Abstract
A study of the effect of aggregate size on the resuscitation of dormant M. smegmatis was conducted by constructing cell aggregates with defined sizes and shapes using dielectrophoresis and monitoring the resuscitation process under controlled laboratorial conditions in a long-term cell feeding system. Differently sized cell aggregates were created on the surface of indium tin oxide coated microelectrodes, their heights and shapes controlled by the strength of the induced electric field and the shape of the microelectrodes. Both two-dimensional (ring-patterned) and three-dimensional cell aggregates were produced. The cell aggregates were maintained under sterile conditions at 37 °C for up to 14 days by continuously flushing Sauton’s medium through the chamber. Resuscitation of dormant M. smegmatis was evaluated by the production of the fluorescent dye 5-cyano-2,3-ditolyltetrazolium chloride. The results confirm that the resuscitation of dormant M. smegmatis is triggered by the accumulation of a resuscitation promoting factor inside the aggregates by diffusion limitation.
INTRODUCTION
Tuberculosis (TB) is caused by the pathogenic, slowly growing non-spore-forming bacterium Mycobacterium tuberculosis (M. tuberculosis), which has been identified and described for more than 200 years.1 TB is still a leading infectious disease, which has killed more people in the world than any other single bacterial infection today.2, 3 The TB bacterium in its dormant form, responsible for latent TB, can be blamed as it causes great difficulties in diagnosis and subsequently achieving successful antibiotic treatment of the infection.
Dormancy is a reversible state of low metabolic activity, which some bacteria use to survive long periods of environmental stress. In nonsporulating bacteria, dormancy is often accompanied by the development of a “nonculturable” (NC) phenotype characterized by the inability of the dormant bacteria to produce colonies on solid media.4 To better understand the mechanisms by which mycobacteria control the use of dormancy in order to survive environmental stresses, the NC state of Mycobacterium smegmatis (M. smegmatis) is often investigated as a model system. M. smegmatis is a fast growing nonpathogenic relative of M. tuberculosis and has been used extensively as a safer and faster growing alternative to M. tuberculosis in the study of the dormant NC state of mycobacteria.5, 6
When old stationary phase batch cultures of M. smegmatis are stored in an appropriate medium, the number of culturable cells declines from 106 cells ml−1 to less than 10 cells ml−1, as assessed by the Colony Forming Unit number within 72 h post inoculation,6 indicating the formation of dormant (NC) cells. The formation of NC M. smegmatis cells is accompanied by the accumulation of an inhibitory substance in the growth medium. Cocultivation with active Micrococcus luteus (M. luteus) cells or inoculation of M. smegmatis in supernatant of actively growing M. luteus can trigger the resuscitation of NC M. smegmatis.6 The “resuscitation promoting factor” (RPF) that is involved has been successfully isolated from supernatant of M. luteus and characterized by Mukamolova et al.7 RPFs have been found to be a family of proteins whose homologous genes are widespread among the guanine-cytosine (GC)-rich Gram-positive bacteria, including streptomycetes, corynebacteria, and mycobacteria.8 They stimulate cell growth and cell multiplication at very low concentrations and are active toward different strains of bacteria.7 The analysis of the structure of the RPFs has revealed that they may be peptidoglycan hydrolases and may actively participate in the modification of the thickened cell wall of the NC cells during resuscitation.9, 10 Such a mechanism has been found to be more favorable if there is physical contact between the cells involved,10 which may well play a role in granuloma formation by M. tuberculosis in the body. The recent discovery of the ability of M. tuberculosis cells to produce biofilms with drug-tolerant bacteria inside is a further indication that cell-cell contacts may be a very important aspect of TB pathogenesis and anti-TB chemotherapy.11
To date, most in vitro experiments on bacterial cell dormancy have been done on suspensions, in which the cells are present as single cells or very small aggregates. This is unrepresentative of the cells’ natural state, as most cells in nature live in an aggregated state. Aggregation has a strong influence on a cell’s physiological state as it influences the transport of nutrients toward the cells and the removal of products. It has been experimentally proven12 that the formation of bacterial microaggregates in the lag phase is crucial for the initiation of multiplication of cells under conditions inappropriate for growth. Microcryptic growth of cells and accumulation of nutrients within aggregates has been postulated to play a role.12 Similarly, organization of bacterial cells in microcolonies could be an important factor for efficient intercellular communications mediated by low molecular weight autoinducers.13 Signaling molecules, such as the RPFs, can be expected to accumulate to higher concentrations within multicellular aggregates in a similar way. As RPF induces the resuscitation of dormant cells, it can therefore be expected that the resuscitation of dormant cells in larger aggregates will be quicker.
Of the methods available for the reproducible construction of microbial aggregates with a defined size, the induced movement of polarizable particles in nonuniform electric fields, dielectrophoresis (DEP) is one of the most useful.14 The formation of cell aggregates with DEP, the movement of particles in nonuniform electric fields,15 involves suspension of the cells in a low conductivity medium, and the attraction of cells to (or repulsion from, depending on the frequency of the electric field and the medium conductivity) high electric field regions between microelectrodes.16 This method can be used to create aggregates of different shapes and sizes,17, 18 with different cell types in different positions. Studies with bacteria have included investigations of the exchange of metabolites and bacterial signaling molecules, such as N-acyl homoserine lactone (AHL).19, 20, 21 Here, we will discuss its use in the study of bacterial dormancy.
MATERIALS AND METHODS
Organism and media
Mycobacterium smegmatis strain mc2155 (M. smegmatis w-t) and its derivative harboring the plasmid pAGR carrying the RPF gene [M. smegmatis (pAGR)] (Ref. 6) were maintained, precultured, and subcultured as described previously,22 except the antibiotic Hygromycin B was added at a final concentration of 50 μg ml−1 in nutrient agar (NA), the nutrient broth E, and modified Hartman’s-de Bont medium as supplement for M. smegmatis (pAGR) strain. Dormant M. smegmatis w-t and M. smegmatis (pAGR) cells were resuscitated in Sauton’s medium at 37 °C. Per liter Sauton’s medium contains 0.5 g KH2PO4, 1.4 g MgSO4⋅7H2O, 4 g L-asparagine, 60 ml glycerol, 0.05 g ferric-ammonium citrate, 2.0 g sodium citrate, and 100 μl of 1% ZnSO4 solution.
Micrococcus luteus (M. luteus) was maintained on NA at 5–7 °C. Active cultures were obtained by growing the cells in nutrient broth (Oxoid Ltd., UK) at 30 °C in an orbital incubator shaker for 24 h.
Construction of the chambers
Microelectrodes were made from indium tin oxide (ITO) covered glass microscope slides (Delta Technologies, USA) using photolithography as described previously.23 ITO is conductive, transparent, and does not fluoresce, facilitating observation of the aggregates with a fluorescence microscope. Two different designs were used.
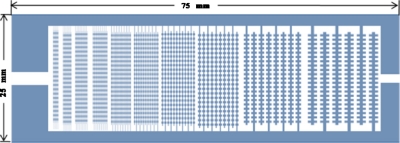
The “all-in-one” (AIO) microelectrode was based on a design by Venkatesh and Markx,17 and is shown in Fig. 1. The electrode design has ten regions with interdigitated oppositely castellated electrodes with characteristic dimensions of 25, 50, 75, 100, 125, 150, 175, 200, 225, and 250 μm. The AIO electrode design allows one to produce three-dimensional (3D) aggregates of different sizes in a single chamber and expose them to the same environmental conditions.
Figure 1.
Diagram of AIO interdigitated castellated electrodes with characteristic dimensions from 25 to 250 μm.
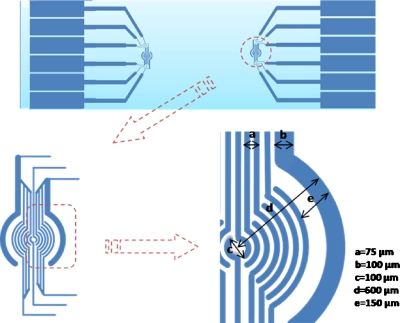
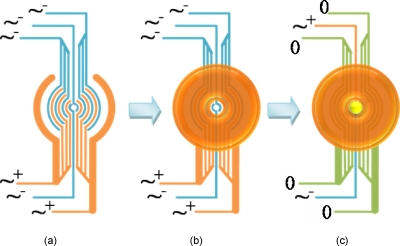
A “ring-patterned” electrode was used to construct two-dimensional (2D) layers with different micro-organisms in different positions on the glass surface, as shown in Fig. 2. The main body of this electrode pattern consisted of 16 half radial rings, which could each be addressed individually. The electrodes were wired up in two clusters, i.e., the core and the periphery. The core consisted of two half rings with a diameter of 100 μm and an electrode width of 25 μm. The periphery consisted of 14 half rings; the diameters of the rings were in the range of 200–1200 μm, the 12 inner electrodes had a width of 25 μm, and the outermost electrode a width of 75 μm.
Figure 2.
Design of the ring-patterned microelectrode for the construction of 2D aggregates.
Feeding chambers, as shown in Fig. 3, were constructed on the top of ITO microelectrodes of both designs using self-adhesive plastic covers from Sigma-Aldrich, UK (Hybri-well Press-seal hybridization chambers of 21×41×0.15 or 21×41×0.25 mm3). The chambers had a height of 150–300 μm, using layers of insulating tape underneath the self-adhesive plastic cover to vary the chamber height. Electric connections were made using standard friction locker heads with extended metal fingers (RS components, U.K.). Peeled leads and individual metal fingers from the friction heads were held together by silicon tubes with a diameter of 0.8 mm (Tygon). The silicon tube also isolated each metal finger from each other to avoid accidental electrical shortcuts. By doing this, it was possible to make up to six connections to individual wires within a 1.8 cm space. A multiswitch was used to apply signals to the microelectrodes. Electrical signals were generated using TG120 Thurlby-Thandar signal generators. Inlet and outlet points were formed with two short 1 mm syringe needles; the whole chamber was sealed with two component fixer (RS). A disposable intravenous cannula was fixed close to the end of the inlet syringe needle, which created an injection port with a no-return valve for the addition of chemicals. The chambers were sterilized by pumping sodium hypochlorite solution through the system for at least 6 h. Sterilized de-ionized water was then pumped through the chamber for another 6–10 h to remove the sodium hypochlorite.
Figure 3.
Closed feeding chamber built on the top of an AIO microelectrode fitted with an injection port.
Construction of 3D aggregates
In the case of dormant M. smegmatis, any remaining active M. smegmatis would give a false positive result. Although there was no evidence of active cells being present in the dormant cells suspension was put through a separation process in which, as a precaution, before the resuscitation experiment was commenced, the dormant cell suspension was set through a separation process, in which any remaining active M. smegmatis cells would be removed. The method is described in more detail elsewhere.22 In short, two dielectrophoretic separation chambers with interdigitated, oppositely castellated electrodes with a characteristic size of 30 μm were connected in series, and an electric field was generated between the electrodes by applying a signal of 120 kHz, 20 Vp.p.. The cells, suspended in a 0.5M sorbitol solution with a conductivity of 2 μS cm−1, were made to flow through the chamber. Under the conditions given any remaining active cells would be strongly attracted to high field regions on the surface of the electrodes and be kept there. The dormant cells were less strongly attracted by the electric field and were carried away by the medium flow, from which they could be collected.
Samples of dormant M. smegmatis (pAGR), a mixture of dormant M. smegmatis w-t and active M. luteus (50:1), active M. luteus, and dormant M. smegmatis w-t cell were prepared, washed, and resuspended in 0.5M sorbitol solution with 0.05% Tween-80. The cell suspensions were pumped into a previously sterilized AIO, feeding chambers with a flow rate of around 10 μl min−1. An electric field was generated between the electrodes by applying a 1 MHz, 20 Vp.p. signal for at least 60 min until all of the microelectrodes arrays were saturated with cells. Cells, which were not attracted onto the surface of electrodes, were removed from the chambers by a flow of 0.5M sorbitol with a flow rate at 10 μl min−1. 200 μl 0.0025% W∕V polyethylenimine (PEI) solution was then pumped into the chambers at a flow rate of 10 μl min−1 to immobilize the cells.24 The PEI solution was kept in the chambers for 30 min and then the PEI was rinsed away with a gentle flow of 0.5M sorbitol. The electric field was removed when all of the cells were fully immobilized. Each experiment was done in quadruplicate.
Characterization of aggregate size and shape
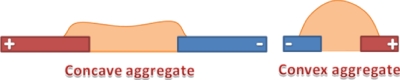
To characterize the size and shape of the 3D cell aggregates, a suspension of M. smegmatis w-t active cells was prepared, washed several times, and resuspended in 0.5M sorbitol solution with 0.05% Tween-80. The cell suspension was then pumped into an AIO chamber with a chamber height of 300 μm using a peristaltic pump with a flow rate of 5 μl min−1, the electrodes were energized, and the flow continued for at least 45 min until a matrix of uniform cell aggregates was formed at the electrodes. The shape and height of randomly picked aggregates at all ten electrode regions were then measured microscopically by working out the difference between the focal point at the edge of the aggregate and at different points on the aggregate surface. At least ten aggregates were chosen from each region of the electrode arrays and the average heights of aggregates and the standard deviation were plotted against the characteristic sizes of the electrodes in each region at different electric field strengths. Aggregates were classified18 as concave or convex on the basis of the ratios of the height of the aggregate at the center of the aggregate relative to that at the electrode edges (see Fig. 4).
Figure 4.
Illustration of the different aggregate shapes formed. A concave aggregate would have a ratio of aggregate height at the edge of the electrode to that at the center that is larger than 1.
Construction of 2D cell aggregates
To make 2D aggregates, washed 72-h-old dormant M. smegmatis w-t cells were first introduced into a chamber with ring-patterned electrodes. All of the microelectrodes were energized with a 1 MHz, 20 Vp.p. electric field, as shown in Fig. 5a. A cell aggregate in the shape of a ring was formed in the peripheral region, as shown in Fig. 5b, leaving the center core region empty. Cell suspension was pumped into the chamber with a flow rate of 25 μl min−1; under these conditions, it was possible to generate very thin layers, which were only one or two cell layers thick. 0.0025% (W∕V) PEI was then introduced into the chamber to immobilize the dormant M. smegmatis w-t cells (30 min); any nonadherent cells and superfluous PEI were washed away with some 0.5M sorbitol solution. The second cell suspension, active M. luteus, was then pumped into the chamber with the center electrode energized, as shown in Fig. 5c. As only the core electrodes were energized, the M. luteus cells formed a single layer which filled the center of electrode array (and its connecting wire), shown in bright yellow. A gentle medium flow was used to remove the nonattracted cells. M. luteus cells were then immobilized (0.0025% W∕V PEI solution for 30 min) before the electric field was removed from the core electrodes. Two identical ring-patterned microelectrodes were fabricated on each slide. As each experiment was done in triplicate, six ring-patterned cell aggregates were made in total.
Figure 5.
Procedures used for patterning and immobilizing two strains of microorganisms in a 2D pattern. (a) Energized electrodes before introduction of first cell type. (b) Cells are attracted to high field regions and accumulate in outer rings after their introduction; cells are then immobilized. (c) On energizing the two inner electrodes and grounding the outer electrodes, cells of the second cell type preferentially accumulate at the center; cells are then immobilized.
Resuscitation
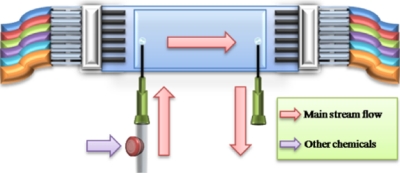
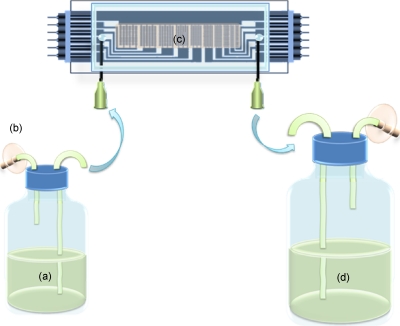
To study the resuscitation of dormant cells, a system was used as in Fig. 6. Feed and waste collection tanks were made with from universal glass bottles. Air filters and liquid transfer tubes were fitted onto the screw lids of the universal glass bottles and sealed with silicon rubber to keep the medium fully sterilized and avoid cross contamination during the resuscitation. The resuscitation of dormant M. smegmatis cells was carried out at 37 °C. Sauton’s medium was pumped continuously at a rate of 50 μl min−1 through the chamber for up to 15 days. The activity of the cells in the aggregates was checked every 24 h by measuring the conversion of 5-cyano-2,3-ditolyltetrazolium chloride (CTC) into fluorescent formazan. To do this, the chambers were temporarily removed from the feeding system, and 1 ml of 4 mM CTC solution in 1M glucose was added to the chamber using a 1 ml syringe. After the injection, the inlet and outlet points of each chamber were joined by a piece of shorted silicon tube to seal the CTC solution inside the chamber, and the chambers were then put back into the incubator for 90 min. Fluorescence in the chambers was observed using a Nikon eclipse E600, UK, epifluorescence microscope fitted with a Nikon Coolpix 4500 digital camera. Images were analyzed using the image analysis package SIGMASCAN PRO 5. After pictures were taken, the chambers were reconnected into the feeding system and fresh Sauton’s medium was pumped through.
Figure 6.
Diagram of the system used for studying the resuscitation of dormant M. smegmatis. Fresh Sauton’s medium was pumped into the chamber at a flow rate of 50 μl min−1 for 8–12 days; the chamber was kept at 37 °C and resuscitation was checked daily. (a) Feed tank with Sauton’s medium; (b) air filter; (c) microelectrode chamber with cell aggregates; (d) waste collection.
RESULTS
Formation of aggregates at differently sized castellated microelectrodes
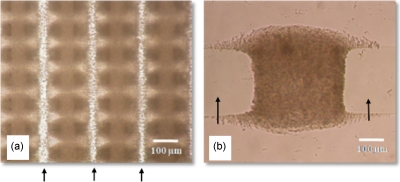
To study the formation of aggregates, washed M. smegmatis w-t cell suspension was pumped into an AIO chamber with a peristaltic pump with a flow rate of 5 μl min−1 and attracted to the electrodes using a 1 MHz signal and different voltages. Typical aggregates are shown in Fig. 7.
Figure 7.
Aggregates of M. smegmatis w-t cells in an AIO chamber with electrodes with characteristic sizes ranging from 25 to 250 μm. The applied signal was 1 MHz, 20 Vp.p.. Dark areas are formed by cells; the arrows point at the ITO electrodes.
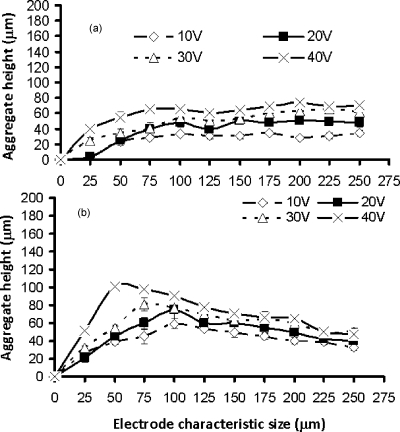
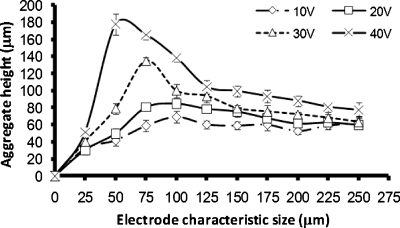
The aggregation process is complex,17, 18 and many factors, such as the electrode size and geometry, the medium conductivity, the flow rate, the chamber dimensions, the parameters of the preset electric field, the cell type, and their physiological states can have a significant impact on the aggregate height and shape formed.17, 18 Aggregate formation started at the highest electric field regions at the edges of the electrodes. Gradually, as more and more cells were attracted, the spaces in between two opposite castellations were filled, often forming “pearl chains” of cells aligned with the field lines. In the smaller electrode regions, the aggregates were formed very quickly, while it took a longer time at the larger electrode regions as the electric fields were smaller. After the cells had fully bridged the gaps in between the fingers, the cells started to pack on the top of each other. With the extended aggregation time, the aggregates formed at the smaller electrodes and higher voltages started to merge, forming first lines and then large and continuous biofilms. At the larger electrodes, the individual aggregates remained separate; see also Fig. 8. The shape of the aggregates at all electrode sizes was initially concave (“saddle shaped”), but soon became convex at the smaller electrodes and at higher voltages as more cells accumulated in the aggregate; at the larger electrodes and lower voltages, this process took much longer. After 45 min, the aggregate height at the edge of microelectrodes became relatively constant [see Fig. 9a]. The height in the center of the aggregates kept increasing over a period of up to 6 h [compare Figs. 9b, 10]. After 6 h of aggregation, all aggregates had become convex.
Figure 8.
Close-up of aggregates of active M. smegmatis w-t cells. The applied signal was 1 MHz, 20 Vp.p..; aggregates are shown 30 min after the start of the aggregation process. Darker areas indicate the presence of cells; the arrows point at the ITO electrodes. (a) Typical biofilm with overlapping small aggregates in the 50 μm region. (b) Single aggregate formed in the 250 μm region.
Figure 9.
The height of M. smegmatis w-t aggregates at different characteristic sizes of the microelectrodes. The applied electric signal was 1 MHz, 20 Vp.p.. (a) Height at the edges of the castellations, 45 min after the start of the aggregation process; (b) height at the center of the aggregates, 45 min after the start of the aggregation process.
Figure 10.
The height of M. smegmatis w-t aggregates at different characteristic sizes of the microelectrodes at the center of the aggregates, 6 h after the start of the aggregation process. The applied electric signal was 1 MHz, 20Vp.p..
Resuscitation in 3D aggregates
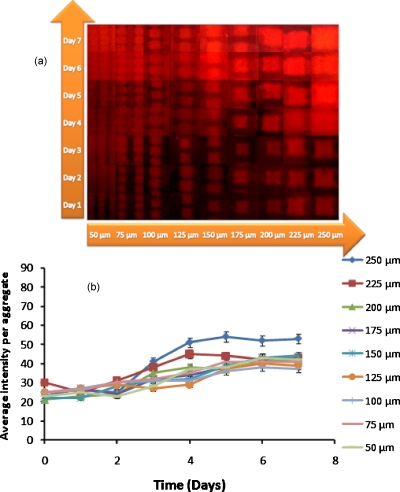
NC cells of M. smegmatis w-t cannot be resuscitated in liquid medium until exogeneous RPF is added. However, NC cells of M. smegmatis harboring a plasmid with RPF gene (M. smegmatis pAGR) are able to spontaneously resuscitate in fresh Sauton’s medium.6 Samples of dormant M. smegmatis w-t, active M. luteus, M. smegmatis (pAGR) and a 50:1 mixture of dormant M. smegmatis w-t and active M. luteus were prepared in 0.5M sorbitol. Aggregates of different sizes in the range of 50–250 μm were formed with DEP, and after their immobilization, the cells were kept at 37 °C, while fresh Sauton’s medium was continuously pumped through. Resuscitation was measured by monitoring the formation of formazan. Results are shown in Figs. 111213.
Figure 11.
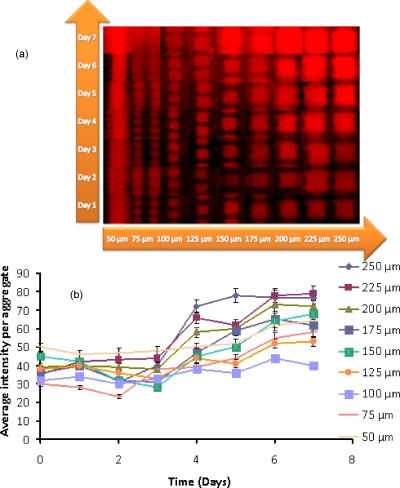
Resuscitation of dormant M. smegmatis (pAGR). (a) Composite picture showing fluorescent images of formazan formation; (b) average fluorescence intensity per aggregate as a function of incubation time.
Figure 12.
Resuscitation of a 50:1 mixture of dormant M. smegmatis w-t and active M. luteus. (a) Composite picture showing fluorescent images of the formazan formation; (b) average fluorescence intensity per aggregate as a function of the incubation time.
Figure 13.
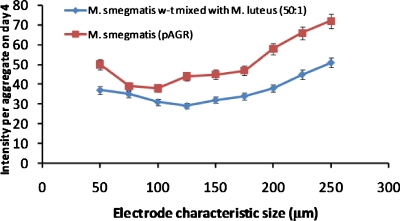
Average fluorescence intensity per aggregate at day 4 of dormant M. smegmatis (pAGR) and of a 50:1 mixture of dormant M. smegmatis w-t mixed with active M. luteus as a function of aggregate size.
Cell activity was first observed in the largest aggregates (made with the 250 and 225 μm electrodes) containing dormant M. smegmatis (pAGR), or a mixture of dormant M. smegmatis w-t and active M. luteus, on the fourth day of the incubation. This time period is similar to that for resuscitation in multiwell plates.6 With increasing incubation time, the smaller sized aggregates gradually became fluorescent as well. It took around 7 days for all of the aggregates of dormant M. smegmatis (pAGR) and of the mixture of dormant M. smegmatis w-t and active M. luteus cells to become fluorescent; the intensity then remained constant up to 12 days. Aggregates made with only dormant M. smegmatis w-t did not become fluorescent even after 15 days of incubation, while aggregates made with only active M. luteus expressed only very weak fluorescence throughout the whole feeding period.
It can be noticed in Fig. 13 that dormant M. smegmatis (pAGR) expressed a stronger fluorescent signal than the mixed dormant M. smegmatis w-t and M. luteus samples throughout the whole of the feeding period. A possible explanation for this is that the number of active M. luteus cells, which secrete RPF, and therefore, initiate the resuscitation process, did not increase during the experiment because M. luteus grows poorly on Sauton’s medium. RPF levels in mixed M. smegmatis w-t and M. luteus aggregates are therefore relatively constant. M. smegmatis (pAGR) cells, on the other hand, can secrete RPF themselves. Thus, once the resuscitation has started, the increased activity of M. smegmatis (pAGR) leads to higher RPF levels, which in turn accelerates the resuscitation process.
Resuscitation in 2D aggregates
The previous experiments suggested that the accumulation of RPF by diffusion limitation plays a role in the resuscitation of cells in aggregates. However, larger aggregates also contain more cells and it was, therefore, necessary to show that it was not the cell number in the larger aggregates that caused an increase in the resuscitation but the accumulation of a resuscitation agent inside the aggregate by diffusion limitation. For this purpose, a ring system was devised which could be used to create what are essentially 2D aggregates on a surface. All cells in the 2D aggregates are exposed to similar conditions as the cells in the 3D aggregates (including a similar exposure to DEP, immobilization procedure, and culturing conditions). However, diffusion limitation is unlikely to occur in the 2D aggregates.
To create 2D aggregates, a ring-based system was developed, as shown in Fig. 2. Dormant M. smegmatis w-t cells were first made to form aggregates on the outer rings; this cell suspension was pumped into the chamber with a high flow rate of 25 μl min−1 to generate thin aggregates, only a few cell layers thick. After the cells had been immobilized with PEI, the second cell suspension, active M. luteus, was pumped into the chamber to form an aggregate in the center of the structure. The M. luteus cells were then also immobilized with PEI before the electric field was removed. An example of a finished 2D aggregate is shown in Fig. 14. Measurement of the height of the cell aggregations formed in the 2D system showed that the average height for the dormant M. smegmatis w-t aggregated in the outer rings was around 10 μm while the aggregate of active M. luteus was around 50–60 μm high.
Figure 14.
Bright field images taken during the construction 2D aggregates consisting of dormant M. smegmatis w-t and active M. luteus cells. (a) Dormant M. smegmatis were immobilized in the outer rings; the center of the structure was left empty; (b) same system after immobilization of M. luteus in the center.
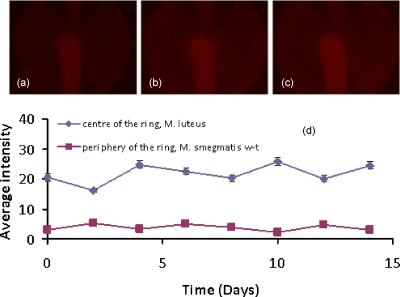
The cells in the 2D aggregates were fed a constant flow of fresh Sauton’s medium for 15 days. Despite this, as shown in Fig. 15, the dormant M. smegmatis w-t cells could not be resuscitated by coculture with M. luteus cells in the 2D system, as no cell growth or activity occurred during the period of observation.
Figure 15.
Assessment of the resuscitation of M. smegmatis over time in a 2D system with no diffusion limitation. (a) Fluorescence image of system after the first day, (b) fourth day, and (c) fifteenth day. (d) Average intensity for the two different regions in the aggregate as a function of the incubation time.
CONCLUSIONS
It has been shown, by forming 2D and 3D bacterial cell aggregates of different sizes, that the accumulation by diffusion limitation of RPFs plays a role in the resuscitation of M. smegmatis. The formation of larger 3D aggregates, in which diffusion limitation of mass transfer to the outside would be stronger, led to earlier resuscitation of the cells in the aggregates. Control experiments were performed with 2D aggregates, in which a similar number of cells were exposed to similar experimental procedures and conditions. No resuscitation was observed in these flat (2D) aggregates. In these aggregates little diffusion limitation can be expected to occur, and little RPF to accumulate.
The results highlight the important roles multicellularity and intercellular communication play in the resuscitation and subsequent growth of NC cells. The similarity of M. smegmatis to M. tuberculosis suggests that a similar accumulation of RPF may occur in aggregates of M. tuberculosisin vivo. Further experiments will be needed to confirm this.
ACKNOWLEDGMENTS
We wish to thank the School of Chemical Engineering and Analytical Science for a scholarship for K.Z., and NATO for funds for exchange visits. We also wish to thank Dr. Hatfield and Mr. M. McGowan of the School of Electronic and Electrical Engineering of the University of Manchester for the use of the clean rooms for electrode manufacture. We wish to thank Dr. Mike Winson, Aberystwyth, for helpful discussions.
References
- McCarthy O. R., J. R. Soc. Med. 94, 413 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. R. and Musser J. M., Trends Microbiol. 9, 547 (2001). 10.1016/S0966-842X(01)02228-4 [DOI] [PubMed] [Google Scholar]
- Kochi A., Tubercle 72, 1 (1991). 10.1016/0041-3879(91)90017-M [DOI] [PubMed] [Google Scholar]
- Young M., Mukamolova G. V., and Kaprelyants A. S., in Mycobacterial Molecular Microbiology, edited by Parish T. (Horizon Scientific, Norfolk, UK, 2005), Chap. 8. [Google Scholar]
- Shires K. and Steyn L., Mol. Microbiol. 39, 994 (2001). 10.1046/j.1365-2958.2001.02291.x [DOI] [PubMed] [Google Scholar]
- Shleeva M. O., Mukamolova V. G., Young M., Williams D. H., and Kaprelyants S. A., Microbiology 150, 1687 (2004). 10.1099/mic.0.26893-0 [DOI] [PubMed] [Google Scholar]
- Mukamolova G. V., Turapov O. A., Young D. I., Kaprelyants A. S., Kell D. B., and Young M., Mol. Microbiol. 46, 623 (2002). 10.1046/j.1365-2958.2002.03184.x [DOI] [PubMed] [Google Scholar]
- Mukamolova G. V., Yanopolskaya N. D., Kell D. B., and Kaprelyants A. S., Antonie van Leeuwenhoek 73, 237 (1998). 10.1023/A:1000881918216 [DOI] [PubMed] [Google Scholar]
- Cohen-Gonsaud M., Barthe P., Bagneris C., Henderson B., Ward J., Roumestand C., and Keep N. H., Nat. Struct. Mol. Biol. 12, 270 (2005). 10.1038/nsmb905 [DOI] [PubMed] [Google Scholar]
- Mukamolova G. V., Murzin A. G., Salina E. G., Demina G. R., Kell D. B., Kaprelyants A. S., and Young M., Mol. Microbiol. 59, 84 (2006). 10.1111/j.1365-2958.2005.04930.x [DOI] [PubMed] [Google Scholar]
- Ojha A. K., Baughn A. D., Sambandan D., Hsu T., Trivelli X., Guerardel Y., Alahari A., Kremer L., Jacobs W. R., and Hatfull G. F., Mol. Microbiol. 69, 164 (2008). 10.1111/j.1365-2958.2008.06274.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloshin S. A., Shleeva M. O., Syroeshkin A. V., and Kapreliants A. S., Mikrobiologiia 74, 489 (2005). [PubMed] [Google Scholar]
- Hense B. A., Kuttler C., Müller J., Rothballer M., Hartmann A., and Kreft J. U., Nat. Rev. Microbiol. 5, 230 (2007). 10.1038/nrmicro1600 [DOI] [PubMed] [Google Scholar]
- Markx G. H., Andrews J. S., and Mason V. P., Trends Biotechnol. 22, 417 (2004). 10.1016/j.tibtech.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Alp B., Stephens G. M., and Markx G. H., Enzyme Microb. Technol. 31, 35 (2002). 10.1016/S0141-0229(02)00071-6 [DOI] [Google Scholar]
- Pohl H. A., Dielectrophoresis (Cambridge University Press, Cambridge, 1978). [Google Scholar]
- Venkatesh A. G. and Markx G. H., J. Phys. D: Appl. Phys. 40, 106 (2007). 10.1088/0022-3727/40/1/S15 [DOI] [Google Scholar]
- Sebastian A., Venkatesh A. G., and Markx G. H., Electrophoresis 28, 3821 (2007). 10.1002/elps.200700019 [DOI] [PubMed] [Google Scholar]
- Andrews J. S., Mason V. P., Thompson I. P., Stephens G. M., and Markx G. H., J. Microbiol. Methods 64, 96 (2006). 10.1016/j.mimet.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Ramirez C. A., Andrews J. S., Kookos I., Stephens G. M., Thompson I. P., and Markx G. H., in Biofilms: Persistence and Ubiquity, edited by McBain A., Allison D., Pratten J., Spratt D., Upton M., and Verran J. (The Biofilm Club, Manchester, 2005), p. 427. [Google Scholar]
- Mason V. P., Markx G. H., Thompson I. P., Andrews J. S., and Manefield M., FEMS Microbiol. Lett. 244, 121 (2005). 10.1016/j.femsle.2005.01.031 [DOI] [PubMed] [Google Scholar]
- Zhu K., Kaprelyants A. S., Salina E. G., and Markx G. H., “Separation by dielectrophoresis of dormant and nondormant bacterial cells of Mycobacterium smegmatis,” Biomicrofluidics (in press). [DOI] [PMC free article] [PubMed]
- Flores-Rodriguez N. and Markx G. H., J. Micromech. Microeng. 16, 349 (2006). 10.1088/0960-1317/16/2/020 [DOI] [Google Scholar]
- Verduzco-Luque C. E., Alp B., Stephens G. M., and Markx G. H., Biotechnol. Bioeng. 83, 39 (2003). 10.1002/bit.10646 [DOI] [PubMed] [Google Scholar]