Abstract
The MUC1 C-terminal transmembrane subunit (MUC1-C) oncoprotein is a direct activator of the canonical nuclear factor-κB (NF-κB) RelA/p65 pathway and is aberrantly expressed in human multiple myeloma cells. However, it is not known whether multiple myeloma cells are sensitive to the disruption of MUC1-C function for survival. The present studies demonstrate that peptide inhibitors of MUC1-C oligomerization block growth of human multiple myeloma cells in vitro. Inhibition of MUC1-C function also blocked the interaction between MUC1-C and NF-κB p65 and activation of the NF-κB pathway. In addition, inhibition of MUC1-C in multiple myeloma cells was associated with activation of the intrinsic apoptotic pathway and induction of late apoptosis/necrosis. Primary multiple myeloma cells, but not normal B-cells, were also sensitive to MUC1-C inhibition. Significantly, treatment of established U266 multiple myeloma xenografts growing in nude mice with a lead candidate MUC1-C inhibitor resulted in complete tumor regression and lack of recurrence. These findings indicate that multiple myeloma cells are dependent on intact MUC1-C function for constitutive activation of the canonical NF-κB pathway and for their growth and survival.
The nuclear factor-κB (NF-κB) pathway is constitutively activated at high frequency in human multiple myeloma cells by mechanisms that are largely unknown (Chauhan et al., 1996; Hideshima et al., 2001). The NF-κB proteins (RelA/p65, RelB, c-Rel, NF-κB1/p50, and NF-κB/p52) are ubiquitously expressed transcription factors that localize to the cytoplasm in complexes with members of the IκB family of inhibitor proteins (Hayden and Ghosh, 2008). In response to stimulation, the high-molecular-weight IκB kinase (IKKα, IKKβ, and IKKγ) complex phosphorylates IκB proteins and induces their ubiquitination and degradation. In turn, NF-κB is released for nuclear translocation and activation of NF-κB target genes that contribute to inflammatory responses, cellular proliferation, and survival (Karin and Lin, 2002). In the canonical NF-κB pathway, NF-κB RelA/p65-IκBα complexes shuttle between the nucleus and cytoplasm (Hayden and Ghosh, 2008). Activation of this pathway, for example in the response to tumor necrosis factor-α, induces IKKβ-mediated phosphorylation and degradation of IκBα with a shift in targeting of NF-κB p65 to the nucleus. Significantly, down-regulation of the constitutively activated canonical NF-κB pathway in multiple myeloma cells by diverse agents that block IKKβ is associated with the inhibition of growth and induction of death (Hideshima et al., 2002, 2006, 2009; Annunziata et al., 2007; Jourdan et al., 2007). Mutations in genes encoding positive and negative regulators of canonical NF-κB signaling have been identified in a subset of multiple myeloma cells (Annunziata et al., 2007). Moreover, noncanonical NF-κB signaling involving IKKα as an upstream effector of NF-κB2/p52 and RelB is activated in certain multiple myeloma cells as a consequence of mutations in genes that regulate this pathway (Annunziata et al., 2007; Keats et al., 2007). These observations have suggested that both the canonical and noncanonical NF-κB pathways may contribute to the malignant multiple myeloma phenotype (Hideshima et al., 2009). However, the findings that most multiple myeloma cells are sensitive to agents that target IKKβ have provided support for the importance of the canonical NF-κB pathway in maintaining their growth and survival.
The MUC1 oncoprotein is aberrantly expressed by most, if not all, multiple myeloma cell lines and primary patient samples (Takahashi et al., 1994; Burton et al., 1999; Treon et al., 1999; Paydas et al., 2001; Cloosen et al., 2006; Baldus et al., 2007; Kawano et al., 2008). MUC1 consists of two subunits that form a heterodimeric complex at the cell membrane (Kufe, 2009). The extracellular MUC1 N-terminal subunit is the mucin component of the heterodimer. The MUC1 C-terminal transmembrane subunit (MUC1-C) has a 58-amino acid extracellular domain that interacts with galectin-3 and functions as a cell surface receptor (Ramasamy et al., 2007; Kufe, 2009). MUC1-C also consists of a 72-amino acid cytoplasmic domain that is sufficient for inducing transformation (Huang et al., 2005). In addition to its localization at the cell membrane, MUC1-C accumulates in the cytoplasm and is targeted to the nucleus of multiple myeloma cells (Li et al., 2003). Of potential functional importance, silencing of MUC1 expression in multiple myeloma cells is associated with increased sensitivity to the induction of apoptosis (Kawano et al., 2008). It is noteworthy that the MUC1-C cytoplasmic domain binds directly to IKKβ and contributes to activation of the IKK complex (Ahmad et al., 2007). Moreover, the MUC1-C cytoplasmic domain binds directly to NF-κB p65 and blocks the interaction of NF-κB p65 and its inhibitor IκBα, thus further promoting the activation of the canonical NF-κB pathway (Ahmad et al., 2009). The interaction between MUC1-C and NF-κB p65 is detectable on the promoters of NF-κB target genes. For example, MUC1-C-NF-κB p65 complexes occupy the Bcl-xL gene promoter, in which MUC1-C contributes to NF-κB-mediated induction of Bcl-xL expression (Ahmad et al., 2009). The MUC1-C cytoplasmic domain contains a CQC motif that is necessary for its oligomerization and thereby localization of MUC1-C to the nucleus (Leng et al., 2007). Inhibition of MUC1-C oligomerization in epithelial cells with a peptide drug, designated GO-201, blocks the interaction between MUC1-C and NF-κB p65 and decreases the expression of NF-κB target genes (Ahmad et al., 2009). These findings have provided support for a model in which MUC1-C is a direct activator of NF-κB p65, and that targeting of MUC1-C function blocks activation of the canonical NF-κB pathway.
In the present work, we show that peptide inhibitors of MUC1-C oligomerization block constitutive activation of NF-κB p65 and induce death of multiple myeloma cells in vitro. The results also demonstrate that inhibiting MUC1-C is highly effective in the treatment of human multiple myeloma xenografts in nude mice. The findings indicate that disrupting MUC1-C function has a dominant-negative effect on multiple myeloma growth and survival.
Materials and Methods
Cell Culture.
Human U266, RPMI8226, KMS28PE, MM.1R, and H929 multiple myeloma cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. Primary multiple myeloma cells and normal B cells were prepared as described previously (Ryan et al., 2005; Vasir et al., 2005) and suspended in RPMI 1640 medium containing 10% human AB serum (Sigma-Aldrich, St. Louis, MO), penicillin, and streptomycin. Cells were treated with the GO-201, GO-203, CP-1, and CP-2 peptides synthesized by AnaSpec Inc. (San Jose, CA). Viability was determined by trypan blue exclusion.
Analysis of Cell Cycle Distribution and Apoptosis/Necrosis.
Cells were fixed in 80% ethanol and incubated in PBS containing 40 μg/ml RNase and 5 μg/ml propidium iodide (PI). Cell cycle distribution was determined by flow cytometry. Cells were also incubated with PI/annexin V (BD Biosciences Pharmingen, San Diego, CA) and analyzed by flow cytometry.
Immunoprecipitation and Immunoblotting.
Whole-cell and nuclear lysates were prepared from subconfluent cells as described previously (Ahmad et al., 2009; Joshi et al., 2009). Soluble proteins were precipitated with anti-MUC1-C (Ab5; Lab Vision, Fremont, CA). The immunoprecipitates and cell lysates were subjected to immunoblotting with anti-NF-κB p65 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-MUC1-C, anti-lamin B (EMD Biosciences, San Diego, CA), anti-β-actin (Sigma-Aldrich), anti-Bcl-xL (Santa Cruz Biotechnology), anti-caspase-9 (Cell Signaling Technology, Danvers, MA), anti-PKCδ (Santa Cruz Biotechnology) and anti-PARP (Cell Signaling Technology). Immune complexes were detected with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Chromatin Immunoprecipitation Assays.
Soluble chromatin was prepared as described previously (Ahmad et al., 2009) and precipitated with anti-p65 or a control nonimmune IgG. For polymerase chain reaction, 2 μl from a 50-μl DNA extraction was used with 25 to 35 cycles of amplification.
Measurement of Reactive Oxygen Species Levels.
Cells were incubated with 2 μM hydroethidine (HE) (Polysciences, Warrington, PA) for 20 min at room temperature. Conversion of HE to ethidium was measured by excitation at 470 nm and emission at 590 nm (Yin et al., 2003).
Multiple Myeloma Tumor Xenograft Model.
Four to 6-week-old BALB/c nu/nu mice (Charles River Laboratories, Wilmington, MA) were injected subcutaneously with 107 U266 cells in the flank. When tumors were ∼100 mm3, the mice were pair-matched into control and treatment groups of 10 mice each, excluding those with tumors not within 15% of the mean volume. PBS (control vehicle), 30 mg/kg GO-203, and 30 mg/kg CP-2 were administered by intraperitoneal injection each day for 21 days. Another group was treated with 30 mg/kg GO-203 each day for 5 days/week for 3 weeks. Tumor volume (V) was calculated using the formula V = L2 × W/2, where L and W are the larger and smaller diameters, respectively. Tumors were evaluated by staining with H&E.
Results
Inhibition of MUC1-C Function Suppresses Growth of Multiple Myeloma Cells.
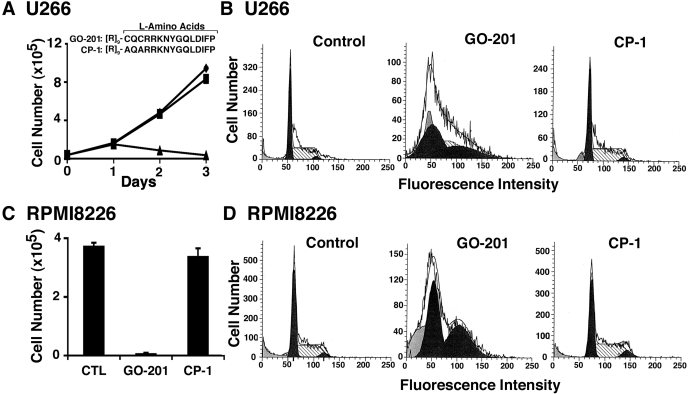
U266, RPMI8226, and KMS28PE multiple myeloma cells express the MUC1 N-terminal and MUC1-C subunits as determined by flow cytometry and immunoblot analysis (Supplemental Fig. S1) (Kawano et al., 2008). To assess sensitivity to inhibition of MUC1-C, U266 cells were treated with GO-201, a peptide inhibitor of MUC1-C oligomerization that was derived from the MUC1-C cytoplasmic domain (CQCRRKNYGQLDIFP; all l-amino acids) and linked at the N terminus to nine arginine residues ([R]9) for cell permeability (Fig. 1A) (Raina et al., 2009). As a control, cells were also treated with the CP-1 peptide ([R]9-AQARRKNYGQLDIFP) that is ineffective in blocking MUC1-C oligomerization (Fig. 1A) (Raina et al., 2009). GO-201, but not CP-1, inhibited U266 cell growth (Fig. 1A). In concert with this response, GO-201 treatment was associated with a substantial arrest of cells in G1 and G2 phases (Fig. 1B). RPMI8226 cells also responded to GP-201 and not to CP-1, with decreases in growth and accumulation of cells in G1 and G2 phases (Fig. 1, C and D; Supplemental Fig. S2A). Moreover, similar results were obtained with KMS28PE cells (Supplemental Fig. S2B). These findings indicated that targeting MUC1-C oligomerization inhibits the growth of multiple myeloma cells.
Fig. 1.
GO-201 induces the arrest of U266 and RPMI8226 cell growth. A, amino acid sequences of GO-201 and CP-1 linked to a poly(arginine) transduction domain (Raina et al., 2009). U266 cells were left untreated (♦) and treated with 5 μM GO-201 (▲) or CP-1 (■) each day for 3 days. Viable cell number was determined by trypan blue exclusion. B, U266 cells were left untreated (Control) and treated with 5 μM GO-201 or CP-1 each day for 3 days. Cells were fixed and analyzed for cell cycle distribution by flow cytometry. C and D, RPMI8226 cells were left untreated (CTL) and treated with 5 μM GO-201 or CP-1 each day for 3 days. Viable cell number as determined by trypan blue exclusion is expressed as the mean ± S.D. of three determinations (C). Cells were analyzed for cell cycle distribution (D).
GO-201 Inhibits NF-κB Activation and Induces the Death of Multiple Myeloma Cells.
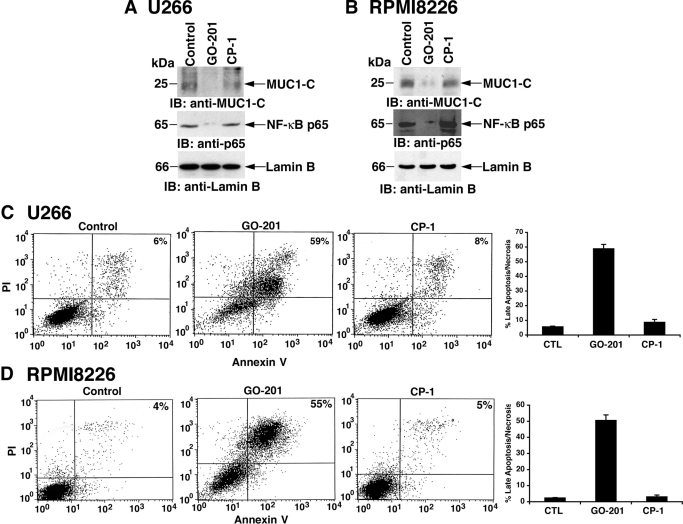
Previous studies have demonstrated that MUC1-C interacts directly with NF-κB RelA p65 and that inhibition of MUC1-C function with GO-201 in breast cancer cells blocks targeting of MUC1-C and NF-κB p65 to the nucleus (Ahmad et al., 2009). Consistent with these effects, treatment of U266 cells with GO-201, and not CP-1, decreased nuclear levels of both MUC1-C and NF-κB p65 (Fig. 2A). A similar down-regulation of nuclear MUC1-C and NF-κB p65 was observed when RPMI8226 cells were treated with GO-201 (Fig. 2B). These results and the dependence of multiple myeloma cells on the NF-κB p65 pathway for survival raised the possibility that GO-201 could induce cell death. Indeed, treatment of U266 cells with GO-201 was associated with both PI and annexin V staining, consistent with the induction of late apoptosis/necrosis (Fig. 2C, left). Similar effects with approximately 60% of the GO-201-treated U266 cells exhibiting a late apoptotic/necrotic response were obtained in repetitive experiments (Fig. 2C, right). In addition and importantly for the specificity of GO-201, treatment with CP-1 had little if any effect (Fig. 2C). RPMI8226 cells also responded to GO-201 and not CP-1 with the induction of late apoptosis/necrosis (Fig. 2D, left), which was confirmed in repetitive experiments (Fig. 2D, right). GO-201 treatment of KMS28PE cells was further associated with decreases in nuclear MUC1-C and NF-κB p65 (Supplemental Fig. S3A) and late apoptotic/necrotic death (Supplemental Fig. S3B), indicating that this response to the inhibition of MUC1-C function is observed in diverse multiple myeloma cells.
Fig. 2.
GO-201 blocks nuclear targeting of NF-κB p65 and induces late apoptosis/necrosis. A and B, U266 (A) and RPMI8226 (B) cells were left untreated (Control) and treated with 5 μM GO-201 or CP-1 each day for 2 days. Nuclear lysates were immunoblotted with the indicated antibodies. C and D, U266 (C) and RPMI8226 (D) cells were left untreated (CTL) and treated with 5 μM GO-201 or CP-1 each day for 3 days. Cells were stained with PI/annexin V and analyzed by flow cytometry (left). The percentage of cells positive for both PI and annexin V is indicated in the top right quadrants. The results are expressed as the percentage (mean ± S.D. of three determinations) of late apoptotic/necrotic cells (right).
Effects of GO-203 on Growth of Multiple Myeloma Cells.
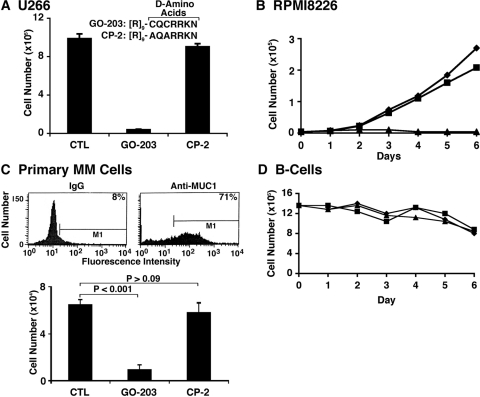
GO-201 has potential disadvantages as a therapeutic agent in that l-amino acids are susceptible to degradation by proteases in plasma and the tumor microenvironment. Consequently, we synthesized a shorter configuration with d-amino acids, designated GO-203 ([R]9-CQCRRKN), to circumvent proteolytic degradation and thereby increase stability (Fig. 3A). As found with GO-201, GO-203 was effective in inhibiting the growth of U266 (Fig. 3A) and RPMI8226 (Fig. 3B) cells. GO-203 was also effective in inducing late apoptosis/necrosis of these cells (data not shown). By contrast, the d-amino acid control CP-2 ([R]9-AQARRKN) had no effect on the growth or survival of U266 and RPMI8226 cells (Fig. 3, A and B). To further assess the activity of GO-203 against other multiple myeloma cells, we studied the MUC1-positive MM.1R and H929 lines. Treatment of MM.1R cells with GO-203 and not CP-2 was associated with the inhibition of growth (Supplemental Fig. S4A) and the induction of late apoptosis/necrosis (data not shown). Similar effects were obtained in the response of H929 cells to GO-203 exposure (Supplemental Fig. S4B). These results indicate that the U266, RPMI8226, KMS28PE, MM.1R, and H929 cell lines studied each responded to MUC1-C inhibition with loss of survival. Nonetheless, this broad activity does not exclude the possibility that other multiple myeloma cell lines will be resistant to the effects of GO-203. Analysis of primary multiple myeloma cells by flow cytometry further demonstrated the expression of MUC1 in more than 70% of the population (Fig. 3C, top). Short-term culture of the primary multiple myeloma cells further showed that viable cell number is significantly decreased in response to GO-203 treatment compared with that obtained with CP-2 (Fig. 3C, bottom). By contrast, GO-203 had no apparent effect on viability of normal B cells (Fig. 3D). These findings indicate that GO-203 is active against multiple myeloma cell lines and primary cells.
Fig. 3.
GO-203 is an effective inhibitor of multiple myeloma cell growth and survival. A, d-amino acid sequences of GO-203 and CP-2 linked to a poly(arginine) transduction domain. U266 cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 4 days. Viable cell number as determined by trypan blue exclusion is expressed as the mean ± S.D. of three determinations. B, RPMI8226 cells were left untreated (♦) and treated with 5 μM GO-203 (▲) or CP-2 (■) each day for the indicated days. Viable cell number was determined by trypan blue exclusion. C, primary multiple myeloma cells were isolated from the bone marrow of a patient with >90% CD138+ cells, incubated with a control IgG or an anti-MUC1 antibody and analyzed by flow cytometry (top). The percentage of MUC1-positive cells is indicated. The primary multiple myeloma cells were left untreated (CTL) and treated with 5 μM GO-203 or CP-2 each day for 6 days. Viable cell number as assessed by trypan blue exclusion is expressed as the mean ± S.D. of three determinations (bottom). P values were determined by the Student's t test. D, normal peripheral blood B-cells were left untreated (♦) and treated with 5 μM (■) or 10 μM (▲) GO-203 each day for the indicated days. Viable cell number was determined by trypan blue exclusion.
GO-203 Blocks Activation of the NF-κB Pathway.
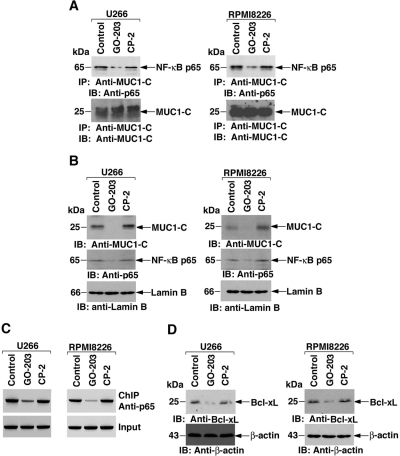
The demonstration that GO-201 decreases nuclear MUC1-C and NF-κB p65 levels provided the basis for more detailed studies on the effects of GO-203 on activation of the NF-κB pathway. MUC1-C associates with NF-κB p65 in human carcinoma cells (Ahmad et al., 2009). Coprecipitation studies demonstrated that MUC1-C also associates with NF-κB p65 in U266 cells (Fig. 4A, left). Moreover, this interaction was decreased by the treatment of U266 cells with GO-203 and not CP-2 (Fig. 4A, left). The association of MUC1-C and NF-κB p65 was also detectable in RPMI8226 cells and was down-regulated by GO-203 treatment (Fig. 4A, right). As found with GO-201, nuclear targeting of MUC1-C and NF-κB p65 was decreased by GO-203 in U266 (Fig. 4B, left) and RPMI8226 (Fig. 4B, right) cells. Previous work showed that MUC1-C promotes NF-κB p65 occupancy of the Bcl-xL gene promoter and increases Bcl-xL expression (Ahmad et al., 2009). To determine whether GO-203 affects occupancy of the Bcl-xL promoter by NF-κB p65, we performed chromatin immunoprecipitation assays of the NF-κB-responsive element (GGGACTGCCC; −367 to −358) (Grillot et al., 1997). In U266 cells, occupancy of the Bcl-xL promoter by NF-κB p65 was decreased in the response to GO-203 treatment (Fig. 4C, left). Similar results were obtained in chromatin immunoprecipitation studies of GO-203-treated RPMI8226 cells (Fig. 4C, right). Moreover, in concert with this decrease in NF-κB p65 occupancy of the Bcl-xL promoter, GO-203 treatment was associated with down-regulation of Bcl-xL expression (Fig. 4D). These findings demonstrate that inhibition of MUC1-C blocks constitutive activation of the NF-κB p65 pathway in multiple myeloma cells.
Fig. 4.
GO-203 inhibits constitutive activation of the NF-κB p65 pathway. U266 (left) and RPMI8226 (right) cells were left untreated (Control) and treated with 5 μM GO-203 or CP-2 each day for 2 days. A, anti-MUC1-C precipitates were immunoblotted with anti-p65 and anti-MUC1-C. B, nuclear lysates were immunoblotted with the indicated antibodies. C, soluble chromatin was precipitated with anti-p65. The final DNA extractions were amplified by polymerase chain reaction with pairs of primers that cover the NF-κB response element (−597 to −304) in the Bcl-xL promoter. D, Whole-cell lysates were immunoblotted with the indicated antibodies.
GO-203 Induces Activation of the Intrinsic Apoptotic Pathway.
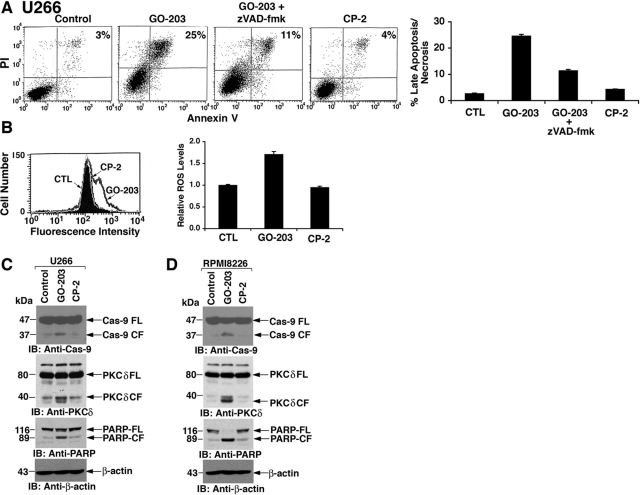
NF-κB promotes cell survival through the induction of target genes, such as Bcl-xL, that attenuate the induction of apoptosis (Luo et al., 2005; Dutta et al., 2006). The demonstration that GO-203 inhibits the NF-κB p65 pathway thus prompted the assessment of whether GO-203 induces multiple myeloma cell death by activating the apoptotic pathway. In this regard, staining of GO-203-treated U266 cells with PI and annexin V supported the induction of late apoptosis/necrosis (Fig. 5A). Concurrent exposure of the U266 cells to zVAD-fmk to block caspase activation demonstrated a >50% decrease in cells staining with both PI and annexin V (Fig. 5A). The response of RPMI8226 cells to GO-203 was similarly inhibited by zVAD-fmk, indicating that GO-203 induces death, at least in large part, by an apoptotic mechanism (Supplemental Fig. S4C). NF-κB also blocks cell death by inducing genes that encode antioxidant proteins (Luo et al., 2005). In addition, MUC1 attenuates stress-induced disruption of redox balance (Yin et al., 2003). In concert with inhibition of MUC1-C function and NF-κB p65 activation, treatment of U266 cells with GO-203 and not CP-2 was associated with increases in reactive oxygen species (ROS) levels (Fig. 5B, left) that were confirmed in repetitive experiments (Fig. 5B, right). GO-203 treatment of U266 cells was also associated with activation of caspase-9 and cleavage of PKCδ and PARP, which are caspase-3 substrates (Fig. 5C). RPMI8226 cells similarly responded to GO-203 with activation of caspase-9 and PKCδ and PARP cleavage (Fig. 5D), consistent with induction of the intrinsic apoptotic pathway.
Fig. 5.
GO-203 induces activation of the intrinsic apoptotic pathway. A, U266 cells were left untreated (Control) and treated with 5 μM GO-203 or 5 μM CP-2 each day for 3 days. For the GO-203-treated cells, 5 μM zVAD-fmk was added during the last 24 h. Cells were stained with PI/annexin V and analyzed by flow cytometry. The percentage of cells positive for both PI and annexin V is indicated in the top right quadrants (left). The results are expressed as the percentage (mean ± S.D. of three determinations) of late apoptotic/necrotic cells (right). B, U266 cells were left untreated (CTL) and treated with 5 μM GO-203 or 5 μM CP-2 each day for 3 days. ROS levels were determined by oxidation of HE and flow cytometry (left). The results are expressed as relative ROS levels (mean ± S.D. of three determinations) compared with that obtained with control cells (right). C and D, U266 (C) and RPMI8226 (D) cells were left untreated (Control) and treated with 5 μM GO-203 or CP-2 each day for 3 days. Lysates were immunoblotted with the indicated antibodies.
Activity of GO-203 against U266 Tumor Xenografts in Mice.
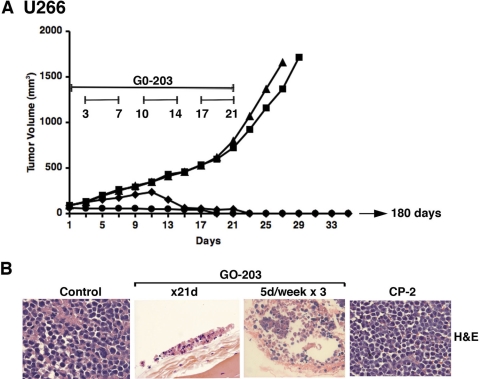
To assess antitumor activity of GO-203, U266 tumor xenograft (∼100 mm3) cells were established in the flanks of nude mice. Intraperitoneal administration of GO-203 at 30 mg/kg/day for 21 days completely inhibited growth compared with that obtained with the vehicle (PBS) (Fig. 6A). Dosing of GO-203 on a different schedule at 30 mg/kg/day for 5 days per week for 3 weeks was associated with a partial slowing of growth over 12 days and then a progressive decrease in tumor volume (Fig. 6A). No further treatment was administered after day 21. However, the tumors treated on both GO-203 schedules continued to regress and were no longer palpable by day 28 (Fig. 6A). In contrast to these results, treatment with CP-2 at 30 mg/kg/day for 21 days had no apparent effect on tumor growth (Fig. 6A). On day 28, one mouse from each GO-203 treatment group was sacrificed to assess the tumor implantation site. No remaining tumor was evident visually at the site or by spread to other organs. Histological examination of the implantation sites from both GO-203 treatment groups showed small foci of remaining tumor cells with pyknotic nuclei and loss of cellular architecture (Fig. 6B). Mice in both GO-203-treated groups were followed for 180 days without evidence for reappearance of tumor.
Fig. 6.
GO-203 induces complete regressions of U266 tumors. BALB/c nu/nu mice were injected subcutaneously in the flank with 107 U266 cells. The mice were pair-matched when the tumors were ∼100 mm3. Treatment groups consisted of 10 mice injected intraperitoneally with PBS (vehicle control; ■), 30 mg/kg GO-203 (●), or 30 mg/kg CP-2 (▲) each day for 21 days. Another group was treated with 30 mg/kg GO-203 administered each day for 5 days per week for 3 weeks (♦). Mice were weighed twice weekly, and tumor measurements were performed every 2 days. There was no weight loss in any of the groups. The results are expressed as the mean tumor volume with a S.E. of <15% (A). There was no evidence for recurrence in the two GO-203 treatment groups at 180 days. U266 tumors and tumor implantation sites harvested on day 28 were stained with H&E (B).
Discussion
MUC1-C Contributes to Constitutive Activation of the Canonical NF-κB Pathway in Multiple Myeloma Cells.
The mechanisms responsible for constitutive activation of the canonical NF-κB pathway in multiple myeloma cell lines, and primary patient samples are largely not known. Certain multiple myeloma cells have increased NF-κB-inducing kinase expression or inactivating TRAF3 mutations that can contribute to NF-κB p65 activation (Annunziata et al., 2007). However, the identification of additional effectors that activate the canonical NF-κB pathway in multiple myeloma cells has remained elusive. Indeed, the present studies demonstrate that inhibitors of MUC1-C oligomerization are effective in down-regulating NF-κB p65 activation in diverse multiple myeloma cell lines. Aberrant expression of MUC1 in multiple myeloma cells (Takahashi et al., 1994; Burton et al., 1999; Treon et al., 1999; Paydas et al., 2001; Cloosen et al., 2006; Baldus et al., 2007; Kawano et al., 2008) is associated with the accumulation of the MUC1-C subunit in the cytoplasm and nucleus (Li et al., 2003). MUC1-C contributes to the activation of the NF-κB pathway through direct binding to NF-κB p65 (Ahmad et al., 2007). The present results demonstrate that inhibition of MUC1-C with GO-203 blocks the binding of MUC1-C to NF-κB p65. We also found that inhibition of MUC1-C function is associated with decreased nuclear targeting of both MUC1-C and NF-κB p65. The decrease in nuclear MUC1-C is explained by the demonstration that MUC1-C oligomerization, which is blocked by targeting the CQC motif with GO-201 and GO-203, is necessary for its nuclear transport (Leng et al., 2007). Recent evidence obtained from carcinoma cells has supported a role for MUC1-C in promoting nuclear localization of NF-κB p65 and its occupancy on the promoters of target genes (Ahmad et al., 2009). In multiple myeloma cells, silencing MUC1 was associated with decreased nuclear targeting of NF-κB p65 (Kawano et al., 2008). Moreover, the present results demonstrate that inhibition of MUC1-C with GO-203 decreases nuclear NF-κB p65 levels and occupancy of p65 on the Bcl-xL promoter. These findings indicate that MUC1-C oligomerization contributes to constitutive activation of the canonical NF-κB pathway in multiple myeloma cells.
Inhibition of MUC1-C Induces Late Apoptosis/Necrosis of Multiple Myeloma Cells.
Sensitivity of different multiple myeloma cell lines to MLN120B, an IKKβ inhibitor, varies widely in terms of growth arrest and induction of apoptosis (Annunziata et al., 2007; Hideshima et al., 2009). The divergent response of multiple myeloma cells to IKKβ inhibition may be dictated by addiction to other signals, for example, activation of the noncanonical NF-κB pathway (Hideshima et al., 2009). Alternatively, direct activation of NF-κB p65 by MUC1-C could circumvent, at least in part, the strategy of inhibiting upstream signals conferred by IKKβ. In this context, MUC1-C competes with IκBα for binding to NF-κB p65 and thereby can bypass IKKβ-mediated IκBα degradation for activation of the pathway (Ahmad et al., 2009). The present results further demonstrate that multiple myeloma cells with predominantly canonical NF-κB activation (RPMI8226, H929) exhibit similar responses to GO-203-induced growth arrest and death as those with up-regulation of both the canonical and noncanonical pathways (U266, MM.1R) (Hideshima et al., 2009). In addition to its interaction with NF-κB p65, MUC1-C blocks stress-induced increases in ROS (Yin et al., 2003, 2007, 2009) and is targeted to the mitochondrial outer membrane, in which it attenuates the release of proapoptotic effectors (Ren et al., 2004). In concert with these effects, inhibition of MUC1-C function in multiple myeloma cells with GO-203 was associated with a disruption of redox balance. GO-203 treatment was also associated with down-regulation of Bcl-xL expression and activation of the intrinsic apoptotic pathway. Moreover, inhibition of MUC1-C in diverse multiple myeloma cell lines was associated with PI and annexin V staining, consistent with induction of late apoptosis/necrosis. Inhibition of caspases with zVAD-fmk partially blocked GO-203-induced cell death, indicating that apoptosis is a predominant response of multiple myeloma cells to this agent. By contrast, breast and prostate cancer cells responded to MUC1-C inhibition with increases in ROS levels and the induction of necrotic cell death (Joshi et al., 2009; Raina et al., 2009). Given the difficulties in distinguishing late apoptosis from necrosis (McGahon et al., 1995), the present results thus do not exclude the possibility that some of the multiple myeloma cells may have died by a necrotic response. In addition, although multiple myeloma cells are dependent on the canonical NF-κB pathway for growth and survival, the response to MUC1-C inhibition could include down-regulation of other signals that, in concert with loss of NF-κB p65 activation, contribute to cell death. Targeting dependence on specific oncoproteins has been associated with circumventing mutations and/or activation of alternative pathways (Weinstein and Joe, 2008). Therefore, further studies will be needed to determine whether targeting MUC1-C and thereby constitutive NF-κB activation in multiple myeloma cells will result in the emergence of resistance.
Blocking MUC1-C Oligomerization Has a Dominant-Negative Effect on Multiple Myeloma Cell Survival.
Silencing of MUC1 in KMS28PE multiple myeloma cells had no apparent effect on survival (Kawano et al., 2008). By contrast, the present results demonstrate that inhibition of MUC1-C oligomerization induces death of KMS28PE and other multiple myeloma cell lines. These distinct observations indicate that sudden blocking of MUC1-C oligomer formation has a dominant-negative effect that is not found with MUC1-C silencing. In this context, stable expression of an oligomerization-defective MUC1-C mutant in carcinoma cells blocks colony formation and tumorigenicity, consistent with a dominant-negative function for transformation (Leng et al., 2007). In addition and importantly, these peptide inhibitors of MUC1-C have had little if any effect on growth and survival of MUC1-negative cells, supporting the specificity of this approach (Joshi et al., 2009; Raina et al., 2009). The development of inhibitors that directly block MUC1-C oligomerization has thus made it possible to assess dependence on MUC1-C function for multiple myeloma cell growth and survival. Death of multiple myeloma cell lines and primary cells to MUC1-C inhibition with GO-201 in vitro thus provided the first evidence for sensitivity to disruption of MUC1-C function. However, because peptides with l-amino acids are susceptible to degradation by proteases, we studied the effects of converting the GO-201 sequence to d-amino acids and decreasing the number of amino acids after the critical CQC motif. We found that GO-203 retained activity and, importantly, was more stable than GO-201 in mouse and human plasma (D. Raina, J. Supko, M. Kosugi, R. Ahmad, G. Panchamoorthy, X. He, N. Zvereva, S. Kharbanda, and D. Kufe, unpublished data). Toxicity studies of GO-203 in mice further indicated that this agent has little if any effect on normal organ function, including hematopoietic parameters. In the present work, nude mice bearing U266 xenografts were treated daily with GO-203 based on a plasma half-life of ∼12 h for this agent. In addition, experience with MUC1-C inhibitors in breast and prostate cancer models indicated that daily treatment for 21 days is needed to achieve complete regressions (Joshi et al., 2009; Raina et al., 2009). Indeed, GO-203 treatment of U266 tumor xenografts each day for 21 days or 5 days per week for 3 weeks was associated with slowing of growth and then complete disappearance after stopping therapy. By contrast, CP-2 treatment had no effect on U266 tumor growth. These findings and those obtained from in vitro studies thus indicate that certain multiple myeloma cells are sensitive to the disruption of MUC1-C function for their growth and survival. In this regard, MUC1-C could represent a target for the treatment of patients with multiple myeloma with the clinical development of cell-penetrating peptides, such as GO-203, or small molecules that are designed to disrupt MUC1-C oligomerization.
Supplementary Material
Acknowledgments
We thank Dr. Teru Hideshima for helpful advice.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This study was supported by the Multiple Myeloma Research Foundation; the Leukemia Lymphoma Society; and the National Institutes of Health National Cancer Institute [Grant CA42802].
Disclosure of potential conflicts of interest: D.K. is an equity holder of and consultant to Genus Oncology. S.K. is an employee of Genus Oncology.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.065011.
- NF-κB
- nuclear factor κB
- IKK
- IκB kinase
- MUC1
- mucin 1
- MUC1-C
- MUC1 C-terminal subunit
- ROS
- reactive oxygen species
- PKCδ
- protein kinase C-δ
- PARP
- poly(ADP-ribose) polymerase
- PI
- propidium iodide
- PBS
- phosphate-buffered saline
- HE
- hydroethidine
- H&E
- hematoxylin and eosin
- zVAD-FMK
- N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone.
References
- Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, Kufe D. (2009) MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer Res 69:7013–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, Kufe D. (2007) MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nat Cell Biol 9:1419–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, et al. (2007) Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12:115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldus SE, Palmen C, Thiele J. (2007) MUC1 (EMA) expressing plasma cells in bone marrow infiltrated by plasma cell myeloma. Histol Histopathol 22:889–893 [DOI] [PubMed] [Google Scholar]
- Burton J, Mishina D, Cardillo T, Lew K, Rubin A, Goldenberg DM, Gold DV. (1999) Epithelial mucin-1 (MUC1) expression and MA5 anti-MUC1 monoclonal antibody targeting in multiple myeloma. Clin Cancer Res 5 (10 Suppl):3065S–3072S [PubMed] [Google Scholar]
- Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA, Anderson KC. (1996) Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood 87:1104–1112 [PubMed] [Google Scholar]
- Cloosen S, Gratama J, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, von Mensdorff-Pouilly S, Tarp MA, Mandel U, Clausen H, Germeraad WT, et al. (2006) Cancer specific Mucin-1 glycoforms are expressed on multiple myeloma. Br J Haematol 135:513–516 [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. (2006) Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 25:6800–6816 [DOI] [PubMed] [Google Scholar]
- Grillot DA, González-García M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF, Nuñez G. (1997) Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol 158:4750–4757 [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362 [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Kiziltepe T, Ikeda H, Okawa Y, Podar K, Raje N, Protopopov A, Munshi NC, Richardson PG, et al. (2009) Biologic sequelae of I{kappa}B kinase (IKK) inhibition in multiple myeloma: therapeutic implications. Blood 113:5228–5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, et al. (2002) NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem 277:16639–16647 [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Schlossman R, Richardson P, Anderson KC. (2001) The role of tumor necrosis factor alpha in the pathophysiology of human multiple myeloma: therapeutic applications. Oncogene 20:4519–4527 [DOI] [PubMed] [Google Scholar]
- Hideshima T, Neri P, Tassone P, Yasui H, Ishitsuka K, Raje N, Chauhan D, Podar K, Mitsiades C, Dang L, et al. (2006) MLN120B, a novel IkappaB kinase beta inhibitor, blocks multiple myeloma cell growth in vitro and in vivo. Clin Cancer Res 12:5887–5894 [DOI] [PubMed] [Google Scholar]
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. (2005) MUC1 oncoprotein blocks glycogen synthase kinase 3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res 65:10413–10422 [DOI] [PubMed] [Google Scholar]
- Joshi MD, Ahmad R, Yin L, Raina D, Rajabi H, Bubley G, Kharbanda S, Kufe D. (2009) MUC1 oncoprotein is a druggable target in human prostate cancer cells. Mol Cancer Ther 8:3056–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan M, Moreaux J, Vos JD, Hose D, Mahtouk K, Abouladze M, Robert N, Baudard M, Rème T, Romanelli A, et al. (2007) Targeting NF-kappaB pathway with an IKK2 inhibitor induces inhibition of multiple myeloma cell growth. Br J Haematol 138:160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Lin A. (2002) NF-kappaB at the crossroads of life and death. Nat Immunol 3:221–227 [DOI] [PubMed] [Google Scholar]
- Kawano T, Ahmad R, Nogi H, Agata N, Anderson K, Kufe D. (2008) MUC1 oncoprotein promotes growth and survival of human multiple myeloma cells. Int J Oncol 33:153–159 [PMC free article] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, et al. (2007) Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell 12:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufe DW. (2009) Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer 9:874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, Kufe D. (2007) Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem 282:19321–19330 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen W, Ren J, Yu WH, Li Q, Yoshida K, Kufe D. (2003) DF3/MUC1 signaling in multiple myeloma cells is regulated by interleukin-7. Cancer Biol Ther 2:187–193 [DOI] [PubMed] [Google Scholar]
- Luo JL, Kamata H, Karin M. (2005) IKK/NF-kappaB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest 115:2625–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR. (1995) The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol 46:153–185 [DOI] [PubMed] [Google Scholar]
- Paydaş S, Sahin B, Gönlüşen G, Hazar B, Zorludemir S. (2001) MUC1 expression in plasmacytoma. Leuk Res 25:221–225 [DOI] [PubMed] [Google Scholar]
- Raina D, Ahmad R, Joshi MD, Yin L, Wu Z, Kawano T, Vasir B, Avigan D, Kharbanda S, Kufe D. (2009) Direct targeting of the mucin 1 oncoprotein blocks survival and tumorigenicity of human breast carcinoma cells. Cancer Res 69:5133–5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. (2007) The MUC1 and galectin-3 oncoproteins function in a microRNA-dependent regulatory loop. Mol Cell 27:992–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. (2004) Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell 5:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan EP, Pollock SJ, Pollack SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. (2005) Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol 174:2619–2626 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Makiguchi Y, Hinoda Y, Kakiuchi H, Nakagawa N, Imai K, Yachi A. (1994) Expression of MUC1 on myeloma cells and induction of HLA-unrestricted CTL against MUC1 from a multiple myeloma patient. J Immunol 153:2102–2109 [PubMed] [Google Scholar]
- Treon SP, Mollick JA, Urashima M, Teoh G, Chauhan D, Ogata A, Raje N, Hilgers JH, Nadler L, Belch AR, et al. (1999) Muc-1 core protein is expressed on multiple myeloma cells and is induced by dexamethasone. Blood 93:1287–1298 [PubMed] [Google Scholar]
- Vasir B, Borges V, Wu Z, Grosman D, Rosenblatt J, Irie M, Anderson K, Kufe D, Avigan D. (2005) Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br J Haematol 129:687–700 [DOI] [PubMed] [Google Scholar]
- Weinstein IB, Joe A. (2008) Oncogene addiction. Cancer Res 68:3077–3080 [DOI] [PubMed] [Google Scholar]
- Yin L, Kharbanda S, Kufe D. (2007) Mucin 1 oncoprotein blocks hypoxia-inducible factor 1alpha activation in a survival response to hypoxia. J Biol Chem 282:257–266 [DOI] [PubMed] [Google Scholar]
- Yin L, Kharbanda S, Kufe D. (2009) MUC1 oncoprotein promotes autophagy in a survival response to glucose deprivation. Int J Oncol 34:1691–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Li Y, Ren J, Kuwahara H, Kufe D. (2003) Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem 278:35458–35464 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.