio between the Mr values of the chromosome and the plasmid. A copy number of 29 was obtained from a culture of E. coli HB101 harboring pBR322 grown to a culture density of 1.6 X 10(9) CFU . ml-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Antonov P. P., Ivanov I. G., Markov G. G. Heterogeneity of Streptomyces DNA. FEBS Lett. 1977 Jul 1;79(1):151–154. doi: 10.1016/0014-5793(77)80372-4. [DOI] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON E. T., McCARTHY B. J. A general method for the isolation of RNA complementary to DNA. Proc Natl Acad Sci U S A. 1962 Aug;48:1390–1397. doi: 10.1073/pnas.48.8.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- Benigni R., Petrov P. A., Carere A. Estimate of the genome size by renaturation studies in Streptomyces. Appl Microbiol. 1975 Aug;30(2):324–326. doi: 10.1128/am.30.2.324-326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- Colman A., Byers M. J., Primrose S. B., Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978 Nov 2;91(1):303–310. doi: 10.1111/j.1432-1033.1978.tb20966.x. [DOI] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman S. E., Hershberger C. L. Amplified DNA in Streptomyces fradiae. J Bacteriol. 1983 Aug;155(2):459–466. doi: 10.1128/jb.155.2.459-466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Johnson T. R., Ilan J. Large-scale isolation of plasmid DNA and purification of lambda phage DNA using hydroxylapatite chromatography. Anal Biochem. 1983 Jul 1;132(1):20–25. doi: 10.1016/0003-2697(83)90420-7. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Strohl W. R. Beggiatoa, Thiothrix, and Thioploca. Annu Rev Microbiol. 1983;37:341–367. doi: 10.1146/annurev.mi.37.100183.002013. [DOI] [PubMed] [Google Scholar]
- Liautard J. P. Rapid separation of single-stranded DNA from double-stranded DNA by reversed-phase high-performance liquid chromatography. J Chromatogr. 1984 Feb 17;285(1):221–225. doi: 10.1016/s0021-9673(01)87756-6. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., BERNE K. I., THOMAS C. A., Jr ELECTRON MICROSCOPY OF DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:648–649. doi: 10.1016/s0022-2836(65)80019-5. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Ono H., Hintermann G., Crameri R., Wallis G., Hütter R. Reiterated DNA sequences in a mutant strain of Streptomyces glaucescens and cloning of the sequence in Escherichia coli. Mol Gen Genet. 1982;186(1):106–110. doi: 10.1007/BF00422920. [DOI] [PubMed] [Google Scholar]
- Orlova V. A., Danilenko V. N. Mul'tiplikatsiia fragmenta DNK u Streptomyces antibioticus--produtsent oleandomitsina. Antibiotiki. 1983 Mar;28(3):163–167. [PubMed] [Google Scholar]
- Robinson M., Lewis E., Napier E. Occurrence of reiterated DNA sequences in strains of Streptomyces produced by an interspecific protoplast fusion. Mol Gen Genet. 1981;182(2):336–340. doi: 10.1007/BF00269680. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Deletion and amplification of DNA sequences in melanin-negative variants of Streptomyces reticuli. Mol Gen Genet. 1983;189(3):501–505. doi: 10.1007/BF00325917. [DOI] [PubMed] [Google Scholar]
- Schrempf H. Plasmid loss and changes within the chromosomal DNA of Streptomyces reticuli. J Bacteriol. 1982 Aug;151(2):701–707. doi: 10.1128/jb.151.2.701-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Sen A. A rapid method for the purification of extrachromosomal DNA from eukaryotic cells. J Biol Chem. 1978 Oct 10;253(19):6654–6656. [PubMed] [Google Scholar]
- Stackebrandt E., Woese C. R. The phylogeny of prokaryotes. Microbiol Sci. 1984 Aug;1(5):117–122. [PubMed] [Google Scholar]
- Strohl W. R., Cannon G. C., Shively J. M., Güde H., Hook L. A., Lane C. M., Larkin J. M. Heterotrophic carbon metabolism by Beggiatoa alba. J Bacteriol. 1981 Nov;148(2):572–583. doi: 10.1128/jb.148.2.572-583.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. R., Larkin J. M. Enumeration, isolation, and characterization of beggiatoa from freshwater sediments. Appl Environ Microbiol. 1978 Nov;36(5):755–770. doi: 10.1128/aem.36.5.755-770.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wood N. B., Rake A. V., Shapiro L. Structure of Caulobacter deoxyribonucleic acid. J Bacteriol. 1976 Jun;126(3):1305–1315. doi: 10.1128/jb.126.3.1305-1315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Reexamination of the genome size of myxobacteria, including the use of a new method for genome size analysis. J Bacteriol. 1981 Mar;145(3):1257–1265. doi: 10.1128/jb.145.3.1257-1265.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee T., Inouye M. Two-dimensional DNA electrophoresis applied to the study of DNA methylation and the analysis of genome size in Myxococcus xanthus. J Mol Biol. 1982 Jan 15;154(2):181–196. doi: 10.1016/0022-2836(82)90059-6. [DOI] [PubMed] [Google Scholar]


