Abstract
Stimulation of α1-adrenoreceptors (α1-AR) acutely alters ion channel behavior via several signaling pathways [calcium and protein kinase C (PKC)]. Little is known about sustained α1-adrenergic/PKC signaling and channel regulation as may occur during cardiovascular disease states. Here we describe the effects of prolonged α1A-AR and PKC activity on human ether-a-go-go-related gene (HERG) K+ channels (Kv11.1) expressed in a heterologous expression system. Stimulation of α1A-AR with phenylephrine or direct activation of PKC with phorbol ester increased HERG channel protein abundance and K+ current density in a time- and dose-dependent manner. Channel augmentation reached a steady-state plateau within 24 h with a 2- to 6-fold induction. Phorbol ester and moderate α1A-AR stimulation enhanced HERG abundance in a PKC-dependent fashion but with stronger α1A-adrenergic stimulation; protein kinase A (PKA)-dependent activity also contributed. Comparable channel induction of other cardiac K+ channels was not seen in this system. Comparison of wild-type HERG and channels with either mutated PKC phosphorylation sites (HERGΔPKC) or mutated PKA phosphorylation sites (HERGΔPKA) suggested that the mechanisms of augmentation of HERG by the two kinases were partially overlapping. The PKC-dependent effect was largely due to enhanced synthetic rates. Stimulation of α1-AR in cultured rat neonatal cardiac myocytes also enhanced the abundance of ERG channels. These findings show that α1A-AR stimulation is capable of influencing the balance of HERG channel synthesis and degradation via multiple signaling pathways, a process that may have relevance in cardiac diseases and treatment.
The human ether-a-go-go-related gene (HERG) encodes the pore-forming subunit of the channel responsible for the rapidly activating delayed rectifier K+ current, Ikr (Sanguinetti et al., 1995). HERG is the gene subject to mutations in the hereditary long QT syndrome, locus LQT2 (Curran et al., 1995). Acquired long QT physiology has also been associated with HERG, particularly in cases of inadvertent proarrhythmic drug effects (Kamiya et al., 2006). In both hereditary and acquired long QT syndrome involving HERG, the ultimate result is due to a reduction in IKr current density, or “repolarization reserve” (Thomas et al., 2003a; Roden, 2004). Dynamic regulation of the HERG channel in common acquired cardiovascular conditions is not well understood. Furthermore, even less is certain about how HERG participates in sudden arrhythmic death and cardiac electrical remodeling in chronic heart disease.
Tachyarrhythmia leading to sudden cardiac death complicates a variety of common chronic heart diseases. Hyperadrenergic tone with elevated circulating catecholamine hormones is a hallmark of a wide variety of cardiovascular conditions. Among the physiological or disease states characterized by chronically elevated circulating catecholamines are myocardial ischemia (Singh, 2002) and chronic heart failure (Eisenhofer et al., 1996). Initially, the adrenergic stimulation is compensatory for disturbed supply-demand issues in cardiac output; however, as the disease progresses, it may become maladaptive. In the case of α-adrenergic receptors, continuously altered signaling through protein kinase C (PKC) may contribute to the pathogenesis of acquired heart disease and may play a role in electrical remodeling (Tomaselli and Marbán, 1999; Nerbonne and Guo, 2002; Murphy and Frishman, 2005). Electrical remodeling is the process by which specific protein expression profiles that occur under a variety of environmental stimuli (hemodynamic demands) lead to altered ion channel abundance, spatial distribution, and regulation. These changes may affect action potential morphology, synchrony of excitation, and propagation. Any electrophysiological changes such as these may lead to susceptibility to cardiac rhythm disturbances. Most studies to date have focused on altered ion channel expression (primarily at the RNA level). Alterations in channel protein translation, assembly, processing, and degradation as mechanisms of electrical remodeling have been less studied.
The present study was designed to investigate the effects of prolonged α-adrenergic receptor stimulation upon HERG channels. Sustained α1A-adrenergic stimulation caused a profound augmentation of HERG channel protein that was largely PKC-dependent, involved direct phosphorylation of the channel, and was achieved by enhanced synthesis or translation rates. These findings newly link a signaling pathway that may play an important role in the maintenance of cardiac electrical stability.
Materials and Methods
Cell Culture and Transfection.
HEK293 cell lines were cultured in RPMI 1640 medium and supplemented with l-glutamine, 10% fetal calf serum (HyClone, Waltham, MA), and penicillin-streptomycin (Gibco, Carlsbad, CA). Cultured cells were maintained in 5% CO2/95% humidified air at 37°C. For initial studies using transient expression, 4 μg of C-terminal myc-tagged HERG plasmid (in pCI-NEO) (McDonald et al., 1997) plus 4 μg of α1A-adrenergic receptor (in pCDNA3) were transiently cotransfected into HEK293 cells together with 2 μg of green fluorescent protein cDNA by electroporation. Cells were washed and resuspended in a buffer mimicking cytoplasm (120 mM KCl, 0.15 mM CaCl2, 10 mM K2HPO4/KH2PO4, 25 mM HEPES, 2 mM EGTA, 5 mM MgCl2, 2 mM ATP, and 5 mM glutathione, pH 7.6) and were electroporated in a 2-mm gap cuvette using a Gene Pulser Xcell (Bio-Rad, Hercules, CA) with the following settings: voltage, 110 V; and pulse length, 15 ms. After electroporation, the cells were plated sparsely and grown on sterile glass coverslips within 100-mm tissue culture dishes. Cells were used for electrophysiological studies 24 to 72 h after electroporation.
In subsequent experiments, stably transfected cells were generated. HERG (wild type, PKC mutant-HERG, or PKA mutant-HERG) plasmid DNA was linearized and transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, cells were sparsely replated and grown under the selective pressure of 500 μg/ml G418 added to the culture media. Ten to 14 days later, G418-resistant colonies appeared and were isolated, suspended by trypsinization, and subjected to serial dilution into 96-well dishes to obtain clonal cell lines. G418-resistant clonal lines were confirmed by immunoblot and patch-clamp analyses.
The α1-AR cDNA in the pEF6/V5-His vector (Invitrogen) was used to stably express the receptor in the HEK-HERG cells. After transfection, as above, stable clones were selected by blasticidin (5 μg/ml) containing medium and detected by Western blot analysis using anti-V5-horseradish peroxidase antibody (Invitrogen) to confirm the α1AR-V5-His stable expression (HEK-HERG/α1AR cell).
Isolation of Rat Neonatal Cardiac Myocytes.
Rat neonatal cardiac myocytes were prepared by following the protocol of Sadoshima's group (Sadoshima et al., 1992) with minor modifications. In brief, after washing extracted neonatal rat hearts with ice-cold PBS, the ventricles were separated and minced. The tissue was then digested six times for 15 min each with 0.12% collagenase type II (Worthington Biochemicals, Freehold, NJ) in PBS at 37°C; the enzyme was then neutralized with 15% horse serum, and the cells were collected by centrifugation. The resulting pellet was resuspended in buffer (in grams per liter: 6.80 NaCl, 4.80 HEPES, 0.14 NaH2PO4, 0.60 glucose, 0.40 KCl, and 0.205 MgSO4 · 7H20), layered over a 45%/64% Percoll step gradient, and centrifuged at 3000 rpm for 30 min using Sorvall TJ-25 centrifuge (Sorvall, Newton, CT). The myocytes were collected from the gradient interface, washed three times with ADS (116 mM NaCl, 5.4 mM KCl, 5 mM glucose, 10 mM NaH2PO4, 0.4 mM MgSO4, and 20 mM HEPES, pH 7.3), and plated in media [per liter of Dulbecco's modified Eagle's medium/F-12: 0.33 g of sodium pyruvate, 0.72 g of glucose, 17.6 g of ascorbic acid, 15 mM HEPES, pH 7.6, 2 g of bovine serum albumin fraction V, 4 mg of transferrin, 0.4 μmol selenite, fatty acid supplement (Sigma-Aldrich, St. Louis, MO), and 2.44g NaHCO3] supplemented with 5% horse serum and 100 μM BrdU. After 24 h, the cells received fresh serum/BrdU-free media.
Western Blot.
HEK293 or rat neonatal cardiac myocytes were prepared for immunoblot analyses by washing adherent cells with ice-cold PBS and then adding ice-cold lysis buffer (150 mM NaCl, 25 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% Nonidet P-40, 0.4% deoxycholic acid, and EDTA-free protease inhibitor cocktail tablets; Roche Diagnostics, Indianapolis, IN). Lysates were then cleared by centrifugation at 16,000g for 5 min at 4°C. The cleared supernatants were assayed for total protein content (Bio-Rad Protein Assay), and equal amounts (50–100 μg) of cell lysate protein were subjected to SDS-PAGE analysis. Protein samples were combined with 4× SDS-PAGE sample buffer [4% (w/v) SDS, 40% glycerol, 20% (v/v) β-mercaptoethanol, 0.004% (w/v) bromphenol blue, and 125 mM Tris buffer, pH 6.8] incubated for 5 min at room temperature, separated on a 7.5% SDS-PAGE, and electrophoretically transferred onto 0.2 μm nitrocellulose membrane (Bio-Rad Laboratories). Membranes were blocked in 10% nonfat dry milk and 0.05% Tween 20 in TBS for 1 h at room temperature and incubated with appropriate primary antibodies at 1:250 to 1:1000 dilution in 5% dry milk and 0.05% Tween 20 in TBS for 1 h at room temperature. Secondary antibodies conjugated to either horseradish peroxidase or infrared-fluorescence IRDye (Rockland Immunochemicals, Gilbertsville, PA) were incubated with the blots at a concentration of 1:10,000 to 1:50,000 in 0.05% Tween 20/TBS at room temperature for 1 h and then washed. Antibody detection was performed by either chemiluminescence (Super-Signal West Pico Chemiluminescent Substrate; Pierce, Rockford, IL) with multiple exposures to ensure the linearity of signal intensity or with the Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE). Identical results were obtained with either system. All gels in figures are representative of three to six independent experiments.
Antibodies.
Anti-Kv11.1 (ERG) extracellular was from Alomone Labs (Jerusalem, Israel) or Santa Cruz Biotechnology (Santa Cruz, CA). Anti-myc mouse monoclonal 9E10, anti-myc rabbit polyclonal A-14, anti-Hsc70 goat polyclonal, anti-Hsp70 mouse monoclonal, and anti-Hsp90 mouse monoclonal antibodies were from Santa Cruz Biotechnology. Anti-tubulin mouse monoclonal antibody was from Sigma-Aldrich. Anti-HERG antibodies were either from rabbit serum immunized with purified HERG N terminus as reported previously (Kagan et al., 2002) or from mouse immunized with a mix of purified recombinant HERG fragments from the C terminus (each fragment was 100 amino acids in length).
Immunofluorescent Microscopy Analysis.
Immunofluorescence staining of HERG, calnexin, and GM130 was performed after fixation of cells in 4% paraformaldehyde and permeabilization with 0.3% Triton X-100. Images were acquired using an Olympus IX70 microscope with an X60 PlanApo objective (Olympus, Tokyo, Japan) and a Photometrics Censys cooled charge-coupled device camera (Photometrics, Tucson, AZ). Images were deconvoluted to reduce fluorescence interference from beyond the focal plane with Powerhazebuster (Vaytek, Fairfield, IA), and all images were displayed with Adobe Photoshop (Adobe Systems, Mountain View, CA). HERG colocalization with subcellular markers and HERG intensity levels were measured using the Image Correlation Analysis plug-in for ImageJ software (http://rsbweb.nih.gov/ij/). Pearson's correlation quotient (ranging from 0 to 1) and intensity correlation quotient (ranging from −0.5 to 0.5) were obtained as a quantification of HERG colocalization.
Patch-Clamp Recording.
Cells on coverslips were taken directly from the cell culture incubator and placed in an acrylic/polystyrene perfusion chamber (Warner Instruments, Hamden, CT) for electrophysiological measurements. Patch pipettes were pulled and polished to obtain a tip resistance of 2–3 MΩ in the patch-clamp solutions. All experiments were carried out at room temperature (20–22°C). Cells were studied on an inverted microscope equipped with electronic patch-pipette micromanipulators and epifluorescence optics for green fluorescent protein (transfected cells). Axopatch 200B patch-clamp amplifiers (Molecular Devices, Sunnyvale, CA) were used for voltage-clamp measurements. The series resistance was approximately 9 to 10 MΩ. Voltage-clamp protocols were controlled via personal computer using pClamp8 acquisition and analysis software. To elicit HERG K+ currents, depolarizing voltage pulses were applied to various levels from a holding potential of −70 mV for 4.5 s followed by stepwise repolarization to −40 mV and then to −120 mV to measure outward tail currents. Signals were analog-filtered at 2000 Hz and sampled at 5 to 10,000 Hz. Voltage-dependent activation data were fitted to Boltzmann equation I = 1/(1 + exp[(Vh − V)/k]), where I is the relative tail current amplitude, V is the applied membrane voltage, Vh is the voltage at half-maximal activation, and k is the slope factor. To compare the effects before and after the administration of reagents, current amplitude was normalized to the control group before the application of drugs.
For whole-cell voltage-clamp, the pipette solution consisted of 126 mM KCl, 2 mM MgSO4, 0.5 mM CaCl2, 5 mM EGTA, 4 mM Mg-ATP, and 25 mM HEPES, pH 7.2 (osmolality, 280 ± 10 mOsmol/kg). External bath solution consisted of 150 mM NaCl, 1.8 mM CaCl2, 4 mM KCl, 1 mM MgCl2, 5 mM glucose, and 10 mM HEPES, pH 7.4 (osmolality, 320 ± 10 mOsmol/kg).
Analysis of mRNA.
Total RNA from HEK-HERG cells and rat neonatal cardiac myocytes was extracted by TRIzol reagent (Invitrogen) and digested with DNAase. cDNA synthesis was performed with Superscript (Invitrogen). We then used the Mx3000P Real-Time PCR System with the Brilliant SYBR Green qPCR kit (Stratagene, La Jolla, CA) to quantify HERG mRNA (glyceraldehyde-3-phosphate dehydrogenase was used for normalization). Negative controls consisted of TRIzol- and DNase I-digested cell samples omitting the reverse transcriptase (HERG forward primer, 5′-TCA ACC TGC GAG ATA CCA ACA TG-3′; and HERG reverse primer, 5′-CTG GCT GCT CCG TGT CCT T-3′. The PCR program is 95°C for 10 min, then 95°C for 30 s, 60°C for 30 s, and 72°C for 60 s, for 50 cycles.
Pulse-Chase and Metabolic Labeling.
Cell proteins were metabolically labeled with [35S]cysteine/methionine (Trans-label; MP Biomedials, Solon, OH) for pulse-chase studies as described previously (Chen et al., 2009). To estimate the rate of HERG synthesis, cells were incubated in cysteine/methionine-free media for 1 h followed by the fresh RPMI 1640 medium containing 1600 μCi/ml [35S]cysteine/methionine for various intervals. Labeling was stopped by the addition of ice-cold detergent lysis buffer. Cell lysates were precleared with Ultra-Link Protein G-agarose (Pierce) for 30 min at 4°C. Supernatant was collected by centrifugation (8000 rpm for 1 min) and incubated with 8 μg of anti-myc (A14G; Santa Cruz Biotechnology) for 1 h. As a control, nonspecific rabbit IgG was used. Antibody-antigen complexes were precipitated with 15 μl of protein G-agarose for 3 h at 4°C. After thorough washing (three times) with PBS, proteins were eluted from the resin with 4× Laemmli sample buffer and subjected to SDS-PAGE. Specific labeling of HERG was measured by the detection of 35S signal from immunoprecipitated channels and compared with both total cellular protein uptake of 35S and labeling of the housekeeping protein tubulin. For total protein 35S uptake, samples taken from cell lysates after HERG precipitation were run on SDS-PAGE dissolved in scintillation fluid for β-emission counting. For tubulin normalization, anti-tubulin antibodies were used in the immunoprecipitation, and densitometry of autoradiography gels was measured.
Reagents.
Phenylephrine and CPT-cAMP were purchased from Sigma-Aldrich. Phorbol 12-myristate 13-acetate (PMA), 5-methylurapidil (5-MU), chelerythrine, bisindolylmaleimide I, 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (Gö6976), and 3-[(8S)-8-[(dimethylamino)methyl]-6,7,8, 9-tetrahydropyrido[1,2-a]indol-10-yl]-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione hydrochloride (Ro 32-0432) were purchased from Calbiochem (San Diego, CA). Collagenase type II was from Worthington, and Protease inhibitor cocktail was from Roche Diagnostics. PMA, CPT-cAMP, chelerythrine, bisindolylmaleimide I, Gö6976, and Ro 32-0432 were dissolved in DMSO as stock solutions and used at the desired final concentrations such that the final DMSO concentration was <0.5%.
Statistics.
Values presented are means ± S.E. Analysis of variance was used for statistical analysis of the data, and p values of <0.05 were considered to be significant.
Results
Sustained α1-Adrenergic Stimulation Increases HERG Protein Abundance in HEK293 Cells.
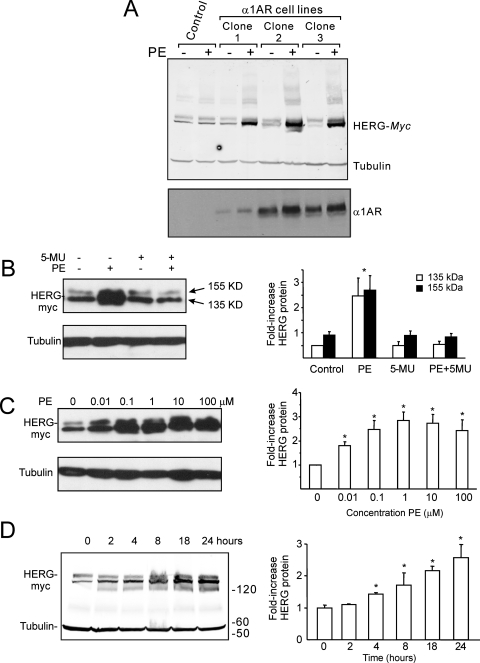
To examine the effect of α1A-adrenergic stimulation on HERG channel abundance, cDNAs of α1A-adrenergic receptor and HERG channels were stably coexpressed in HEK293 cells. As shown in Fig. 1A, treatment with PE (1 μM), an α1-adrenergic receptor agonist, for 24 h markedly increased HERG protein abundance in both immature (∼130 kDa) and mature forms of HERG (∼150 kDa). These effects were abolished by 5-MU (1 μM), a selective α1A-adrenergic receptor antagonist (Fig. 1B), showing the specificity of the α1A-adrenergic receptor-mediated effect.
Fig. 1.
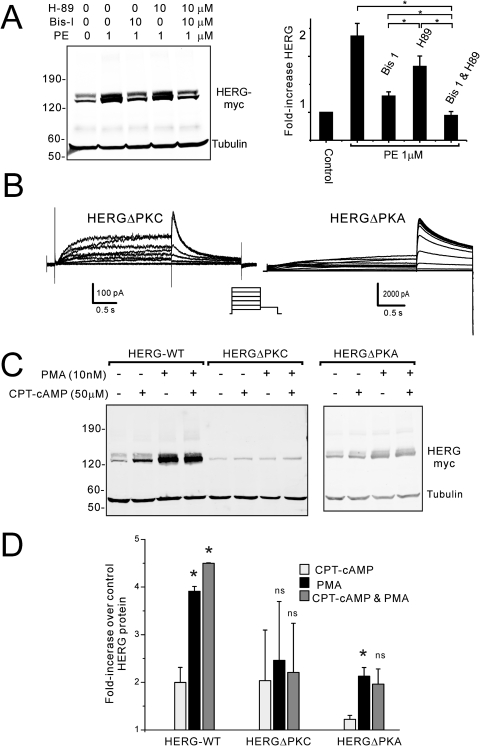
α-1A-Adrenergic augmentation of HERG protein. A, immunoblots from clonal HEK cell lines stably expressing both HERG and the α-1A-adrenergic receptor with and without 24-h phenylephrine treatment with tubulin loading control and immunoblots for the α-1A-adrenergic receptor. B, the specific α-1A-adrenergic receptor antagonist 5-MU blocked the phenylephrine-dependent augmentation of HERG protein (histogram provides summary data for different treatment groups with separate bars for the immature 135- and mature 155-kDa forms). C, clone 1 from A treated with differing concentrations of phenylephrine applied for 24 h. Bottom, tubulin loading control. Histogram shows the average increase in HERG. D, clone 1 from A treated with 1 μM PE for varying durations. Summary data are from four to five separate experiments; ∗, significantly different from control values with p < 0.05.
The α1A-adrenergic effect on HERG protein was concentration-dependent (Fig. 1C) with a saturation greater than 1 μM. The time course of the effect of PE was shown in Fig. 1D. PE at 1 μM increased HERG protein abundance in a time-dependent manner with detectable effect observed within 4 h. The absolute degree of HERG protein induction by α1A-adrenergic was somewhat variable over periods of months but always showed a minimum of 2-fold increase after 24 h of treatment. Accordingly, subsequent results show only experiments performed on cells under identical conditions and within a short time frame (days to weeks).
K+ Current Density through HERG Channels Is Enhanced by Prolonged α1-Adrenergic Stimulation in HEK293 Cells.
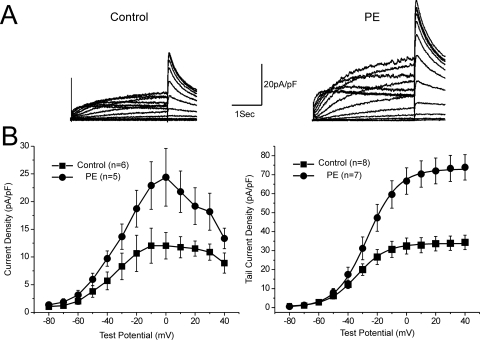
To investigate whether α1-adrenergic activation produced a concomitant increase in current through the HERG channels we subjected cells to voltage-clamp analysis via whole-cell patch-clamp technique. After 24 h of treatment with 1 μM PE, the HERG current density increased significantly (Fig. 2). The maximal current density as measured in I-V relationship increased from 13.4 ± 3.5 to 27.5 ± 5.4 pA/pF (p < 0.05, n = 6). PE also increased the density of the tail currents (control, 34.4 ± 3.8 pA/pF at +40 mV, n = 8; PE, 73.9 ± 6.7 pA/pF, n = 7, p < 0.001). There was a trend toward a depolarizing shift in the voltage-dependence activation with sustained α1A-AR stimulation that did not reach significance (Control Vh, −33.6 ± 2.6 mV; PE Vh, −26.9 ± 2.6 mV).
Fig. 2.
α-Adrenergic augmentation of HERG potassium current density. A, traces show representative current traces from HEK cells stably expressing HERG in response to a series of depolarizing voltage steps. Left, traces from untreated cells; right, traces from a cell expressing the α-adrenergic receptor and treated with phenylephrine for 24 h. B, bottom left, graph showing the isochronal current-voltage relationship during the depolarizing steps (with current normalized to cell capacitance). Bottom right, graph showing the voltage-dependent activation curves as measured at the maximal tail current (normalized to cell capacitance) plotted against the preceding depolarizing step voltage.
α1-Adrenergic Receptor-Dependent Augmentation of HERG Protein Involves Activation of PKC.
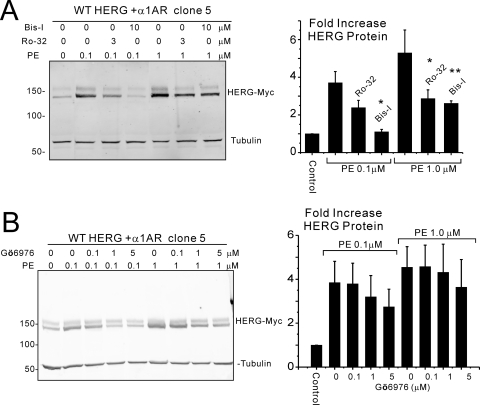
Activation of α1A-adrenergic receptor is classically coupled to Gαq/Gα11 and, as such, activates phospholipase C, Ca2+, and PKC signaling pathways. To determine whether α1A-AR-dependent augmentation of HERG channel protein is mediated by PKC, bisindolylmaleimide I (Bis-I) and Ro 32-0432, pharmacological inhibitors of PKC, were used. Twenty-four-hour treatment of HEK cells stably expressing HERG and α1A-AR with 0.1 μM PE increased HERG protein abundance, and the effect was attenuated by Ro 32-0432 at 3 μM and abolished by 10 μM Bis-I when the inhibitors were added 1 h before treatment with PE and kept in the media (Fig. 3A). When the concentration of PE was increased to 1 μM (a saturating concentration, in terms of HERG augmentation, Fig. 1C), both inhibitors were only partially capable of preventing the increase in HERG protein. A third PKC inhibitor, Gö6976, yielded similar results, suggesting that PKC was not the sole pathway for α1A-AR-dependent effects on HERG but that additional signaling may be involved (Fig. 3B).
Fig. 3.
PKC involvement in α1A-adrenergic augmentation of HERG channel protein. A, immunoblots from a clonal cell line stably expressing HERG and α1A-AR. PE treatment for 24 h increases HERG abundance, an effect that is reduced by Ro 32-0432 (Ro-32) and abolished by Bis-I when PE concentration is 0.1 μM. When the PE concentration is increased to 1 μM, both PKC inhibitors only partially prevent the augmentation of HERG (right histogram shows densitometry results; ∗, p < 0.02; ∗∗, p < 0.002, n = 3.). B, similar conditions as in A showing inhibition of the receptor-mediated augmentation of HERG by another PKC inhibitor, Gö6976. A similar pattern of incomplete inhibition is seen when receptors are maximally stimulated.
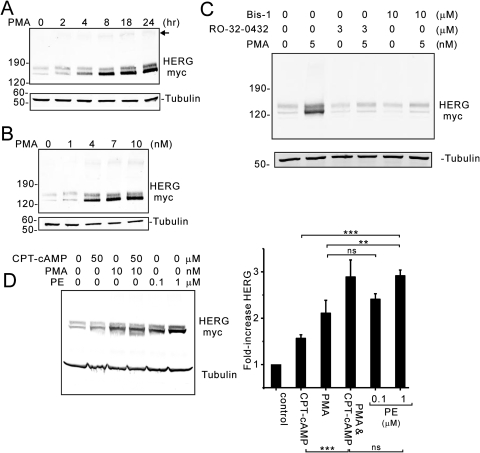
To directly examine PKC-dependent effects on HERG channel abundance, we treated HEK cells stably expressing HERG with the phorbol ester PMA (Fig. 4). Similar to the results with α1A-AR stimulation, prolonged treatment with PMA increased HERG protein abundance in a time- and dose-dependent fashion (Fig. 4, A and B). PMA-dependent increases in HERG were detected as early as 2 to 4 h (when 10 nM was used) and with concentrations between 1 and 10 nM at the 24-h point. Both Ro 32-0432 and Bis-I were capable of completely preventing PMA-dependent effects on HERG (Fig. 4C). These results further support the interpretation that α1A-AR stimulation is, in part, mediated via PKC stimulation, although additional signaling mechanisms may also contribute.
Fig. 4.
Direct PKC activation enhances HERG protein abundance. Immunoblot analysis of HEK cells stably expressing HERG in response to PMA treatment. A, shows changes in HERG with increasing duration of treatment with PMA at 10 nM. B, shows changes in HERG with increasing concentration of PMA treatment for 24 h. C, two PKC inhibitors, bisindolylmaleimide I and Ro 32-0432, block the PMA-dependent increase in HERG protein. D, a comparison of α1A-AR receptor stimulation (PE) is made with direct PKC activation (PMA) (n = 3; ∗, p < 0.05; ∗∗, p < 0.025; ∗∗∗, p < 0.01).
To further explore possible signaling mechanisms for α1A-AR-mediated regulation of HERG protein, we compared the degree of channel augmentation in cells treated with either PMA or PE (Fig. 4D). We consistently observed that α1A-AR stimulation produced a greater augmentation of channel abundance than did phorbol ester. This result further suggested that α1A-AR signaling enhanced HERG channel abundance by additional pathways from PKC. Because α1A-AR couples to Gαq, which then activates phospholipase C, additional signaling changes occur: elevation of cytoplasmic Ca2+, and transient consumption of PIP2. These two processes did not seem to be involved in the receptor-mediated effect on HERG channel protein abundance because preincubation of cells with either the Ca2+ chelator BAPTA-acetoxymethyl ester or phosphatidylinositol 3-kinase inhibitors [100 nM wortmannin or 10 μM 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride (LY294002)] that would prevent the repletion of PIP2 failed to inhibit the α1A-AR effect (data not shown).
PKC- and PKA-Mediated Phosphorylation of the HERG Channel Is Required for α1A-AR-Mediated Increase in HERG Protein Abundance.
In view of previous work on the short-term regulation of HERG gating by α1A-AR that suggested cross-signaling between the receptor and PKC plus PKA (Kiehn et al., 1998), we sought to investigate a possible role for PKA in the present findings. When cells stably expressing HERG and α1A-AR were treated with a high concentration of PE (1 μM), the effect on HERG protein was partially blocked by Bis-I and, to a lesser extent, by the PKA inhibitor N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline (H89). The combination of Bis-I plus H89, however, completely inhibited α1A-AR stimulation-dependent augmentation of HERG protein (Fig. 5A).
Fig. 5.
Phosphorylation of HERG channels by PKC and PKA affect protein abundance. A, inhibition of PKC (with Bis-I) reduces the α1A-AR-mediated increase in HERG more than inhibition of PKA (with H-89), and inhibition of both kinases completely prevents the effect; ∗, p < 0.05, n = 5. B, representative current traces from HEK cells expressing either HERGΔPKC (left) or HERGΔPKA (right). C, immunoblot of HERG, HERGΔPKC, and HERGΔPKA before and after treatment of cells with either PMA or CPT-cAMP. D, summary densitometry data from HERG immunoblot analysis shown in C (∗, significance compared with CPT-cAMP-treated cells, p < 0.05, n = 2). The effects of PKA and PKC stimulation on HERG abundance are partially cumulative. Direct phosphorylation of the channel protein largely accounts for the kinase effects on HERG.
To distinguish between kinase-mediated regulation of channel abundance by direct phosphorylation and phosphorylation of an intermediate regulator, we used an HERG mutant in which 17 of the 18 PROSITE-predicted PKC acceptor serines/threonines were changed to alanine (HERGΔPKC) (Fig. 5B) (Thomas et al., 2003b). HEK cells stably expressing HERGΔPKC were subjected to PMA treatment for 24 h, and the increase in channel protein abundance was greatly decreased compared with wild-type HERG (Fig. 5, C and D). We have shown previously a PKA-dependent augmentation of HERG abundance with enhanced protein synthesis as the mechanism (Chen et al., 2009). Two of the predicted PKC sites (Ser890 and Thr895) are also known PKA phosphorylation sites on HERG (Cui et al., 2000). To investigate whether the effects of PKC were identical with or overlapped with those of PKA-mediated increase in HERG channels, we used a combination of pharmacological and mutation analyses. When cells stably expressing wild-type HERG were treated with the membrane-permeable cAMP analog CPT-cAMP, the augmentation was not as great as that with PMA treatment. Moreover, when both reagents were used in combination, the effect was partially additive. The HERGΔPKC mutant was augmented by CPT-cAMP, but the additivity with PMA was no longer seen. A mutant HERG in which the four acceptor serines/threonines were changed to alanine (HERGΔPKA) (Cui et al., 2000) showed an elimination of CPT-cAMP-mediated increase and a reduced but present enhancement with PMA. Again, combination of PMA and CPT-cAMP failed to show additivity in augmenting the HERGΔPKA mutant.
One caveat for the HERGΔPKC results is that the baseline expression levels are relatively low. This mutant harbors many changes that may have unexpected effects in channel protein folding, assembly, and trafficking—any of which can alter its abundance. Accordingly, we are cautious in relying completely on this mutant for our interpretations. Nevertheless, the combined data suggest that there is partial overlap in phosphorylation sites within HERG responsible for PKC- and PKA-mediated enhancement in channel protein. There is, however, a clear quantitative and qualitative difference between the PKC- and PKA-mediated effects. Moreover, enhancement of HERG protein by α1A-AR stimulation seems to involve cross-talk of PKC and PKA pathways. There remains a diminished but present enhancement of HERGΔPKC with PMA that suggests indirect effects of the kinase on channel, additional unrecognized PKC sites, or that the 18th PROSITE-predicted PKC site (Thr74) may play a role in the effect. The T74A mutation was not used because of the fact that previous studies showed that this abolished all channel activity (Thomas et al., 2003b).
Mechanism of α1A-AR and PKC Augmentation of HERG Channel Protein.
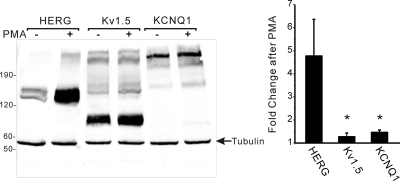
There are several potential ways that receptor stimulation and PKC could enhance the abundance of HERG protein: altered transcription, accelerated translation, or reduced degradation. To assess altered transcription, we isolated RNA from HEK cells stably expressing HERG and α1A-AR under control conditions or after treatment with 1 μM PE or 10 nM PMA for 24 h. Real-time PCR analysis showed that there were no significant increases in HERG mRNA with either stimulus (PMA-treated cells expressed 60% as much HERG mRNA compared with controls, and PE-treated cells expressed 109% compared with controls, n = 4, not significant). Because the HERG cDNA is under the control of the CMV promoter, we investigated whether there were comparable changes in two other cardiac tetrameric voltage-gated K+ channels—KCNQ1 and Kv1.5—whose cDNAs were under the control of the same promoter, epitope-tagged with myc, and were stably transfected into HEK cells. When these cells were treated with 10 nM PMA for 24 h, they failed to show a comparable increase in channel abundance, as did HERG-expressing cells (Fig. 6). Thus, the regulation of transcription did not seem to be the mechanism responsible for increased HERG. Moreover, the effect of PKC on channel abundance was specific for HERG compared with KCNQ1 and Kv1.5.
Fig. 6.
PKC-mediated effects on cardiac K+ channels Kv1.5 and KCNQ1. Anti-myc immunoblot analysis of HEK cells stably expressing myc-epitope-tagged HERG, Kv1.5, or KCNQ1 after treatment with control vehicle or PMA 10 nM for 24 h. Histogram shows the relative PMA-dependent change in channel protein abundance (n = 2, ∗, p < 0.05).
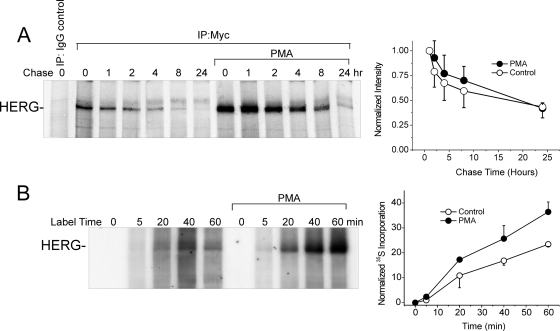
To address regulation of protein synthesis or degradation we performed [35S]cysteine/methionine-labeling. To measure HERG degradation, we determined the stability or half-life using metabolic labeling and pulse-chase experiments followed by autoradiography of immunoprecipitated 35S-labeled HERG protein. Cells stably expressing HERG were pretreated with PMA (10 nM) for 1 h before pulse chase. The half-life of HERG in these experiments was 17 to 18 h, values similar to those published in other cultured cell types (McDonald et al., 1997; Ficker et al., 2003, Chen et al., 2009). PMA treatment did not change the half-life despite an apparent initial stabilization in PMA-treated cells in the first 12 h (Fig. 7A).
Fig. 7.
PKC effects on synthesis and stability of HERG protein. A, pulse chase of HERG labeled with [35S]cysteine/methionine with and without 10 nM PMA treatment. Graph to the right shows a time-dependent decrease in 35S-HERG normalized to the initial incorporated amount at the beginning of the chase period (n = 4). B, early incorporation of [35S]cysteine/methionine in HERG shown in the first 60 min of labeling with and without PMA treatment. Right, graph represents the densitometry data normalized to total cellular 35S incorporation showing time-dependent new synthesis of HERG (n = 2).
To examine the rate of channel synthesis, we measured [35S]cysteine/methionine incorporation into HERG (relative to that of total cellular proteins and tubulin, whose abundance was not altered by PMA). To normalize HERG synthesis values, the densitometry of the autoradiography of HERG was divided by the rate of total cellular protein and specific tubulin 35S incorporation. Both methods resulted in comparable rates. The rate of 35S incorporation into HERG over a period of 60 min was nearly doubled in cells that had been pretreated with 10 nM PMA (Fig. 7B). Thus, the PKC-dependent augmentation of HERG protein abundance is primarily due to an accelerated synthesis or translation of new channels, although an enhanced stability of channels in the first few hours after they are formed may contribute.
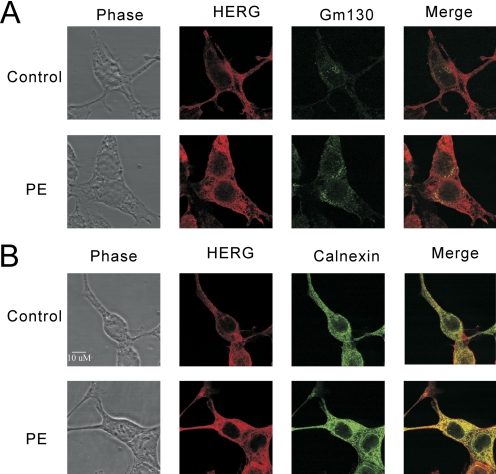
We further examined whether PKC activation had any effect on HERG channel distribution within the cell by performing immunofluorescence confocal microscopy with double labeling of subcellular compartments. Antibody against GM130 was used to define the Golgi, and antibody against calnexin was used for endoplasmic reticulum (ER). PMA treatment clearly enhanced the HERG signal (31–40%, n = 4) but had no detectable effect on calnexin or GM130 (Fig. 8, A and B). When merging of the HERG and subcellular marker signals was done, it was seen that the enhanced HERG signal was present in ER, Golgi, and periphery without obvious preferential accumulation in any one compartment. The degree of colocalization of HERG with calnexin and GM130 was reflected by the general increase in total HERG signal (49% for calnexin, 30% for GM130, as measured by the intensity correlation quotient).
Fig. 8.
Subcellular localization of HERG after 24 h of PKC activation. Confocal immunofluorescence assays with double stating for HERG-myc (red channel) and either the Golgi marker GM130 (A) or the ER marker calnexin (B) (green channel). PMA treatment of 10 nM for 24 h results in globally increased HERG signal in both compartments and on the surface.
Hsp90 and Hsp70 interaction with newly forming HERG channels has been established as a requirement for proper synthesis and trafficking (Ficker et al., 2003). These chaperone interactions have been shown to play an important role in both maturation of normal channels and degradation of misfolded mutant HERG. We sought to determine whether PKC-mediated augmentation of HERG abundance was associated with changes in either of these chaperones by immunoblot analysis. When cells stably expressing HERG were subjected to treatment with increasing concentrations of PMA for 24 h, we observed no change in the abundance of Hsp70, Hsc70, or Hsp90 (data not shown). Thus, changes in chaperone protein abundance did not seem to be responsible for PKC-mediated regulation of HERG protein.
α1-AR Stimulation Effects on ERG Protein Level in Rat Neonatal Cardiac Myocytes.
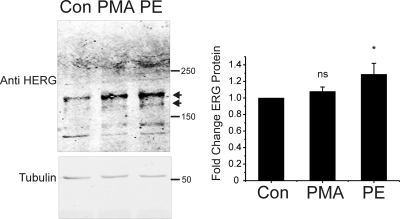
To determine whether sustained stimulation of α1-adrenergic receptor could also increase native ERG protein abundance, we used the rat neonatal cardiac myocyte culture system in which the ERG channel has been shown to be expressed (Guo et al., 2007). Cells were used shortly after isolation in culture and were treated with PMA (10 nM), PE (1 μM), or vehicle (DMSO) for 24 h. As shown in Fig. 9, treatment with PE for 24 h also measurably increased ERG protein abundance (Fig. 9) with a statistically significant average increase in ERG of 29 ± 0.12% (n = 6 when normalized to tubulin). PMA treatment resulted in an increase in ERG protein of 8 ± 0.055% that did not meet statistical significance. This result suggests that native ERG channel protein may also be up-regulated by prolonged stimulation of α1-adrenergic receptor. Because the native ERG gene is under its own promoter rather than the CMV used in heterologous systems, we quantified the amount of ERG mRNA from myocytes under control conditions and 24-h treatment with PMA or PE by real-time PCR. Compared with control, PMA-treated cells expressed 33 ± 30% as much ERG Message (n = 4, not significantly different), and PE-treated cells expressed 82 ± 79% as much (n = 4, not statistically different). These measurements indicate that enhanced transcription was not the mechanism for increased ERG protein in PE-treated cells. On the contrary, it seems that there is a trend toward reduced transcription of ERG, which, taken with the protein abundance, further highlights the enhanced translation of channel protein.
Fig. 9.
α1-AR stimulation enhances ERG channel abundance in rat neonatal cardiac myocytes. Left, anti-HERG immunoblots from isolated rat neonatal cardiac myocytes under control conditions (CON) and after 24 h of treatment with either 10 nM PMA (PMA) or 1 μM PE (PE). ERG channel bands are indicated by arrows. Bottom gel shows tubulin immunoblot from the same gel used to normalize for loading variances. Right, summary data for six experiments with ERG normalized to tubulin densitometry (∗, p < 0.05, n = 6).
Discussion
The results we report here describe the effects of sustained stimulation of α1A-adrenoceptors on the HERG channel protein. With prolonged receptor stimulation in a heterologous expression system the HERG channel protein abundance was markedly increased over 24 h. The increase in protein was largely due to PKC activation and phosphorylation of the channel protein, but at the highest stimulation of α1A-AR, additional signaling pathways contributed, including PKA activation. The mechanism(s) of α1A-AR/PKC-mediated augmentation of channel protein involves post-transcriptional acceleration of channel synthesis or translation. Enhanced mRNA transcription or stability did not seem to be involved in the effect, and the augmentation of channel abundance was specific to HERG compared with two other cardiac K+ channels.
Regulation of ion channels by means of G protein-coupled receptors and downstream kinases is well established. Most studies of receptor- and kinase-mediated regulation have focused on short-term effects on channel gating. Acute PKA-mediated regulation of HERG channel behavior has been shown to involve both direct and indirect signaling (Kiehn et al., 1998; Thomas et al., 1999; Cui et al., 2000, 2001; Kagan et al., 2002). The situation for α1-AR and PKC regulation of HERG is less clear. Acute stimulation of α1A-adrenergic receptors has been shown to decrease HERG channel activity via stimulation of PKC (Thomas et al., 2003b) and consumption of PIP2 (Bian et al., 2004). Several studies showed that activation of PKC by phorbol esters leads to the suppression of HERG current amplitude in heterologous expression systems (Kiehn et al., 1998; Thomas et al., 2003b). The phorbol ester-dependent suppression has been ascribed to accelerated deactivation, slowed activation, depolarizing shift in activation, and reduction in current density. It has also been reported that the phorbol ester-dependent effect was due to PKA activation (Kiehn et al., 1998) and that removal of PKC consensus phosphorylation sites from HERG failed to abolish phorbol ester-mediated regulation, suggesting involvement atypical receptor kinase cross-talk (Thomas et al., 2003b). Another report provided evidence that PKC phosphorylation of C-terminal portions of HERG were necessary for the short-term PKC regulation of the channel (Cockerill et al., 2007). The IKr regulation in cardiac myocytes however, may be more complicated. In guinea pig cardiac myocytes, PKC activation enhanced IKr (Heath and Terrar, 2000). In rabbit cardiac myocytes, α-adrenergic stimulation acutely reduced HERG/IKr primarily because of PIP2 consumption (Bian et al., 2004). All of these studies were conducted within a time frame of minutes, thus mimicking the short-term effects rather than sustained stimulation, as may occur over hours to days.
We recently reported that prolonged stimulation of PKA (hours to days) enhanced HERG channel protein abundance (Chen et al., 2009). The PKA-dependent increase in HERG showed some similarities to those described in this report in that there was an acceleration of synthesis, and the effect occurred over a similar time frame. Notable differences, however, occur with α1A-AR/PKC-dependent changes in HERG. The most notable, for instance, is that the degree of channel protein increase is greater and K+ current increased occurred earlier with α1A-AR/PKC stimulation. Mutation of all PROSITE-predicted PKC sites (with the exception of Thr74) in HERG greatly reduced the phorbol ester-induced augmentation of channel protein but did not completely abolish it, whereas mutation of the four PKA phosphorylation sites completely prevented PKA-dependent effects on the channel. There is some degree of overlap in the PKC- and PKA-dependent effects in that their ability to increase HERG was not entirely additive, and several of the predicted phosphorylation sites within the C terminus of HERG are common to the two kinases. Moreover, at high levels of α1A-AR stimulation, both kinases seemed to play a role in increasing HERG. Taken together, these results suggest that the PKA-dependent regulation of HERG abundance is rather straightforward, whereas that of α1-AR/PKC is more complex and possibly stronger.
Our results indicate that accelerated synthesis or translation of channel protein accounted for the α1A-AR/PKC effect rather than altered transcription rates. However, the exact mechanism(s) of how this signaling pathway changes HERG abundance remains an open question. Phosphorylation of the nascent channels seems to play the largest role, but additional kinase effectors may also contribute because mutation of the PKC consensus sites did not completely suppress the effect. The biosynthesis of HERG seems to be a tenuous process—a concept underscored by the fact that most LQT2 mutations result in misfolded product that never reaches to the cell surface because of either faulty production, early degradation, or mistrafficking (Ficker et al., 2000; Kagan et al., 2000; Thomas et al., 2003; Gong et al., 2005; Anderson et al., 2006). If newly forming proteins contain considerable stretches of hydrophobic segments, they may be prone to aggregation if not properly shielded by chaperones before reaching their final conformation. In this event, translation may be delayed, or the newly synthesized proteins may be processed by quality control mechanisms of the cell, leading to early degradation (Chirico et al., 1988; Meacham et al., 1999). This may occur with some nonmutant proteins under normal circumstances (Schubert et al., 2000; Qian et al., 2006). A possible interpretation of our data is that newly forming HERG protein is made more soluble or less likely to aggregate into nonproductive conformation if the cytoplasmic segments are subject to phosphorylation by either PKA or PKC. Further work is needed to precisely distinguish between this and alternative mechanisms such as the regulation of endosomal recycling (Delisle et al., 2009), lysosomal degradation, and altered chaperone interactions with HERG (despite stable amounts of heat shock proteins) (Ficker et al., 2003).
Activation of α1A-receptor may lead to the activation of various PKC isoforms in cardiac myocytes (Pucéat et al., 1994). The PKC-involved isoforms in protein degradation include PKCδ, PKCε, and PKCζ. Future studies will be necessary to determine which isoform(s) of PKC are involved in the effect of sustained stimulation of α1A-adrenergic receptor on HERG protein abundance. Although signaling through α1-AR is generally ascribed to Gαq/Gα11 and PKC, earlier studies have shown that cAMP generation and stimulation of PKA may also occur to varying degrees in several systems by either direct or indirect pathways (Cotecchia et al., 1990). This seems to be the case in our experiments. Whether signaling cross-talk of α-1AR and cAMP/PKA occurs in cardiac tissue in a similar manner is yet to be determined.
Heterologous expression systems (such as HEK293 cells) provide a malleable approach, enabling precise manipulations to determine molecular mechanisms of protein interactions through biochemistry and electrophysiology. It may not, however, accurately reflect the milieu of the same proteins in vivo. Therefore, we investigated the α1A-adrenergic regulation of HERG/IKr in cardiac tissue and found that sustained α1A receptor stimulation also significantly increased rat cardiomyocyte ERG channel protein expression, albeit to a more modest degree. Further investigation is merited to delineate the signaling events that mediate post-transcriptional regulation of IKr density and channel protein abundance in vivo.
Hyperadrenergic stimulation frequently accompanies cardiovascular disease states and may result in the internalization/down-regulation of β-adrenergic receptors, altered Gαs-mediated cAMP/PKA signaling, switching of β-AR-coupling to Gαi, and up-regulation of α1-AR/Gαq signaling (Ungerer et al., 1993; Rockman et al., 2002; Lohse et al., 2003; Woodcock et al., 2008). How α1-adrenergic receptors affect these situations is less characterized, but they also seem to have altered signaling during hyperstimulation in disease states with differing consequences in terms of heart function (O'Connell et al., 2006; Woodcock et al., 2008). α1-AR, β-AR, PKC, and PKA have each been implicated in the pathogenesis of cardiovascular diseases that may be complicated by ventricular arrhythmias (Lohse et al., 2003). Medical therapies are presently targeted to or are under investigation for each of these proteins. The experience with adrenergic antagonists is the most obvious example, however; there is continued research and interest in developing more specific reagents. How such therapies would specifically affect the long-term regulation of the HERG channel is important because membrane repolarization and risk for sudden arrhythmic death may be affected. Our results suggest a novel regulation of HERG/IKr potassium channels by α1A-adrenoceptor activity that may represent another potential link between stress and potentially life-threatening ventricular arrhythmias. Further investigation into molecular mechanisms of HERG channel regulation is warranted because it may significantly affect the prevention and treatment of cardiac arrhythmias.
Acknowledgments
We thank Dr. Charles Rubin for many helpful discussions and Neo Kay Li for technical assistance.
This work was supported by the National Institutes of Health Division of Blood Diseases and Resources [Grant HL077326]; the Singapore Biomedical Research Council; the University of Heidelberg (Frontiers Program); and the Adumed Foundation.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.062216.
- HERG
- human ether-a-go-go-related gene
- ERG
- ether-a-go-go
- HEK
- human embryonic kidney
- PKA
- protein kinase A
- PKC
- protein kinase C
- PMA
- phorbol 12-myristate 13-acetate
- CPT
- chlorophenylthiol
- α1-AR
- α1-adrenoreceptors
- HERGΔPKC
- human ether-a-go-go-related gene with 17 of 18 possible protein kinase C target sites mutated to alanine
- HERGΔPKA
- human ether-a-go-go-related gene with all four possible protein kinas A target sites mutated to alanine
- PE
- phenylephrine
- PBS
- phosphate-buffered saline
- PAGE
- polyacrylamide gel electrophoresis
- TBS
- Tris-buffered saline
- PCR
- polymerase chain reaction
- PIP2
- phosphatidylinositol bisphosphate
- ER
- endoplasmic reticulum
- Hsp90
- 90-kDa heat shock protein
- 5-MU
- 5-methylurapidil
- Gö6976
- 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole
- Ro 32-0432
- 3-[(8S)-8-[(dimethylamino)methyl]-6,7,8,9-tetrahydropyrido[1,2-a]indol-10-yl]-4-(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione hydrochloride
- H89
- N-[2-(4-bromocinnamylamino)ethyl]-5-isoquinoline
- LY294002
- 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
- Bis-I
- bisindolylmaleimide I
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
- Hsc70
- 70-kDa heat shock protein cognate.
References
- Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, Gong Q, Zhou Z, Ackerman MJ, January CT. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113:365–373 [DOI] [PubMed] [Google Scholar]
- Bian JS, Kagan A, McDonald TV. (2004) Molecular analysis of PIP2 regulation of HERG and IKr. Am J Physiol Heart Circ Physiol 287:H2154–H2163 [DOI] [PubMed] [Google Scholar]
- Chen J, Sroubek J, Krishnan Y, Li Y, Bian J, McDonald TV. (2009) PKA phosphorylation of HERG protein regulates the rate of channel synthesis. Am J Physiol Heart Circ Physiol 296:H1244–H1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. (1988) 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 332:805–810 [DOI] [PubMed] [Google Scholar]
- Cockerill SL, Tobin AB, Torrecilla I, Willars GB, Standen NB, Mitcheson JS. (2007) Modulation of hERG potassium currents in HEK-293 cells by protein kinase C. Evidence for direct phosphorylation of pore forming subunits. J Physiol 581:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S, Kobilka BK, Daniel KW, Nolan RD, Lapetina EY, Caron MG, Lefkowitz RJ, Regan JW. (1990) Multiple second messenger pathways of alpha-adrenergic receptor subtypes expressed in eukaryotic cells. J Biol Chem 265:63–69 [PubMed] [Google Scholar]
- Cui J, Kagan A, Qin D, Mathew J, Melman YF, McDonald TV. (2001) Analysis of the cyclic nucleotide binding domain of the HERG potassium channel and interactions with KCNE2. J Biol Chem 276:17244–17251 [DOI] [PubMed] [Google Scholar]
- Cui J, Melman Y, Palma E, Fishman GI, McDonald TV. (2000) Cyclic AMP regulates the HERG K(+) channel by dual pathways. Curr Biol 10:671–674 [DOI] [PubMed] [Google Scholar]
- Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. (1995) A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell 80:795–803 [DOI] [PubMed] [Google Scholar]
- Delisle BP, Underkofler HA, Moungey BM, Slind JK, Kilby JA, Best JM, Foell JD, Balijepalli RC, Kamp TJ, January CT. (2009) Small GTPase determinants for the Golgi processing and plasmalemmal expression of human ether-a-go-go related (hERG) K+ channels. J Biol Chem 284:2844–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. (1996) Cardiac sympathetic nerve function in congestive heart failure. Circulation 93:1667–1676 [DOI] [PubMed] [Google Scholar]
- Ficker E, Dennis AT, Obejero-Paz CA, Castaldo P, Taglialatela M, Brown AM. (2000) Retention in the endoplasmic reticulum as a mechanism of dominant-negative current suppression in human long QT syndrome. J Mol Cell Cardiol 32:2327–2337 [DOI] [PubMed] [Google Scholar]
- Ficker E, Dennis AT, Wang L, Brown AM. (2003) Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res 92:e87–100 [DOI] [PubMed] [Google Scholar]
- Gong Q, Keeney DR, Molinari M, Zhou Z. (2005) Degradation of trafficking-defective long QT syndrome type II mutant channels by the ubiquitin-proteasome pathway. J Biol Chem 280:19419–19425 [DOI] [PubMed] [Google Scholar]
- Guo J, Massaeli H, Li W, Xu J, Luo T, Shaw J, Kirshenbaum LA, Zhang S. (2007) Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J Pharmacol Exp Ther 321:911–920 [DOI] [PubMed] [Google Scholar]
- Heath BM, Terrar DA. (2000) Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol 522:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan A, Melman YF, Krumerman A, McDonald TV. (2002) 14-3-3 amplifies and prolongs adrenergic stimulation of HERG K+ channel activity. EMBO J 21:1889–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan A, Yu Z, Fishman GI, McDonald TV. (2000) The dominant negative LQT2 mutation A561V reduces wild-type HERG expression. J Biol Chem 275:11241–11248 [DOI] [PubMed] [Google Scholar]
- Kamiya K, Niwa R, Mitcheson JS, Sanguinetti MC. (2006) Molecular determinants of HERG channel block. Mol Pharmacol 69:1709–1716 [DOI] [PubMed] [Google Scholar]
- Kiehn J, Karle C, Thomas D, Yao X, Brachmann J, Kübler W. (1998) HERG potassium channel activation is shifted by phorbol esters via protein kinase A-dependent pathways. J Biol Chem 273:25285–25291 [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. (2003) What is the role of beta-adrenergic signaling in heart failure? Circ Res 93:896–906 [DOI] [PubMed] [Google Scholar]
- McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, Goldstein SA, Fishman GI. (1997) A minK-HERG complex regulates the cardiac potassium current IKr. Nature 388:289–292 [DOI] [PubMed] [Google Scholar]
- Meacham GC, Lu Z, King S, Sorscher E, Tousson A, Cyr DM. (1999) The Hdj-2/Hsc70 chaperone pair facilitates early steps in CFTR biogenesis. EMBO J 18:1492–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S, Frishman WH. (2005) Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev 13:3–12 [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Guo W. (2002) Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol 13:406–409 [DOI] [PubMed] [Google Scholar]
- O'Connell TD, Swigart PM, Rodrigo MC, Ishizaka S, Joho S, Turnbull L, Tecott LH, Baker AJ, Foster E, Grossman W, et al. (2006) Alpha1-adrenergic receptors prevent a maladaptive cardiac response to pressure overload. J Clin Invest 116:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. (1994) Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem 269:16938–16944 [PubMed] [Google Scholar]
- Qian SB, Princiotta MF, Bennink JR, Yewdell JW. (2006) Characterization of rapidly degraded polypeptides in mammalian cells reveals a novel layer of nascent protein quality control. J Biol Chem 281:392–400 [DOI] [PubMed] [Google Scholar]
- Rockman HA, Koch WJ, Lefkowitz RJ. (2002) Seven-transmembrane-spanning receptors and heart function. Nature 415:206–212 [DOI] [PubMed] [Google Scholar]
- Roden DM. (2004) Drug-induced prolongation of the QT interval. N Engl J Med 350:1013–1022 [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. (1992) Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem 267:10551–10560 [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81:299–307 [DOI] [PubMed] [Google Scholar]
- Schubert U, Antón LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. (2000) Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404:770–774 [DOI] [PubMed] [Google Scholar]
- Singh N. (2002) Diabetes, heart rate, and mortality. J Cardiovasc Pharmacol Ther 7:117–129 [DOI] [PubMed] [Google Scholar]
- Thomas D, Kiehn J, Katus HA, Karle CA. (2003a) Defective protein trafficking in hERG-associated hereditary long QT syndrome (LQT2): molecular mechanisms and restoration of intracellular protein processing. Cardiovasc Res 60:235–241 [DOI] [PubMed] [Google Scholar]
- Thomas D, Zhang W, Karle CA, Kathöfer S, Schöls W, Kübler W, Kiehn J. (1999) Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J Biol Chem 274:27457–27462 [DOI] [PubMed] [Google Scholar]
- Thomas D, Zhang W, Wu K, Wimmer AB, Gut B, Wendt-Nordahl G, Kathöfer S, Kreye VA, Katus HA, Schoels W, et al. (2003b) Regulation of HERG potassium channel activation by protein kinase C independent of direct phosphorylation of the channel protein. Cardiovasc Res 59:14–26 [DOI] [PubMed] [Google Scholar]
- Tomaselli GF, Marbán E. (1999) Electrophysiological remodeling in hypertrophy and heart failure. Cardiovasc Res 42:270–283 [DOI] [PubMed] [Google Scholar]
- Ungerer M, Böhm M, Elce JS, Erdmann E, Lohse MJ. (1993) Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87:454–463 [DOI] [PubMed] [Google Scholar]
- Woodcock EA, Du XJ, Reichelt ME, Graham RM. (2008) Cardiac alpha 1-adrenergic drive in pathological remodelling. Cardiovasc Res 77:452–462 [DOI] [PubMed] [Google Scholar]