Abstract
Objectives
This study aimed to determine if CD31 is a novel marker of a circulating angio-vasculogenic cell population and to establish their therapeutic effects on experimental ischemia.
Background
Emerging evidence suggested that therapeutic mechanisms underlying various bone marrow (BM)-derived cells are due to paracrine effects. Furthermore, the vasculogenic potential of these cells is under debate. CD31 is a well known marker for endothelial cells (ECs) but is also expressed in a fraction of peripheral blood (PB) mononuclear cells.
Methods
CD31+ cells were isolated from human PB by magnetic-activated cell sorting (MACS). The gene expression profile was examined by DNA microarray and real-time RT-PCR (qRT-PCR). Various in vitro endothelial differentiation or vasculogenic assays were conducted. Finally, cells were directly implanted into a mouse hindlimb ischemia (HLI) model to test angiogenic-vasculogenic and therapeutic effects.
Results
Fluorescent-activated cell sorter (FACS) analysis revealed that PB-CD31+ cells exhibited endothelial and hematopoietic stem/progenitor markers. CD31+ cells had higher levels of expression of pro-angiogenic genes on microarray and qRT-PCR and generated higher numbers of endothelial progenitor cells (EPCs) compared to CD31− cells. CD31+ cells spontaneously formed vascular tube-like structures and exhibited an endothelial cell phenotype in vitro. In a HLI model, CD31+ cell transplantation augmented blood perfusion and prevented limb loss. Both angiogenic cytokines and capillary density were increased, suggesting CD31+ cells augmented neovascularization.
Conclusions
CD31 is a novel marker that designates circulating angiogenic and vasculogenic cells. These cells are easily isolated from human PB and thus are a novel candidate for treatment of ischemic cardiovascular disease.
Keywords: CD31, endothelial cells, angiogenesis, vasculogenesis, ischemic hindlimb
Ischemic cardiovascular disease is the most prevalent disease in Westernized society. Despite progress in therapies, nearly 80 million are affected yearly from heart attack, stroke and ischemic cardiomyopathy (1). As our knowledge of stem cell biology grows, cell-based therapies have become a promising strategy for tissue regeneration. Transplantation of various bone marrow (BM)-derived cells, including mononuclear cells (MNCs), endothelial progenitor cells (EPCs), mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), were reported to induce therapeutic neovascularization in adult ischemic tissues (2–5). While cell-based therapy has great therapeutic potential, several issues remain. The use of unselected BM cells or BM-MNCs was associated with adverse events including calcification, vascular plaque growth, and worsening of tissue ischemia (6–8). EPCs and MSCs require ex vivo culture to be used clinically. For all BM-derived cells, their ability to (trans)differentiate into ECs, and the magnitude of their vasculogenic potential, has been debated (9–11). Paracrine mechanisms and/or other non-differentiation effects are the primary mediators of BM-derived cell benefit in ischemic cardiovascular disease (3,12–14) and mainly work via augmentation of neovascularization/angiogenesis or improvement of microcirculation.
Based on these new discoveries on the mechanisms and adverse effects of various BM cells over the past decade, in this study, we sought to explore whether there is a specific native population of BM cells which possesses higher paracrine or angiogenic activity, and further, to determine the therapeutic potential of this population for treating ischemic cardiovascular diseases. Additionally, given the controversy regarding the (trans)differentiation, we sought to definitively determine whether BM cells are able to give rise to ECs via vasculogenesis. We used rigorous criteria and new experimental techniques, to address the main points of criticism from the previous studies that refuted the concept of endothelial differentiation from BM cells (9–11,15).
We have found that CD31, a ubiquitous EC marker, is also expressed on the surface of various mouse hematopoietic cell lineages (16) including HSCs (17), and undifferentiated mouse embryonic stem cells (18). CD31, also known as platelet endothelial cell adhesion molecule (PECAM)-1, is a 130-kd cell surface molecule and a member of the immunoglobulin superfamily (19). CD31 plays important role for transendothelial leukocyte migration, cell-cell adhesion and anti-apoptotic signaling (20). However, the therapeutic role of the cells expressing this marker in tissue regeneration is unknown. Based on the conserved expression of CD31 on cells of the hemangioblast lineage, we hypothesized that CD31+ cells in PB have higher angiogenic and/or vasculogenic capabilities.
Through a series of in vitro and in vivo experiments, we found that CD31+ cells isolated from PB showed higher angiogenic activity and vasculogenic potential, effectively improving ischemia in mouse HLI by augmentation of neovascularization.
Methods
An expanded Methods section is available in the Online Appendix.
Isolation of CD31+ and CD31− cells
Flow cytometry
Microarray analysis
Transplantation of the CD31+ and CD31− cells into ischemic hindlimb
Real-time RT-PCR (qRT-PCR) assay
EPC culture assay and immunocytochemistry
Cell adhesion assay
Hematopoietic colony forming unit assays
Histological analysis
Statistical analysis
Results
Endothelial and hematopoietic stem cell characteristics of PB-CD31+ cells
FACS analysis on PB-CD31+ cells showed that > 95% of MACS-isolated CD31+ cells express CD31 (Fig. 1A). Approximately 40% of CD31+ cells expressed CD14, a monocyte/macrophage marker and more than 99% of CD31+ cells expressed CD45, a pan-hematopoietic marker; this suggests that CD31+ cells are not circulating ECs (Fig. 1A and 1C). CD31+ cells preferentially expressed endothelial markers (CD105, CD141, CD144 and von Willebrand Factor [vWF]; p < 0.05) and stem cell or progenitor markers (CD34, CD133, CD117 and KDR [VEGFR-2]; p < 0.05) (Fig. 1B and Online Fig. 1A). To investigate the hematopoietic progenitor cell (HPC) properties, a clonogenic assay was performed. CD31+ cells generated a significantly higher number of hematopoietic colonies compared to CD31− cells such as colony forming unit-erythroid (CFU-E), burst forming unit-erythroid (BFU-E), colony forming unit granulocyte/macrophage (CFU-GM), and colony forming unit-granulocyte/erythroid/macrophage-/megakaryocyte (CFU-GEMM) (p < 0.05) (Fig. 1C and Online Fig. 1B). These data show that CD31+ cells have characteristics of HSC/HPCs as well as ECs.
Figure 1. Hematopoietic and endothelial characteristics of PB CD31+ cells.


(A) MACS-sorted CD31+ and CD31− cells were analyzed by FACS for hematopoietic and EC markers. (B) Quantification of the FACS data for CD31+ and CD31− cells showing enriched expression of monocyte, hematopoietic stem/progenitor and EC markers in CD31+ cells. n = 4 per group. (C) In vitro HPC assay of CD31+ and CD31− cells. HPC activities were higher in the CD31+ group. n = 5 per group. *p < 0.05; **p < 0.01.
Enriched angiogenic, cell adhesion and chemoattraction genes in CD31+ cells
To compare global gene expression patterns between CD31+ and CD31− cells, we carried out microarray analysis. Hierarchical cluster analysis showed that gene expression in CD31+ cells are distinct from CD31− cells in that 749 genes were upregulated and 26 genes were downregulated by more than two-fold in the CD31+ cells compared to CD31− cells (Fig. 2A). Further characterization with gene ontology data base (GO, http://www.geneontolgy.org) demonstrated that genes involved in angiogenesis, cell adhesion, transmembrane structure, chemokine production and reception, and extracellular matrix were highly and preferentially expressed in CD31+ cells (Fig. 2B, Table 1 and Online Table S1 to S3) (21).
Figure 2. The gene expression profile and GO-Scan classification of differentially expressed genes.

(A) Hierarchical cluster analysis. Gene expression profiling of CD31+ and CD31− cells. Each column represents three CD31+ and CD31− cells and each row reflects a probe set. Red signifies increased expression and blue signifies decreased expression. (B) The x axis represents the number of differentially expressed genes in a specific category of annotations from our list of 779 genes (generated using multiple comparisons correction: False-Discovery Rate [FDR] adjusted p-value < 0.05). (C) The expression patterns of multiple angiogenic factors were measured by qRT-PCR. MNC = mononuclear cell. Data are presented as fold difference compared to the MNC group, n = 6 per group. *p < 0.05; **p < 0.01.
Table 1.
Genes associated with angiogenesis significantly up- or down-regulated in CD31+ cells compare to CD31− cells.
| Probe Set ID | Gene Symbol | Description | Fold change |
|---|---|---|---|
| 7932985 | NRP1 | Neuropilin 1 | 11.8 |
| 7977046 | TNFAIP2 | Tumor necrosis factor, alpha-induced protein 2 | 7.4 |
| 7991335 | ANPEP | Alanyl (membrane) aminopeptidase | 6.6 |
| 8095680 | IL8 | Interleukin 8 | 6.5 |
| 8108217 | TGFBI | Transforming growth factor beta-induced | 6.5 |
| 8140556 | HGF | Hepatocyte growth factor | 5.8 |
| 8103399 | PDGFC | Platelet derived growth factor C | 5.2 |
| 8119898 | VEGFA | Vascular endothelial growth factor A | 4.7 |
| 8156278 | EDG3 | Endothelial differentiation, sphingolipid G protein coupled receptor 3 | 4.2 |
| 8017599 | PECAM1 | Platelet/endothelia cell adhesion molecule 1 | 3.9 |
| 7970737 | FLT3 | Fms related tyrosine kinase 3 | 3.5 |
| 8040473 | RHOB | Ras homolog gene family, member B | 3.5 |
| 7973084 | ANG | Angiogenin | 3.2 |
| 7969414 | KLF5 | Kruppel-like factor 5 | 3 |
| 7951686 | IL18 | Interleukin 18 | 2.5 |
| 8096160 | ARHGAP24 | Rho GTPase activating protein 24 | 2.4 |
| 8092552 | IGF2BP2 | Insulin-like growth factor 2 mRNA binding protein 2 | 2.2 |
| 8152297 | ANGPT1 | Angiopoietin 1 | 2.2 |
| 8064978 | JAG1 | Jagged 1 | 2.1 |
| 8137670 | PDGFA | Platelet-derived growth factor alpha polypeptide | 2 |
| 7951351 | PDGFD | Platelet derived growth factor D | −2 |
| 8099471 | FGFBP2 | Fibroblast growth factor binding protein 2 | −2.2 |
To confirm the results of microarray data, we performed qRT-PCR analysis. Major angiogenic factors such as vascular endothelial growth factor (VEGF)-A, fibroblast growth factor (FGF)-2, hepatocyte growth factor (HGF) and angiopoietin (Ang)-1, and an adhesion molecule, VE-cadherin, were more highly expressed in CD31+ cells compared to CD31− cells or MNCs (Fig. 2C). Chemokines such as monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-8, which play important role for neovascularization, were also significantly upregulated (22,23). Collectively, these findings show that CD31+ cells define a population enriched with angiogenic, chemoattractant and cell adhesion genes.
CD31+ cells show higher vasculogenic potential and cell adhesion capacity in vitro
We next investigated the in vitro endothelial differentiation potential of CD31+ cells. First, we performed EPC culture assay. The number of EPCs and EPC colonies were markedly higher in the CD31+ cell group compared to the CD31-cell group (all p < 0.01) (Fig. 3A and 3B). Second, we carried out a cell adhesion assay. The number of adhered cells to various extracellular matrix proteins, fibronectin, vitronectin, collagen I and laminin, was significantly higher in the CD31+ cells than in the CD31− cells (all p < 0.001) (Fig. 3C). In ECs, adhesion capacity is an indicator of cell engraftment and cell survival.
Figure 3. In vitro vasculogenic properties of CD31+ cells.
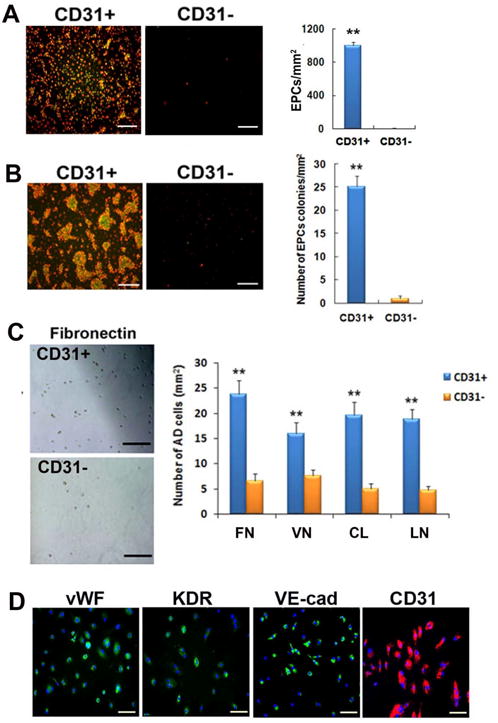
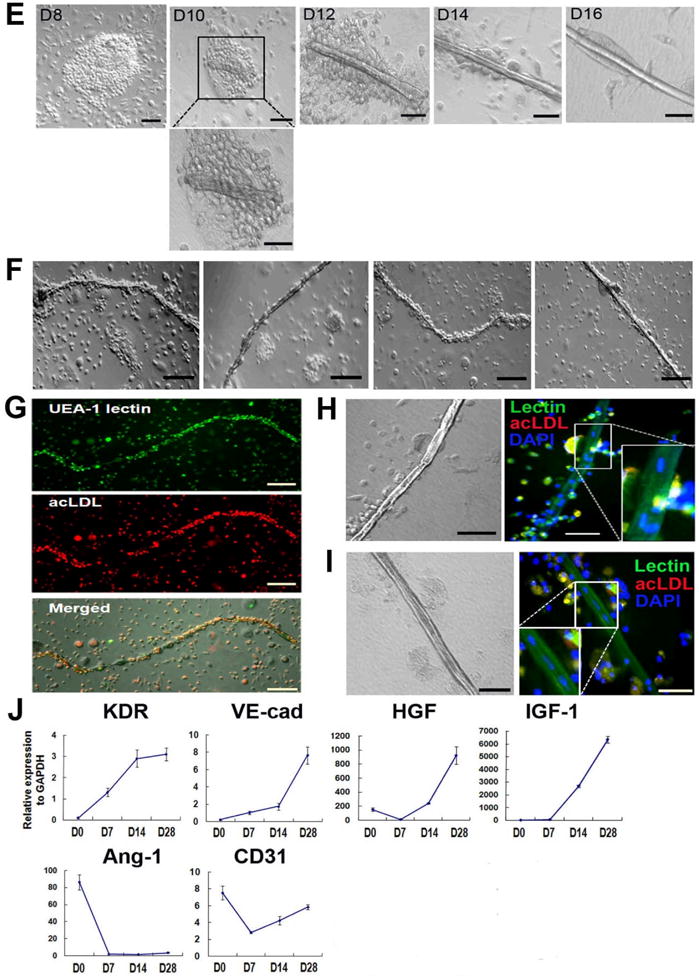
(A, B) Uptake of DiI-acLDL (red fluorescence) and binding to UEA-1 lectin (green fluorescence) identified EPCs. CD31+ cells gave rise to higher number of EPCs (A) and EPC colonies (B). n = 5 per group. **p < 0.01, Bars: 200μm. (C) Cell adhesion assay showed higher adhesion of CD31+ cells to all tested extracellular matrix proteins compared to CD31− cells. FN, fibronectin; CL, type I collagen; VN, vitronectin; LN, laminin. n = 5 per group. Bars: 200 μm. (D) CD31+ cells expressed endothelial proteins vWF, KDR, VE-cadherin and CD31 in culture with EGM-2 for 6 days. Bars: 200 μm. (E) Cultured CD31+ cells formed vascular tube-like structures over the period of 3 wks. In the first week of culture under EGM-2, CD31+ cells were aggregated, at around day 10 the cells started to form tubular structures within the cell clusters, and between days 12 to 16, the cell clusters underwent morphologic changes to form linear tubular structures, mimicking in vivo vasculogenesis. Bars: D8 and D10, 100 μm; D12, D14, D16 and magnified image, 60 μm. (F) Various morphologies of tubular structures. Bars: 200 μm. (G) Tubular structures showing uptake of Dil-acLDL (red fluorescence) and UEA-1 binding (green fluorescence). Bars: 200μm. (H, I) Enlarged images of tubular structures. Phase contrast images (left panels) and fluorescent microscopic images show that the tubular structures are composed of multiple cells, and take up Dil-acLDL and are bound by UEA-1 lectin. Bars: H, 60 μm; I, 100 μm. (J) Temporal expression patterns of multiple angiogenic genes examined by qRT-PCR during cultivation of CD31+ cells over 28 days. n = 5 per group.
To induce EC differentiation, CD31+ cells were cultured in endothelial growth media (EGM)-2, which is endothelia cell basal media (EBM)-2 with 15% FBS and cytokine cocktail (SingleQuots), for 28 days. CD31+ cells exhibited EC markers such as vWF, KDR, VE-cadherin and CD31 by immunocytochemistry (Fig. 3D). CD31− cells were not culturable under these conditions. During this culture, we made an intriguing observation that some CD31+ cells spontaneously formed tubular structures. In the first week of culture, CD31+ cells aggregated, around day 10 the cells started to form tubular structures within the cell clusters, and between day 12 to 16 the cell clusters underwent morphologic changes to form linear tubular structures, mimicking in vivo vasculogenesis (Fig. 3E to 3I). These tubular structures stained positive for lectin and took up Dil-acLDL, characteristics of ECs. We further measured the expression of endothelial and angiogenic genes during the culture of CD31+ cells over 28 days by qRT-PCR. Over 28 days the expression of KDR, VE-cadherin, HGF and insulin-like growth factor (IGF)-1 gradually increased suggesting differentiation into a more vasculogenic and angiogenic phenotype (Fig. 3J). Together, these data demonstrate the vasculogenic and angiogenic potential of CD31+ cells in vitro.
CD31+ cells exert favorable therapeutic effects on hindlimb ischemia
After identifying the angiogenic and vasculogenic characteristics of CD31+ cells in vitro, we investigated the therapeutic potential of CD31+ cells to repair experimentally induced ischemia in vivo. Laser Doppler perfusion image (LDPI) analysis demonstrated that blood perfusion was significantly higher in the CD31+ cell injected limbs versus CD31- cell- or PBS-injected limbs at day 7, 14 and 21 (Fig. 4A and 4B). The CD31+ cell-treated group showed a significantly lower limb loss score than the PBS control group (p < 0.05) (Fig. 4C and 4D). The capillary density and vascular maturation in the ischemic hindlimb adductor muscle after surgery was also significantly higher in the CD31+ group than in the CD31− group or the PBS group (p < 0.01) (Fig. 5A, 5B and Online Fig. 2). We also found that functional capillaries examined after intracardiac injection of ILB4 were significantly higher in the CD31+ group compared to the other groups (Online Fig. 3). In addition TUNEL assay, together with ILB4 staining, demonstrated that transplantation of CD31+ cells significantly reduced endothelial cell apoptosis compared to control groups suggesting protective effects on existing vessels (Online Fig. 4). Collectively, these findings suggest that CD31+ cells can augment functional neovascularization and preserve existing vessels in vivo and are effective for treating ischemic vascular disease.
Figure 4. CD31+ cell transplantation is effective for recovery of ischemia and preservation of tissue in hindlimb ischemia.

(A, B) Recovery of blood perfusion in ischemic hindlimbs. LDPI images (A) and quantitative analysis (B) showed improved blood perfusion in the CD31+ group compared to the CD31−and PBS groups over four weeks after cell transplantation. The blue represents low perfusion and the red represents high perfusion. n = 9 per group **p < 0.01, *p < 0.05, CD31+ vs. PBS. ‡p < 0.01, †p < 0.05, CD31+ vs. CD31−. (C, D) Limb salvage in ischemic hindlimbs. Representative photographs (C) and statistical analysis of limb loss score (D) at day 28 showed significantly higher limb salvage in the CD31+ group compared to the CD31− or the PBS group. Red circles indicate limb loss, foot necrosis, toe necrosis and intact limb. n = 9 per group. *p < 0.05.
Figure 5. CD31+ cell transplantation induced neovascularization in vivo.

(A, B) Capillary density in hindlimb muscles. ILB4 staining for ischemic hindlimb muscles (A) and the quantitative analysis of capillary density (B) demonstrated significantly increased number of capillaries in the CD31+ group compared to the CD31− and PBS group. Bars: A, 100 μm. n = 8 per group. **p < 0.01. (C) CD31+ cell transplantation induced increased expression of angiogenic and chemoattractant factors in tissues measured by qRT-PCR compared to CD31− cell or PBS injection. Data are presented as fold difference to the PBS group n = 5 per group. *p < 0.05; **p < 0.01.
Multiple angiogenic factors are up-regulated after CD31+ cells transplantation
To determine the effects of CD31+ cell transplantation on cytokine expression in ischemic hindlimbs, mice were sacrificed and hindlimb tissues collected. The expression levels of VEGF-A, Ang-1, FGF-2, stromal cell-derived factor (SDF)-1 and CD31, and were significantly increased in the CD31+ cell injected limbs compared to the CD31− cell- or PBS-injected limbs (Fig. 5C). These data suggest that CD31+ cell transplantation upregulates the expression of multiple biological factors associated with neovascularization and bone marrow cell mobilization.
Contribution of CD31+ cells into vasculogenesis
We further sought to determine the potential and magnitude of the contribution of CD31+ cells to ECs generation or vasculogenesis. To track the transplanted cells, we intramuscularly injected 1×106 Dil-labeled CD31+ cells into the ischemic hindlimb of nude mice. Histologic analysis demonstrated that a larger part of the injected CD31+ cells were concentrated in the pericytic or perivascular areas (Fig. 6A to 6D and see Online Video 1) and a smaller population of CD31+ cells exhibited an ECs specific marker, isolectin B4 (ILB4), within the vascular structure at 2–8 weeks (Fig. 6E to 6G and see Online Video 2). This suggests that CD31+ cells generated ECs in vivo. To confirm this, we performed fluorescent in situ hybridization (FISH) using human Y chromosome probe (24,25). FISH data showed that Y chromosome signals from transplanted human CD31+ cells were detected within the nuclei of ECs (Fig. 6I). Collectively, these data indicate that CD31+ cells can give rise to ECs, suggesting their vasculogenic potential.
Figure 6. Engraftment and differentiation of transplanted CD31+ cells into ECs in ischemic hindlimbs.
(A-D) Localization of CD31+ cells in the perivascular regions. Confocal microscopic images showed that Dil-labeled CD31+ cells were more preferentially localized to the pericytic or perivascular areas (arrowheads). One colocalization with ECs is shown by an arrow. Bars: A, C, D, 10 μm; B, 20 μm. (E-G) ECs differentiation of CD31+ cells. Three dimensional z-stacked orthogonal and multipanel images clearly demonstrated that a part of the engrafted CD31+ cells were incorporated into vascular structures and exhibit an EC marker, suggesting endothelial differentiation of CD31+ cells. Green, ILB4; Red, Dil-labeled CD31+ cells; Blue, DAPI. Bars: E, 500 μm; F-G, 10 μm. (H, I) FISH on CD31+ cell transplanted hindlimb tissues. One cell (H, arrowhead) which was localized at the pericytic area exhibited red fluorescence for human Y chromosome. The other cell (I, arrows) exhibited a FISH signal (red) within the nuclei of an ILB4-stained capillary (arrows), suggesting differentiation of injected CD31+ cells into ECs in vivo. ILB4 (green), DAPI (blue). Bars: H, I, 100 μm.
Quantitative analysis of endothelially transdifferentiated CD31+ cells
It has been difficult to quantify the rate of differentiation of BM-derived cells in vivo. Here, to more accurately quantify the number of functional ECs derived from transplanted CD31+ cells, we conducted FACS analysis on enzymatically digested muscle following systemic injection of FITC-labeled ILB4. FACS analysis revealed that 2.2 ± 0.8 % of the cells were double positive for DiI and ILB4 at 4 weeks, suggesting that significant portion of functional ECs were derived from transplanted CD31+ cells (Fig. 7A). Next, to further confirm whether these double positive cells were truly derived from the injected human CD31+ cells, they were subjected to FISH with human Y chromosome. FISH demonstrated that the ILB4- and Dil-positive cells from recipient mice were of human donor origin, confirming differentiation of CD31+ cells into ECs (Fig. 7B).
Figure 7. Verification and quantification of ECs generated from transplanted CD31+ cells.
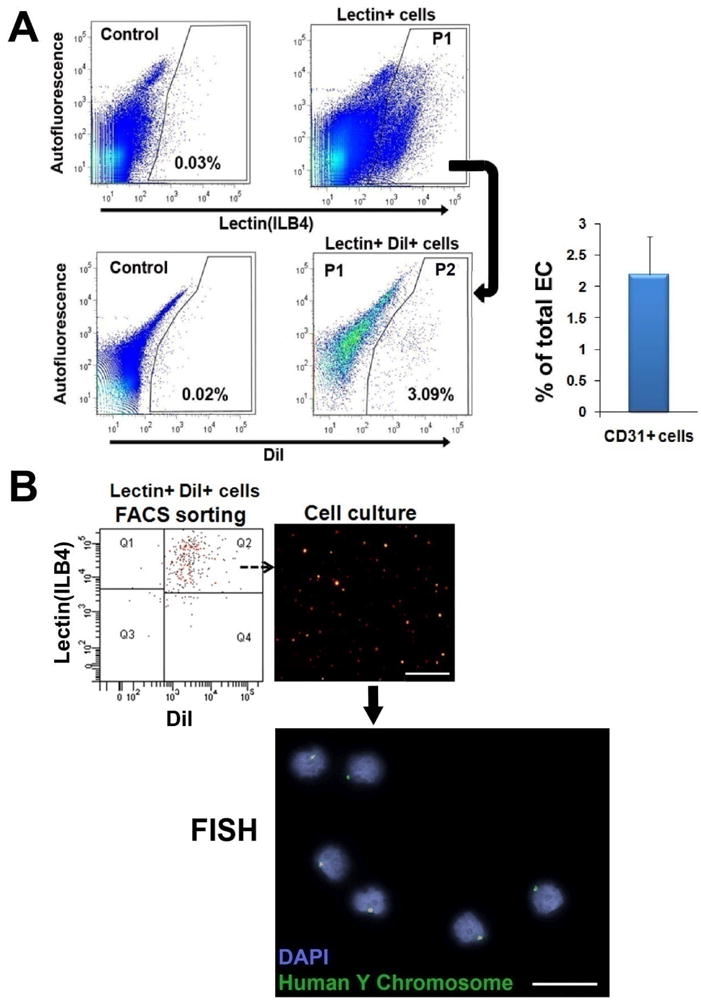
(A) Hindlimb tissues were harvested 4 weeks after injection with DiI-labeled CD31+ cells and digested with an enzyme cocktail. FACS analysis showed that 2.2% of ECs in hindlimb muscles were derived from injected CD31+ cells. P1 gating represents ILB4+ fraction and P2 gating indicates double positive population for DiI (CD31+ cells) and ILB4 (ECs). n = 5. (B) FACS-sorted cells showing double positivity for Dil and ILB4 were subjected to FISH. FISH showed that these double positive cells derived from ischemic hindlimb of nude mice were human origin. Bars: upper B, 200 μm; lower B, 20 μm.
Discussion
In this study, we show that CD31 is a unique and comprehensive marker to represent potent angiogenic and vasculogenic cells in human PB and that these cells are therapeutically effective for recovering tissue from ischemia. First, we demonstrated that human CD31+ cells highly express HSC/HPC and EC markers. Second, CD31+ cells are enriched with pro-angiogenic genes. Third CD31+ cells generated more EPCs compared to CD31− cells and demonstrated EC differentiation and vascular-like tube formation in culture. Fourth, implantation of CD31+ cells into ischemic hindlimb enhanced recovery of tissue from ischemic injury and increased the rate of limb salvage as well as the expression of angiogenic factors. Fifth, implanted CD31+ cells show clear vasculogenic potential in ischemic limb tissues.
Since CD31 is a ubiquitous endothelial marker, we tested the hypothesis that the cells expressing CD31 in PB have angiogenic and/or vasculogenic capabilities. FACS showed that the CD31+ cells expressed HSC/HPC markers and well-known EC markers, suggesting dual properties of CD31+ cells. Unbiased microarray data and qRT-PCR results indicated that angiogenic and other genes beneficial for vascular regeneration are highly enriched in the CD31+ cell population. Another distinguishing feature of CD31+ cells is that they gave rise to EPCs in the culture while CD31− cells did not. CD31+ cells frequently formed vascular-like tubes in culture without the aid of biomaterials such as Matrigel, mimicking the developmental process of vascular tube formation from angioblasts or EPCs (Fig. 3E to 3I). This finding is one of the most unprecedented of this study, supporting the vasculogenic potential of CD31+ cells. These data suggest that, CD31 is an antigenic signpost for robust pro-angiogenic and vasculogenic cells in PB.
In vivo cell transplantation studies further confirmed these results. CD31+ cell transplantation induced higher expression of multiple paracrine factors in the ischemic tissues during the critical period of ischemia repair. Our data suggest that the major mechanism of CD31+ cells would be a non-differentiation mechanism because the rate of endothelial differentiation was too small to account for the magnitude of therapeutic effects seen. CD31+ cells highly expressed crucial angiogenic and arteriogenic factors including VEGF, Ang-1, FGF-2 and MCP-1. Synergistic effects of these angiogenic factors have been reported to induce therapeutic neovascularization (26). Also tissues injected with CD31+ cells expressed high levels of SDF-1 which are known to contribute to a pro-neovascularization environment. For optimal neovascularization, such an environment is required to recruit necessary cell types including monocytes and macrophages (27,28). Another characteristic that may make CD31+ cells particularly effective is their high adhesion capacity. Cell adhesion signals control cell viability and are essential for regulated angiogenesis and vasculogenesis via cell-cell and cell-matrix interactions (9,11,15).
The controversy remains regarding the (trans)differentiation potential of BM-derived cells into ECs. To clarify this, we meticulously evaluated hindlimb muscles using multiple techniques. First, we used 3D reconstruction of confocal microscopic images to examine at least 1200 tissue sections collected from 10 animals from each group. Although most CD31+ cells were localized to the interstitium or perivascular region, some cells were incorporated into vasculature and expressed functional EC markers (Fig. 7A). Second, FISH using Y chromosome probes showed positive donor cell-derived signals within the nuclei of ECs suggesting endothelial differentiation of CD31+ cells. Third, FACS analysis confirmed and quantified ECs derived from CD31+ cells injected into HLI. FACS analysis revealed 2.2% of ECs in hindlimb muscle were derived from implanted CD31+ cells. We used these techniques to avoid sampling errors inherent in histologic analysis. To the best of our knowledge, this is the first study to employ this technology to prove and quantify endothelial differentiation from injected BM cells. Finally, we further confirmed that these FACS-isolated (trans)differentiated ECs are human origin by FISH. These results indicate that a specific population of PB-MNCs can (trans)differentiate into ECs although the differentiation efficiency is low.
Cell selection using the surface antigen CD31 has several advantages over to more specific hematopoietic stem/progenitor markers CD133+ or CD34 (29,30). Approximately 80% of CD133+ cells and 85% of CD34+ cells are contained within the CD31+ cell fraction (Fig. 1 and Online Fig. 1). Thus CD31+ cells can exert most of the beneficial effects of both cell types while still providing additional paracrine or vascularizing effects through other cell types. Clinically, as CD31+ cells occupy around 30% of PB MNCs, these cells do not require mobilization to obtain enough to be used for cell therapy.
In conclusion, PB-derived CD31+ cells contain robust angiogenic and vasculogenic abilities that ameliorate hindlimb ischemia. Although we only showed ischemic limb recovery in this study, this therapy would also be effective for ischemic heart disease given their broad neovascularizing, paracrine and cell adhesion capacity. Accordingly, these cells can serve as a highly promising and novel therapeutic option for ischemic cardiovascular disease. Further investigation will be needed to determine if CD31+ cells are capable of treating human ischemic cardiovascular diseases.
Supplementary Material
Acknowledgments
This work was supported in part by National Institute of Health grants (HL079137, HL084471, HL097353), Research grant (SC4300) from Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, Republic of Korea, Overseas post-doctoral fellowship grant from the Korea Research Foundation, Republic of Korea (MOEHRD, to SW Kim) and Atlanta Clinical Translational Research Institute (ACTSI)-Georgia Tech Emory Collaboration for Regenerative Medicine and Engineering (GTEC) pilot grant.
Abbreviations and Acronyms
- Ang
angiopoietin
- ECs
endothelial cells
- EPCs
endothelial progenitor cells
- FACS
fluorescent-activated cell sorter
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- HLI
hindlimb ischemia
- HPC
hematopoietic progenitor cell
- IL-8
interleukin-8
- MCP-1
monocyte chemoattractant protein-1
- MNCs
mononuclear cells
- PB
peripheral blood
- PECAM
platelet endothelial cell adhesion molecule
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
THERE ARE NO RELATIONSHIP WITH INDUSTRY AND FINANCIAL DISCLOSURE STATEMENTS.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 3.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 4.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 5.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto K, Nishigami K, Nagaya N, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–84. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 7.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–7. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 8.George J, Afek A, Abashidze A, et al. Transfer of endothelial progenitor and bone marrow cells influences atherosclerotic plaque size and composition in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2005;25:2636–41. doi: 10.1161/01.ATV.0000188554.49745.9e. [DOI] [PubMed] [Google Scholar]
- 9.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 10.Zentilin L, Tafuro S, Zacchigna S, et al. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–54. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 11.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 12.Gnecchi M, He H, Liang OD, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 13.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 14.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–9. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 16.Newman PJ, Hillery CA, Albrecht R, et al. Activation-dependent changes in human platelet PECAM-1: phosphorylation, cytoskeletal association, and surface membrane redistribution. J Cell Biol. 1992;119:239–46. doi: 10.1083/jcb.119.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann CI, Bailey AS, Li W, Ferkowicz MJ, Yoder MC, Fleming WH. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104:1010–6. doi: 10.1182/blood-2004-03-0989. [DOI] [PubMed] [Google Scholar]
- 18.Behrem S, Zarkovic K, Eskinja N, Jonjic N. Endoglin is a better marker than CD31 in evaluation of angiogenesis in glioblastoma. Croat Med J. 2005;46:417–22. [PubMed] [Google Scholar]
- 19.Newman PJ, Berndt MC, Gorski J, et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science. 1990;247:1219–22. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- 20.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–64. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–7. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 23.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 24.Chae JK, Kim I, Lim ST, et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler Thromb Vasc Biol. 2000;20:2573–8. doi: 10.1161/01.atv.20.12.2573. [DOI] [PubMed] [Google Scholar]
- 25.Cao R, Brakenhielm E, Pawliuk R, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat Med. 2003;9:604–13. doi: 10.1038/nm848. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita J, Itoh H, Hirashima M, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 27.Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:887–93. doi: 10.1161/01.atv.0000017728.55907.a9. [DOI] [PubMed] [Google Scholar]
- 28.Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006;26:1431–8. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- 29.Kawamoto A, Tkebuchava T, Yamaguchi J, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–8. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 30.Bartunek J, Vanderheyden M, Vandekerckhove B, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: feasibility and safety. Circulation. 2005;112:I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




