Abstract
We previously identified spinophilin as a regulator of α2 adrenergic receptor (α2AR) trafficking and signaling in vitro and in vivo (Science 304:1940–1944, 2004). To assess the generalized role of spinophilin in regulating α2AR functions in vivo, the present study examined the impact of eliminating spinophilin on α2AR-evoked cardiovascular and hypnotic responses, previously demonstrated to be mediated by the α2AAR subtype, after systemic administration of the α2-agonists 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK14,304) and clonidine in spinophilin-null mice. Mice lacking spinophilin expression display dramatically enhanced and prolonged hypotensive, bradycardic, and sedative-hypnotic responses to α2AR stimulation. Whereas these changes in sensitivity to α2AR agonists occur independent of any changes in α2AAR density or intrinsic affinity for agonist in the brains of spinophilin-null mice compared with wild-type control mice, the coupling of the α2AAR to cognate G proteins is enhanced in spinophilin-null mice. Thus, brain preparations from spinophilin-null mice demonstrate enhanced guanine nucleotide regulation of UK14,304 binding and evidence of a larger fraction of α2AAR in the guanine-nucleotide-sensitive higher affinity state compared with those from wild-type mice. These findings suggest that eliminating spinophilin expression in native tissues leads to an enhanced receptor/G protein coupling efficiency that contributes to sensitization of receptor mediated responses in vivo.
The α2-adrenergic receptor (AR) is a prototypical G protein-coupled receptor (GPCR) that couples to the Gi/o subfamily of G proteins (Wang and Limbird, 2007). In native cells, stimulation of the α2AR leads to inhibition of adenylyl cyclase and voltage-gated Ca2+ currents and to activation of receptor-operated K+ currents and mitogen activated protein kinase (Limbird, 1988; Kobilka, 1992; Richman and Regan, 1998; Wang et al., 2004; Lu et al., 2009). Among the three different subtypes of α2AR, α2A-, α2B-, and α2CAR, the α2AAR subtype is the major mediator of the therapeutic effects of α2-adrenergic agents on blood pressure, pain perception, volatile anesthetic sparing, analgesia, and working memory enhancement, as revealed by genetic studies exploiting mice made null or mutant for this receptor subtype (MacMillan et al., 1996; MacDonald et al., 1997; Hein et al., 1999; Kable et al., 2000). Activation of the α2AAR in the nucleus tractus solitarius represents an important central mechanism to lower blood pressure (Sved et al., 1992; MacMillan et al., 1996). In addition, the α2AAR inhibits synaptic firing and induces sedative and hypnotic effects via the locus ceruleus (Lakhlani et al., 1997).
Receptor interacting partners other than heterotrimeric G proteins play pivotal roles in modulating nearly every aspect of GPCR activity, including pharmacological recognition, signaling activation and desensitization, and receptor trafficking among cellular compartments (Bockaert et al., 2004; Gainetdinov et al., 2004; Kenakin, 2004; Rashid et al., 2004; Tilakaratne and Sexton, 2005; Sato et al., 2006). G protein-coupled receptor kinase (GRK) 2 binds to and phosphorylates the agonist-activated α2AR (Jewell-Motz and Liggett, 1996; Pao and Benovic, 2005), which subsequently interacts with arrestin (Wu et al., 1997; DeGraff et al., 2002; Wang and Limbird, 2002). GRK phosphorylation represents one major mechanism for α2AR desensitization after agonist stimulation (Eason et al., 1995; Jewell-Motz and Liggett, 1996; Desai et al., 2006). Whether or not heterologous desensitization pathways (Jones et al., 1990; Jewell-Motz et al., 1998; Liang et al., 1998) and sensitization pathways (Jones et al., 1987; Jones and Bylund, 1988, 1990) reported in vitro also contribute to desensitization of this receptor in vivo is not yet known.
Arrestin is a multifaceted regulator of GPCRs that terminates G protein coupling, mediates receptor internalization, and scaffolds cellular signaling cascades (Reiter and Lefkowitz, 2006; DeWire et al., 2007). Our previous studies identified spinophilin (Allen et al., 1997; Satoh et al., 1998) as an α2AR-interacting partner that regulates multiple aspects of α2AR trafficking and signaling by antagonizing GRK2 interaction with the receptor and subsequent arrestin binding and functions (Wang et al., 2004). Interaction of spinophilin with the α2AR lessens arrestin-dependent internalization of the α2AR (Brady et al., 2003; Wang et al., 2004), and slows the rate of both activation and resensitization of receptor-mediated signaling, presumably through decelerating the receptor internalization/recycling cycle (Wang et al., 2004). In vivo, the reciprocal regulation of the α2AR by spinophilin and arrestin is manifest by the fact that agonist sensitivity of α2AAR-evoked sedative response (as assessed by Rotarod latency) is suppressed in arrestin 3-deficient [Arr3(−/−)] mice but enhanced in spinophilin-null [Sp(−/−)] mice.
Given the diversity of α2AAR functions in vivo, it is not clear whether other α2AAR-mediated responses are also regulated by spinophilin in a similar manner. In the present study, we investigated hypotensive, bradycardic, and hypnotic responses elicited by the α2AAR in mice lacking spinophilin expression. Our data revealed an enhanced and prolonged hypotensive effect and bradycardia in response to the α2-agonists 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine (UK14,304) and clonidine in these mice, as well as enhanced sedative and hypnotic effects. Complementary in vitro studies revealed an enhanced G protein coupling efficiency of the α2AAR in Sp(−/−) mice without changes in receptor density or its intrinsic affinity for the α2-adrenergic agonist compared with wild-type (WT) mice. These findings not only suggest a molecular mechanism that could contribute to the enhanced in vivo responsiveness to activation of the α2AAR in Sp(−/−) mice but also provide additional compelling evidence that α2AAR-G protein interactions, and their signaling consequences, are in constitutive regulation by spinophilin in vivo.
Materials and Methods
Animals
Sp(−/−) and corresponding WT mice were obtained and maintained as described previously (Feng et al., 2000). Mice were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited Animal Resources Program at the University of Alabama at Birmingham in accordance with procedures of the Animal Welfare Act and the 1989 amendments to this Act. All studies with these animals followed protocols approved by University of Alabama Institutional Animal Care and Use Committee.
Coimmunoisolation of Spinophilin and α2AAR from Mouse Brain Lysates
Adult male mice 10 to 12 weeks of age were injected with saline or UK14,304 at 1 mg/kg, the EC50 dose for inducing the sedative response in WT mice (Wang et al., 2004). Thirty minutes after injection, mice were sacrificed, and the whole brain was removed by dissection. The brain was homogenized in a Dounce homogenizer with 10 to 15 strokes on ice in lysis and biological detergent extraction buffer (10 mM Tris Cl, pH 8.0, 1% Nonidet P-40, 10% glycerol, 5 mM EDTA, 5 mM EGTA supplemented with 1 μg/ml soybean trypsin inhibitor, aprotinin, leupeptin, and 0.1 μM phenylmethylsulfonyl fluoride as protease inhibitors). The detergent extract was further homogenized by trituration through a 25-gauge needle 10 times and incubation on ice for 20 min. After centrifugation of the detergent extract at 100,000g at 4°C for 30 min, the supernatant, defined as the detergent-solubilized preparation, was transferred into a microfuge tube and subjected to immunoprecipitation assays with an anti-HA antibody (HA.11; Covance Research Products (Princeton, NJ) as described previously (Wang and Limbird, 2002).
Measurement of Cardiovascular Responses
Cardiovascular responses were measured as described previously (MacMillan et al., 1996; Tan et al., 2002). In brief, male mice (10–12 weeks of age) were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg). The left femoral artery and right jugular vein were catheterized to measure arterial pressure and to administer anesthetic, respectively. Twenty-four hours after this surgery, the systolic, diastolic, and mean arterial blood pressure and heart rate were recorded continually in conscious, freely moving animals by connecting the tubing implanted through left femoral artery to a pressure transducer that is linked to a computerized system, BIOPAC's AcqKnowledge 3.8.2 (BioPac, Goleta, CA). Baseline was established during infusions of saline through the right jugular vein. Twenty minutes later, responses to a bolus injection of 0.1 mg/kg UK14,304 into the right jugular vein were recorded. Previous studies have established that this dose of UK14,304 is insufficient for provoking a sedative response in mice (Tan et al., 2002). Indeed, in this study, no sedative response was observed in the mice as a result of the bolus injection of 0.1 mg/kg UK14,304.
Measurement of Sedative-Hypnotic Responses
Rotarod Latency.
Male mice (10–12 weeks of age) were injected intraperitoneally with saline or different doses of clonidine and then tested for time (seconds) staying on a rotating Rotarod (10 rpm), as described previously (Lakhlani et al., 1997; Tan et al., 2002).
Loss of Righting Reflex.
Male mice (10–12 weeks) were injected intraperitoneally with saline or 5 mg/kg UK14,304, and LORR was evaluated by sleep time as described previously (Lakhlani et al., 1997; Tan et al., 2002).
Radioligand Binding
Saturation binding was performed to assess α2AAR receptor density in mouse brains isolated from WT and Sp(−/−) mice as described previously (MacMillan et al., 1996; Lu et al., 2009). Prazosin (1 μM) was added to block binding of the radiolabeled α2-antagonist, [3H]rauwolscine, to the α2B and α2CAR subtypes in this preparation (MacMillan et al., 1996; Lu et al., 2009).
Competition binding was performed using preparations derived from mouse brains isolated from WT and Sp(−/−) mice to evaluate agonist affinity in the absence or presence of a hydrolysis-resistant GTP analog, 5′-guanylimidodiphosphate (Gpp(NH)p) (MacMillan et al., 1996; Lu et al., 2009). Computer-assisted analyses of the data obtained in these experiments provide a means to indirectly assess G protein coupling to the receptor. G protein interactions with the receptor increase the apparent affinity of the receptor for agonist agents, whereas addition of the hydrolysis-resistant analog of GTP, Gpp(NH)p, reverses these interactions and allows the assessment of the intrinsic affinity of the receptor for agonist in the absence of interactions with G proteins. In the absence of Gpp(NH)p, receptors coupled to G proteins have a higher affinity for agonists and data fit a two-site model, whereas in the presence of Gpp(NH)p, effects of G protein regulation of receptor affinity for agonist are eliminated, apparent receptor affinity is reduced, and data fit a one-site model (De Lean et al., 1980; Samama et al., 1993; Weiss et al., 1996).
All data were analyzed using Prism software (GraphPad Software, San Diego, CA), and the Kd values and percentage of receptors in the higher versus lower affinity states were estimated using nonlinear regression curve fitting.
Results
Spinophilin Preferentially Interacts with the Agonist-Stimulated α2aAR in Mouse Brain.
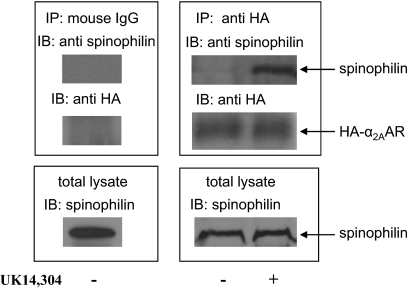
Taking advantage of HA-α2AAR knock-in mice (Lu et al., 2009), we confirmed an endogenous interaction between spinophilin and the α2AAR in mouse brain. As shown in Fig. 1, spinophilin was present in a complex with the HA-α2AAR and could be isolated from the detergent extracts of the mouse brain using an anti-HA antibody. Systemic administration of an α2-agonist UK14,304 at the EC50 dose for inducing the sedative response in mice (1 mg/ml) (Wang et al., 2004) dramatically enhanced the amount of spinophilin coisolated with the HA-α2AAR in brain. These data demonstrate both that an endogenous interaction exists between spinophilin and the α2AAR in mouse brain and that spinophilin preferentially interacts with the agonist-activated form of the α2AAR, consistent with our previous findings examining the α2AAR-spinophilin interaction in in vitro systems (Richman et al., 2001; Wang and Limbird, 2002; Wang et al., 2004).
Fig. 1.
Endogenous interaction of spinophilin with the α2AAR in mouse brain. HA-α2AAR knock-in mice were intraperitoneally injected with saline (control) or UK14,304 (1 mg/kg). Thirty minutes after injection, mouse brains were isolated, homogenized, and coimmunoisolation assays of detergent-solubilized preparations were performed as described under Materials and Methods. Shown are representative blots from three or more independent experiments.
The Hypotensive and Bradycardia Response to α2AR-Agonists Was Enhanced and Prolonged in Spinophilin-Null Mice.
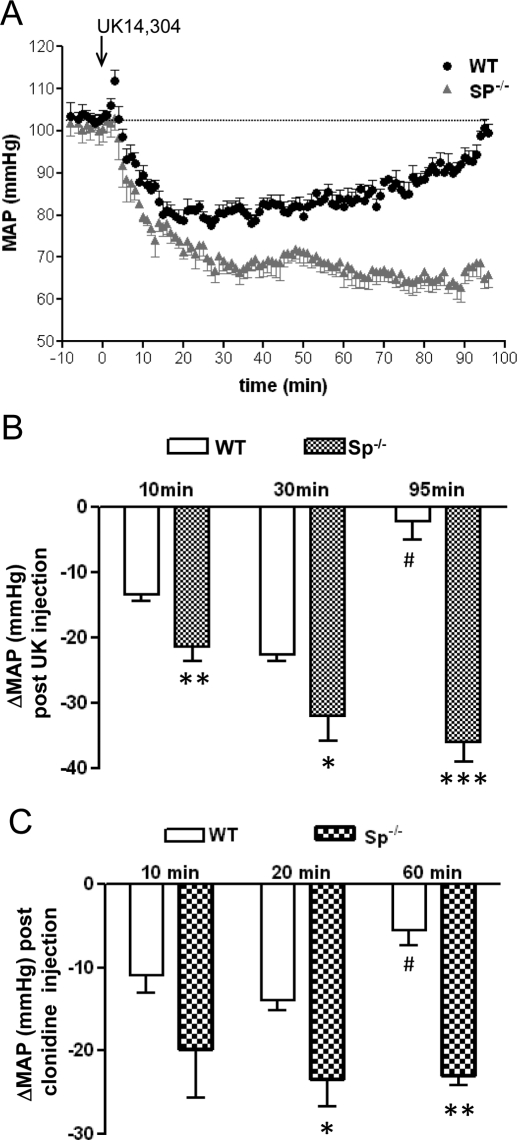
Clinical use of α2AR agonists to lower blood pressure relies on their central actions at the α2AAR subtype (MacMillan et al., 1996; Tan et al., 2002). In conscious, freely moving animals, basal mean arterial pressure and heart rate did not significantly differ between WT and Sp(−/−) mice (Table 1). To avoid inducing sedative responses to mice, 0.1 mg/kg UK14,304 was employed to examine the cardiovascular response to this α2AR agonist (Tan et al., 2002). As shown in Fig. 2, administration of UK14,304 led to a drop in arterial pressure in both WT and Sp(−/−) mice. However, compared with WT mice, Sp(−/−) mice responded to UK14,304 treatment with a steeper and longer lasting decrease in arterial pressure (Fig. 2A). In Sp(−/−) mice, the decrease in blood pressure in response to UK14,304 administration was significantly greater than that in WT mice at 10, 35, and 95 min after bolus UK14,304 administration (Fig. 2B). In addition, although blood pressure in WT mice returned to baseline by 95 min after the UK14,304 injection, in Sp(−/−) mice, the maximum hypotensive response endured for at least 95 min after injection of the α2AR agonist (Fig. 2A). We also analyzed cardiovascular responses to 0.1 mg/kg clonidine administration. Clonidine was able to induce a greater and longer reduction of blood pressure (Fig. 2C) in Sp(−/−) mice compared with WT mice.
TABLE 1.
Basal mean arterial pressure and heart rate in WT and Sp(−/−) mice
Mean arterial pressure (MAP) and heart rate (HR) was measured in awake, freely moving WT and Sp(−/−) mice as described under Materials and Methods. Values represent mean ± S.E.M.; n = 5 for each genotype.
| Genotype | MAP (mm Hg) | HR (beat/min) |
|---|---|---|
| WT | 103 ± 1.6 | 598 ± 32 |
| Sp(−/−) | 101 ± 2.5 | 632 ± 12 |
Fig. 2.
Enhanced and prolonged hypotensive effects are observed in response to α2-agonists in Sp(−/−) mice. A, mean arterial pressure (MAP) was measured in WT and Sp(−/−) mice after injection of UK14,304 (0.1 mg/kg i.v.). B, change in MAP after bolus injection of UK14,304 at the indicated times in WT and Sp(−/−) mice. Values represent mean ± S.E.M. ∗, p < 0.05; ∗∗∗, p < 0.001, comparing Sp(−/−) with WT mice at the same time point. #, p < 0.01, comparing WT at 90 min with WT at 35 min. n = 5 for each genotype. C, change in MAP after bolus injection of clonidine (0.1 mg/kg) at the indicated times in WT and Sp(−/−) mice. Values represent mean ± S.E.M. ∗, p < 0.05; ∗∗, p < 0.01, comparing Sp(−/−) with WT mice at the same time point. #, p < 0.05, comparing WT at 60 min with WT at 20 min. n = 4 for WT and n = 3 for Sp(−/−).
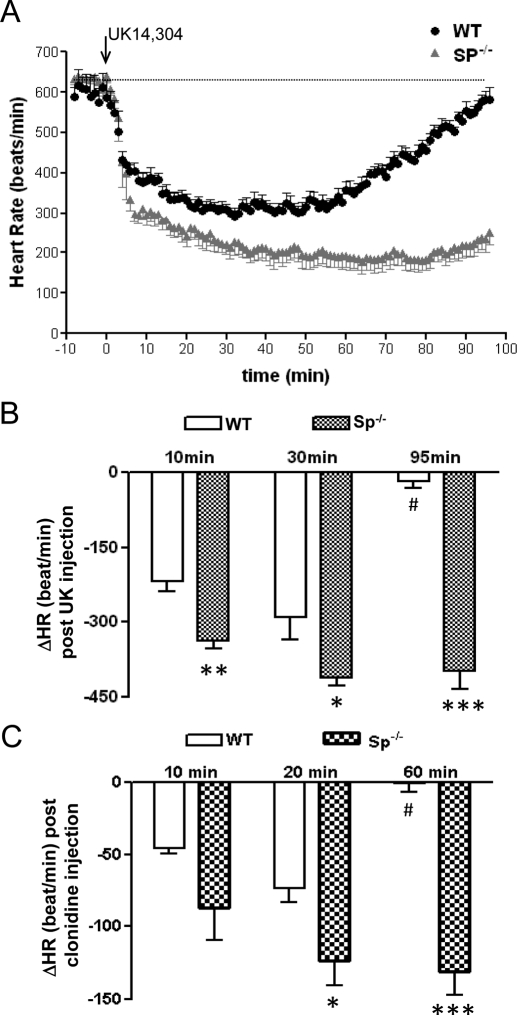
Changes in blood pressure in response to α2AR agonists are accompanied by reflexive changes in heart rate. Thus, we also observed that for both UK14,304 and clonidine, injection of the α2AR agonist induced a more dramatic and prolonged decrease in heart rate in Sp(−/−) mice than in WT mice (Fig. 3, A–C).
Fig. 3.
Increased and prolonged bradycardia are observed in response to the α2-agonist UK14,304 in Sp(−/−) mice. A, heart rate (HR) was evaluated in WT and Sp(−/−) mice after injection of UK14,304 (0.1 mg/kg i.v.) as described under Materials and Methods. B, change in HR after bolus injection of 0.1 mg/kg UK14,304 at the indicated times in WT and Sp(−/−) mice. Values represent mean ± S.E.M. ∗, p < 0.05; ∗∗∗, p < 0.001, comparing Sp(−/−) with WT mice at the same time point. #, p < 0.01, comparing WT at 90 min with WT at 35 min. n = 5 for each genotype. C, change in HR after bolus injection of clonidine (0.1 mg/kg) at the indicated times in WT and Sp(−/−) mice. Values represent mean ± S.E.M. ∗, p < 0.05; ∗∗∗, p < 0.001, comparing Sp(−/−) with WT mice at the same time point. #, p < 0.01, comparing WT at 60 min with WT at 20 min. n = 4 for WT and n = 3 for Sp(−/−).
Sedative-Hypnotic Effects in Response to α2-Agonists Were Enhanced in Mice Lacking Spinophilin.
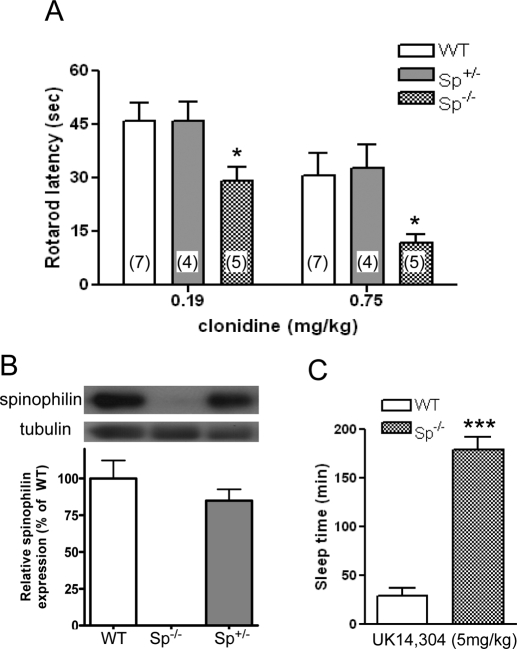
We have shown that Sp(−/−) mice are more sensitive to UK14,304-evoked sedation (Wang et al., 2004) than WT mice as measured by Rotarod latency. Here, we extended our study by testing clonidine, a partial α2AR-agonist, for its ability to induce sedation in WT, Sp(+/−), and Sp(−/−) mice. Because sedative effects in mice require high receptor occupancy (Tan et al., 2002), the partial agonist effects of clonidine are evident in examining sedation, whereas they are not when examining hypotensive responses. As shown in Fig. 4A, the same dose of clonidine induced a significantly stronger sedation (reflected by shorter Rotarod latency) in Sp(−/−) mice than in WT and Sp± mice. We failed to detect any difference in the ability of clonidine in inducing sedation in WT versus Sp± mice (Fig. 4A). Further analysis of the protein level of spinophilin in mice with different genotypes revealed no significant difference between WT and Sp± mice (Fig. 4B).
Fig. 4.
Enhanced sedative/hypnotic effects in response to α2AR stimulation in Sp(−/−) mice. A, hypnotic response was evaluated by Rotarod latency in mice with different genotypes in response to a bolus injection of 0.19 or 0.75 mg/kg clonidine. Values represent mean ± S.E.M. Numbers in parentheses represent the number of mice tested. ∗, p < 0.05. B, relative spinophilin protein content in these mice was detected by Western analysis of brain lysates from WT, Sp(+/−), and Sp(−/−) mice. Values represent mean ± S.E.M. n = 3 for each genotype. C, prolonged sleep-time in response to UK14,304 observed in mice lacking spinophilin expression. Sleep time, estimated by the duration of LORR, was measured for WT and Sp(−/−) mice after injection of 5 mg/kg i.p. UK14,304, as described under Materials and Methods. Values represent mean ± S.E.M. ∗∗∗, p < 0.001, n = 5 for each genotype.
We also examined the hypnotic response evoked by an α2-agonist in WT and Sp(−/−) mice by measuring the sleep time, or LORR, which is known to be mediated by the α2AAR subtype (Lakhlani et al., 1997). After systemic administration of UK14,304 at 5 mg/kg, a dose evoking maximal sedation in WT animals (Wang et al., 2004), both WT and Sp(−/−) mice developed LORR. However, the average sleep time for WT mice was 29 min, whereas the sleep time for Sp(−/−) mice was 179 min, more than 6 times longer (Fig. 4C). These data demonstrate that the absence of the ability of α2AAR to interact with spinophilin leads to a much more prolonged hypnotic response evoked by the α2AR agonists.
The α2AAR Had a Comparable Density in WT and Sp(−/−) Mice.
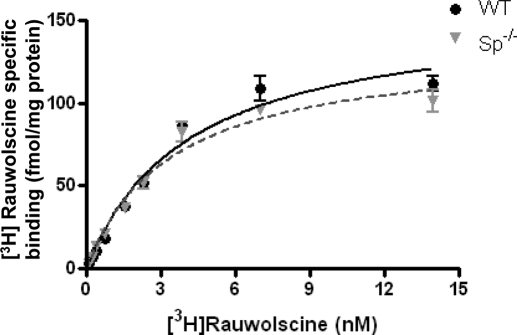
A potential explanation for changes in receptor sensitivity in WT versus Sp(−/−) mice could be that loss of spinophilin expression was accompanied by a change in receptor density, which can affect responsiveness to α2-agonists in vivo (Tan et al., 2002). Saturation binding analysis (Fig. 5) revealed that the density of α2AAR in WT and Sp(−/−) mice was indistinguishable, as was the Kd value for interaction with the radiolabeled α2AR antagonist, [3H] rauwolscine.
Fig. 5.
The density of α2AAR is indistinguishable in particulate brain preparations obtained from WT and Sp(−/−) mice. Saturation binding of [3H]rauwolscine was performed as described under Materials and Methods. Prazosin (1 μM) was added to the incubation to block any binding that would occur to either the α2B or α2CAR subtypes (MacMillan et al., 1996). Values represent mean ± S.E.M.; n = 3 for each genotype. The Bmax values predicted by nonlinear regression fit for α2AAR in WT and Sp(−/−) brain lysates are 156 ± 9.8 and 134 ± 7.7 fmol/mg protein, respectively.
In Sp(−/−) Mice, α2AAR Coupling to G Proteins Was Enhanced Although the Intrinsic Affinity of the α2AAR for Agonist Was Unaltered.
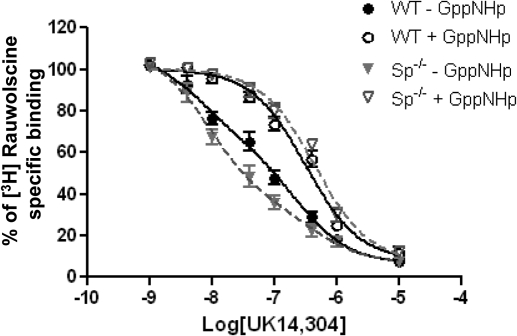
The binding affinity of receptor for an agonist is a critical determinant of the in vivo responsiveness to that agonist. In the framework of the ternary complex model and its extended version (De Lean et al., 1980; Samama et al., 1993; Weiss et al., 1996), receptor affinity for agonist agents is highly regulated by receptor-G protein coupling. Consequently, receptor interactions with agonist in the absence or presence of guanine nucleotides have been widely used as an indirect measure of receptor-G protein coupling efficiency (e.g., in Hausdorff et al., 1990; Kennedy and Limbird, 1993; Zhao et al., 1998; Piñeyro et al., 2005). As shown in Fig. 6 and summarized in Table 2, the ability of UK14,304 to compete for [3H]rauwolscine binding in the presence of Gpp(NH)p was indistinguishable in WT and Sp(−/−) mouse brain membrane preparations. Because Gpp(NH)p eliminates G protein regulation of receptor affinity, these data are interpreted to indicate that the intrinsic affinity of the receptor for this agonist ligand is indistinguishable in WT mice and in Sp(−/−) mice, which was expected, because the α2AAR gene product was not altered in Sp(−/−) mice. In addition, as expected, competition binding profiles obtained in the presence of Gpp(NH)p fit a one-site model, consistent with the absence of multiple affinity states in the presence of the GTP analog, Gpp(NH)p.
Fig. 6.
UK14,304 competition binding curves suggest enhanced coupling of α2AAR to G protein in brain preparations derived from Sp(−/−) mice. Competition binding assays were performed with brain particulate preparations derived from WT or Sp(−/−) mice in the presence or absence of Gpp(NH)p, as indicated. Binding of the [3H]rauwolscine radioligand is given as a percentage of binding without competitors. Curves without GppNHp were best fit via nonlinear regression analysis to two binding sites, whereas curves obtained in incubations performed in the presence of GppNHp were best fit by nonlinear regression analysis to one binding site (see Table 2 for estimates of Kd for each affinity state and percentage of receptors in the higher affinity state for these curves). Values represent mean ± S.E.M. n = 4 for each genotype.
TABLE 2.
Computer-assisted estimates of Kd values of α2AAR affinity states and percentage of α2AAR in the guanine-nucleotide sensitive higher affinity state
UK14,304 competition for [3H]rauwolscine binding was determined in well-washed particulate fractions, to eliminate endogenous guanine nucleotides, in the absence or presence of Gpp(NH)p, as outlined under Materials and Methods. Binding profiles in the absence of Gpp(NH)p were best fit by a two-site model, whereas those obtained in the presence of Gpp(NH)p fit a one-site model. The interaction of the two-state analysis, in terms of receptor interactions with G proteins, is provided in the text.
| Genotype | −Gpp(NH)p |
RH% | +Gpp(NH)p (−logKd) | |
|---|---|---|---|---|
| −logKH | −logKL | |||
| WT | 8.2 ± 0.3 | 6.6 ± 0.2 | 42 ± 8 | 6.5 ± 0.1 |
| Sp(−/−) | 8.1 ± 0.2 | 6.4 ± 0.3 | 72 ± 7* | 6.4 ± 0.1 |
RH% indicates the percentage of receptors at a higher affinity state of agonist.
P < 0.05.
In the absence of exogenously added guanine nucleotide, however, the agonist competition curves for both samples prepared from WT and Sp(−/−) mouse brains fit a two-site model (Fig. 6). The difference in complexity of these curves in the presence (discussed above) versus absence of guanine nucleotides was consistent with the interpretation that this complexity was due, at least in part, to changes in receptor affinity for agonist as a result of receptor interactions with G proteins. This interpretation was further corroborated by the finding that the Kd value for the “lower affinity state” estimated by computer-assisted analysis of these complex curves obtained in the absence of Gpp(NH)p was comparable with that observed for agonist interactions with the receptor when analyzed in the presence of Gpp(NH)p (Table 2). Computer-assisted analysis of these curves also indicated that there was a greater fraction of receptors interacting with G proteins (i.e., greater fraction manifesting a higher affinity state for agonist) in membrane preparations derived from the brain of Sp(−/−) versus WT mice (Table 2). The apparent leftward shift of the UK14,304 agonist competition curve in brain particulate preparations from Sp(−/−) mice was also consistent with the idea that a larger fraction of receptor interacts with G proteins and manifests higher affinity interactions with the agonist ligand than detected in preparations from WT mice (Fig. 6). Taken together, our binding data suggest that endogenous coupling of the α2AAR to G proteins occurred more frequently in mouse brain preparations from Sp(−/−) mice than from WT mice.
Discussion
The present results indicate that spinophilin expression in vivo influences the rate and extent of the α2AAR-mediated decreases in blood pressure, compensatory changes in heart rate, and increased hypnotic effects of two different α2-adrenergic agonists, UK14,304 and clonidine. These alterations in central nervous system responses to α2-adrenergic agonists occur in the absence of changes in α2AAR density and intrinsic affinity for agonist. However, our parallel in vitro studies with whole-brain lysates revealed an enhanced efficiency of α2AAR coupling to G proteins in the brain of Sp(−/−) mice compared with WT mice. The enhanced efficiency of α2AAR-G protein coupling in Sp(−/−) mice could thus explain the enhanced in vivo responses in these mice. Given the discrete distribution of spinophilin in particular brain regions, such as hippocampus and thalamus (Allen et al., 1997), the presence of spinophilin would induce an even more profound uncoupling between α2AAR and G proteins in these regions. Thus the effects on receptor-G protein coupling seen in our studies of whole-brain particulate preparations are probably an underestimate of the extent of endogenous modulation of this coupling in spinophilin-rich brain loci.
The impact of eliminating spinophilin in vivo to enhance receptor-G protein coupling and to sensitize in vivo signaling pathways, such as those that lead to reduced blood pressure, increased bradycardia, and hypnotic-sedative effects, may seem paradoxical, because arrestin interaction with the GRK-phosphorylated receptor leads to desensitization. Thus, one might have anticipated the opposite outcome, for example, in spinophilin-null mice [in which arrestin interaction with the α2AAR would go unopposed (Wang et al., 2004)] enhanced arrestin-mediated desensitization would occur and manifest as diminished sensitivity for agonist and a briefer duration of agonist-mediated effect, exactly the opposite of what was observed. However, it must be remembered that arrestin has multiple roles in the life cycle and signaling of GPCR. For example, arrestin serves as an adapter of GPCR to clathrin-coated pits thus fostering receptor-mediated internalization. In our previous studies, we also showed that α2AAR internalization is significantly accelerated in cells lacking spinophilin expression (Brady et al., 2003; Wang et al., 2004). Because arrestin-clathrin mediated endocytosis serves as a prelude for receptor recycling and replenishment of the surface receptor pool with “re-sensitized” receptors (Wang et al., 2004; Sorkin and vonZastrow, 2009), this role of arrestin could predominate in the systems that we examined in vivo and thus contribute to the enhanced sensitivity and longer duration of α2AAR-activated processes such as lowered blood pressure and bradycardia. Such a role for receptor endocytosis/recycling for signaling has been observed in other systems, as well. For example, clathrin-mediated internalization and receptor recycling has been shown to be essential for sustained EGFR signaling (Sigismund et al., 2008), and recycling of βAR is required for sustained cardiac responsiveness to catecholamine in vivo (Odley et al., 2004). Furthermore, the endosome can serve as a intracellular platform for assembly of signaling complex, either through receptor-associated scaffolding proteins such as arrestin (Violin and Lefkowitz, 2007) or by recruiting signaling molecules to endosomal resident proteins such as Rab5 (Zoncu et al., 2009). Thus, it is possible that yet-to-be confirmed intracellular arrestin-scaffolded α2AAR–provoked signaling pathways also contribute to the enhanced agonist-elicited responses that we evaluated in vivo. Finally, our finding that elimination of spinophilin also leads to enhanced coupled of α2AAR to G proteins may also contribute in a substantive way to the enhanced hypotensive, bradycardic, and sedative/hypnotic responses that we observed in spinophilin-null mice.
In addition to the sedative/hypnotic and hypotensive effects described here, the antinociceptive response evoked by the α2AAR is also enhanced in spinophilin-null mice (Charlton et al., 2008; Nag et al., 2009). Taken together, these studies suggest that functions of the α2AAR in the central and peripheral nervous system are modulated by spinophilin in native settings and are thus perturbed by changes in spinophilin expression in vivo. It is of interest to speculate about the impact of α2AAR-spinophilin interactions on G protein coupling and α2AAR signaling in neurons, because spinophilin is enriched in dendritic spines [the basis for its naming by the Greengard laboratory (Allen et al., 1997)]. This would mean that α2AAR interactions with G proteins (and also with arrestin, for which spinophilin is an endogenous antagonist) would vary considerably in the somatodendritic membrane compared with neuronal terminals.
In conclusion, this study demonstrates that elimination of spinophilin in transgenic mice leads to enhanced G protein coupling to the α2AAR, suggesting that peptidomimetic agents that selectively inhibit α2AAR-spinophilin interactions could enhance α2AAR sensitivity to agonists, allowing treatment of hypertension or modulation of pain perception with reduced doses of α2AR agonists that therefore do not evoke simultaneous sedative or hypnotic side effects.
Acknowledgments
We thank Dr. Paul Greengard (Rockefeller University, New York, NY) for generously providing the spinophilin KO mouse line for this study. We also thank Dr. Ashley Brady (Vanderbilt University) for critical reading of this manuscript.
This work was supported in part by the National Institutes of Health National Institute of Mental Health [Grant MH081917], by the American Heart Association [Grant 0630103N], and by the UAB Neuroscience Core Centers (funded by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS047466, NS057098]). Preliminary studies were funded by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK43879].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.065300.
- AR
- α2-adrenergic receptor
- GPCR
- G protein-coupled receptor
- UK14
- 304, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine
- WT
- wild type
- LORR
- loss of righting reflex
- Gpp(NH)p
- 5′-guanylimidodiphosphate.
References
- Allen PB, Ouimet CC, Greengard P. (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA 94:9956–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Bécamel C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P. (2004) GPCR-interacting proteins (GIPs): nature and functions. Biochem Soc Trans 32:851–855 [DOI] [PubMed] [Google Scholar]
- Brady AE, Wang Q, Colbran RJ, Allen PB, Greengard P, Limbird LE. (2003) Spinophilin stabilizes cell surface expression of alpha 2B-adrenergic receptors. J Biol Chem 278:32405–32412 [DOI] [PubMed] [Google Scholar]
- Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. (2008) Multiple actions of spinophilin regulate mu opioid receptor function. Neuron 58:238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lean A, Stadel JM, Lefkowitz RJ. (1980) A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem 255:7108–7117 [PubMed] [Google Scholar]
- DeGraff JL, Gurevich VV, Benovic JL. (2002) The third intracellular loop of alpha 2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem 277:43247–43252 [DOI] [PubMed] [Google Scholar]
- Desai AN, Salim S, Standifer KM, Eikenburg DC. (2006) Involvement of G protein-coupled receptor kinase (GRK) 3 and GRK2 in down-regulation of the alpha2b-adrenoceptor. J Pharmacol Exp Ther 317:1027–1035 [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. (2007) Beta-arrestins and cell signaling. Annu Rev Physiol 69:483–510 [DOI] [PubMed] [Google Scholar]
- Eason MG, Moreira SP, Liggett SB. (1995) Four consecutive serines in the third intracellular loop are the sites for beta-adrenergic receptor kinase-mediated phosphorylation and desensitization of the alpha 2A-adrenergic receptor. J Biol Chem 270:4681–4688 [DOI] [PubMed] [Google Scholar]
- Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P. (2000) Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97:9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. (2004) Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci 27:107–144 [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Hnatowich M, O'Dowd BF, Caron MG, Lefkowitz RJ. (1990) A mutation of the beta 2-adrenergic receptor impairs agonist activation of adenylyl cyclase without affecting high affinity agonist binding. Distinct molecular determinants of the receptor are involved in physical coupling to and functional activation of Gs. J Biol Chem 265:1388–1393 [PubMed] [Google Scholar]
- Hein L, Limbird LE, Eglen RM, Kobilka BK. (1999) Gene substitution/knockout to delineate the role of alpha 2-adrenoceptor subtypes in mediating central effects of catecholamines and imidazolines. Ann NY Acad Sci 881:265–271 [DOI] [PubMed] [Google Scholar]
- Jewell-Motz EA, Donnelly ET, Eason MG, Liggett SB. (1998) Agonist-mediated downregulation of G alpha i via the alpha 2-adrenergic receptor is targeted by receptor-Gi interaction and is independent of receptor signaling and regulation. Biochemistry 37:15720–15725 [DOI] [PubMed] [Google Scholar]
- Jewell-Motz EA, Liggett SB. (1996) G protein-coupled receptor kinase specificity for phosphorylation and desensitization of alpha2-adrenergic receptor subtypes. J Biol Chem 271:18082–18087 [DOI] [PubMed] [Google Scholar]
- Jones SB, Bylund DB. (1988) Characterization and possible mechanisms of alpha 2-adrenergic receptor-mediated sensitization of forskolin-stimulated cyclic AMP production in HT29 cells. J Biol Chem 263:14236–14244 [PubMed] [Google Scholar]
- Jones SB, Bylund DB. (1990) Effects of alpha 2-adrenergic agonist preincubation on subsequent forskolin-stimulated adenylate cyclase activity and [3H]forskolin binding in membranes from HT29 cells. Biochem Pharmacol 40:871–877 [DOI] [PubMed] [Google Scholar]
- Jones SB, Leone SL, Bylund DB. (1990) Desensitization of the alpha-2 adrenergic receptor in HT29 and opossum kidney cell lines. J Pharmacol Exp Ther 254:294–300 [PubMed] [Google Scholar]
- Jones SB, Toews ML, Turner JT, Bylund DB. (1987) Alpha 2-adrenergic receptor-mediated sensitization of forskolin-stimulated cyclic AMP production. Proc Natl Acad Sci USA 84:1294–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. (2000) In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J Pharmacol Exp Ther 293:1–7 [PubMed] [Google Scholar]
- Kenakin T. (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192 [DOI] [PubMed] [Google Scholar]
- Kennedy ME, Limbird LE. (1993) Mutations of the alpha 2A-adrenergic receptor that eliminate detectable palmitoylation do not perturb receptor-G-protein coupling. J Biol Chem 268:8003–8011 [PubMed] [Google Scholar]
- Kobilka B. (1992) Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci 15:87–114 [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. (1997) Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc Natl Acad Sci USA 94:9950–9955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Eason MG, Jewell-Motz EA, Williams MA, Theiss CT, Dorn GW, 2nd, Liggett SB. (1998) Phosphorylation and functional desensitization of the alpha2A-adrenergic receptor by protein kinase C. Mol Pharmacol 54:44–49 [DOI] [PubMed] [Google Scholar]
- Limbird LE. (1988) Receptors linked to inhibition of adenylate cyclase: additional signaling mechanisms. FASEB J 2:2686–2695 [DOI] [PubMed] [Google Scholar]
- Lu R, Li Y, Zhang Y, Chen Y, Shields AD, Winder DG, Angelotti T, Jiao K, Limbird LE, Zhou Y, et al. (2009) Epitope-tagged receptor knock-in mice reveal that differential desensitization of alpha2-adrenergic responses is because of ligand-selective internalization. J Biol Chem 284:13233–13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. (1997) Gene targeting–homing in on alpha 2-adrenoceptor-subtype function. Trends Pharmacol Sci 18:211–219 [DOI] [PubMed] [Google Scholar]
- MacMillan LB, Hein L, Smith MS, Piascik MT, Limbird LE. (1996) Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science 273:801–803 [DOI] [PubMed] [Google Scholar]
- Nag S, Wang Q, Limbird LE, Mokha SS. (2009) Knockout of spinophilin, an endogenous antagonist of arrestin-dependent alpha2-adrenoceptor functions, enhances receptor-mediated antinociception yet does not eliminate sex-related differences. Behav Brain Res 197:457–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW., 2nd (2004) Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci USA 101:7082–7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao CS, Benovic JL. (2005) Structure/function analysis of alpha2A-adrenergic receptor interaction with G protein-coupled receptor kinase 2. J Biol Chem 280:11052–11058 [DOI] [PubMed] [Google Scholar]
- Piñeyro G, Azzi M, deLéan A, Schiller PW, Bouvier M. (2005) Reciprocal regulation of agonist and inverse agonist signaling efficacy upon short-term treatment of the human delta-opioid receptor with an inverse agonist. Mol Pharmacol 67:336–348 [DOI] [PubMed] [Google Scholar]
- Rashid AJ, O'Dowd BF, George SR. (2004) Minireview: diversity and complexity of signaling through peptidergic G protein-coupled receptors. Endocrinology 145:2645–2652 [DOI] [PubMed] [Google Scholar]
- Reiter E, Lefkowitz RJ. (2006) GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab 17:159–165 [DOI] [PubMed] [Google Scholar]
- Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. (2001) Agonist-regulated interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem 276:15003–15008 [DOI] [PubMed] [Google Scholar]
- Richman JG, Regan JW. (1998) Alpha 2-adrenergic receptors increase cell migration and decrease F-actin labeling in rat aortic smooth muscle cells. Am J Physiol 274:C654–C662 [DOI] [PubMed] [Google Scholar]
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. (1993) A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem 268:4625–4636 [PubMed] [Google Scholar]
- Sato M, Blumer JB, Simon V, Lanier SM. (2006) Accessory proteins for G proteins: partners in signaling. Annu Rev Pharmacol Toxicol 46:151–187 [DOI] [PubMed] [Google Scholar]
- Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, et al. (1998) Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem 273:3470–3475 [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. (2008) Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell 15:209–219 [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. (2009) Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 10:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved AF, Tsukamoto K, Schreihofer AM. (1992) Stimulation of alpha 2-adrenergic receptors in nucleus tractus solitarius is required for the baroreceptor reflex. Brain Res 576:297–303 [DOI] [PubMed] [Google Scholar]
- Tan CM, Wilson MH, MacMillan LB, Kobilka BK, Limbird LE. (2002) Heterozygous alpha 2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci USA 99:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilakaratne N, Sexton PM. (2005) G-protein-coupled receptor-protein interactions: basis for new concepts on receptor structure and function. Clin Exp Pharmacol Physiol 32:979–987 [DOI] [PubMed] [Google Scholar]
- Violin JD, Lefkowitz RJ. (2007) Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci 28:416–422 [DOI] [PubMed] [Google Scholar]
- Wang Q, Limbird LE. (2002) Regulated interactions of the alpha 2A adrenergic receptor with spinophilin, 14-3-3zeta, and arrestin 3. J Biol Chem 277:50589–50596 [DOI] [PubMed] [Google Scholar]
- Wang Q, Limbird LE. (2007) Regulation of alpha2AR trafficking and signaling by interacting proteins. Biochem Pharmacol 73:1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. (2004) Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science 304:1940–1944 [DOI] [PubMed] [Google Scholar]
- Weiss JM, Morgan PH, Lutz MW, Kenakin TP. (1996) The cubic ternary complex receptor-occupancy model. III. Resurrecting efficacy. J Theor Biol 181:381–397 [DOI] [PubMed] [Google Scholar]
- Wu G, Krupnick JG, Benovic JL, Lanier SM. (1997) Interaction of arrestins with intracellular domains of muscarinic and alpha2-adrenergic receptors. J Biol Chem 272:17836–17842 [DOI] [PubMed] [Google Scholar]
- Zhao MM, Gaivin RJ, Perez DM. (1998) The third extracellular loop of the beta2-adrenergic receptor can modulate receptor/G protein affinity. Mol Pharmacol 53:524–529 [DOI] [PubMed] [Google Scholar]
- Zoncu R, Perera RM, Balkin DM, Pirruccello M, Toomre D, De Camilli P. (2009) A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136:1110–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]