Abstract
There is great interest in highly sensitive separation methods capable of quickly isolating a particular cell type within a single manipulation step prior to their analysis. We present a cell sorting device based on the opposition of dielectrophoretic forces that discriminates between cell types according to their dielectric properties, such as the membrane permittivity and the cytoplasm conductivity. The forces are generated by an array of electrodes located in both sidewalls of a main flow channel. Cells with different dielectric responses perceive different force magnitudes and are, therefore, continuously focused to different equilibrium positions in the flow channel, thus avoiding the need of a specific cell labeling as discriminating factor. We relate the cells’ dielectric response to their output position in the downstream channel. Using this microfluidic platform that integrates a method of continuous-flow cell separation based on multiple frequency dielectrophoresis, we succeeded in sorting viable from nonviable yeast with nearly 100% purity. The method also allowed to increase the infection rate of a cell culture up to 50% of parasitemia percentage, which facilitates the study of the parasite cycle. Finally, we prove the versatility of our device by synchronizing a yeast cell culture at a particular phase of the cell cycle avoiding the use of metabolic agents interfering with the cells’ physiology.
INTRODUCTION
There is a growing demand for separation technology. Chemical and biochemical analyses are largely based on chromatographic separation, while fluorescence activated cell sorting1 (FACS) has made much of the success of modern immunology.2 Chromatography and FACS illustrate two major approaches to separation: batch separation in case of chromatography and continuous separation in case of the FACS. Continuously working sorting approaches should be particularly valuable for miniaturized devices since the continuous operation should allow easy integration. Dielectrophoresis (DEP)-based separation is a method based on the electric force exerted on dielectric particles, such as biological cells in the presence of a nonuniform ac field. DEP-based separation techniques offer many advantages and are suitable for laboratory-on-a-chip applications in the biological and medical fields.3 DEP has been previously used in continuously operating microfluidic applications, such as flow cytometry4 and cell dipping.5 Furthermore, DEP-based separation methods require a standard technology of inexpensive and well-established microfabrication processes; they are easily interfaced to fluidic control6, 7 to inject the sample and to standard electronics for the electrical signals generation. DEP probes both the cell interior and the cell membrane3 and can therefore discriminate between cells on the basis of physical or biochemical phenomena occurring inside the cells or at the cell surfaces in a noninvasive manner.8 Finally, DEP sorting does not usually require any cell modification or labeling, which can be critical in applications, such as stem cell research, where no cellular reaction should be induced by receptor binding of the label. Our strategy is therefore to provide a miniaturized continuous-flow cell separation method in a microfluidic platform. The method is based on multiple frequency dielectrophoresis9 (MFDEP), where forces arising from electric fields with different frequency components act on cells flowing in the microfluidic device. The combination of several dielectrophoretic forces at multiple frequencies discriminates between different cell types and physiological states. We propose the use of an equilibrium-based dielectrophoretic sorting strategy; by imposing lateral forces on cells flowing down a main channel, a unique equilibrium position exists for each cell type. Flowing down the channel, the different cells are progressively centered on their respective equilibrium lines, and hence, sorted. The choice to obtain an equilibrium by opposing two DEP forces rather than using DEP in combination with a conductivity gradient,10, 11 viscous drag,12 or gravity13 is motivated by the goal of simplicity: using DEP only, additional constraints, such as the need to precisely control flow rates, are avoided. The equilibrium-based approach has also important advantages over the traditionally used isomotive force field approach.14, 15 First, the residence time of the cells in the active element is not critical. Indeed, as long as the cells spend enough time in the active element to be focused toward their equilibrium position, a cell-to-cell variation in the residence time due to the parabolic flow profile is unimportant. Second, no hydrodynamic or other focusing strategy prior to the sorting element is needed, greatly facilitating device operation. Finally, exerting dielectric forces on cells leads to their spatial separation, and hence, their lateral position contains information about the intrinsic cell properties. Therefore, our device is not only an efficient cell sorter but is also capable of measuring the dielectric response of the cells by the cell position readout. Typically, when using signals of two different frequencies, the equilibrium position depends on the ratio of the forces at the two frequencies, which we term opacity. The device then acts as an opacity meter.6
RESULTS AND DISCUSSION
Sorting principle: Multiple frequency-DEP and potential wells (equilibrium lines)
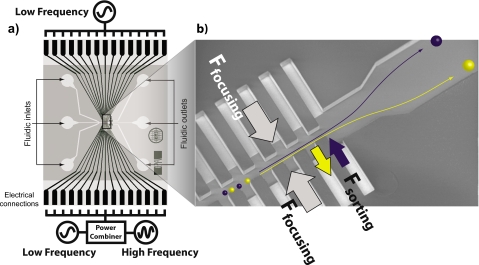
We use arrays of microelectrode chambers, termed liquid electrodes that border the main channel to produce the dielectrophoretic forces, as shown in Fig. 1. For cell sorting purposes, we use generally two low frequency signals (typically in the range of 50–150 kHz), one from each side, which focus the particles toward the channel midline, where the force potential minimum is located. We then superimpose from one side a high frequency signal (typically in the megahertz range), which is responsible for the separation. The high frequency signal is chosen in such a manner that it shifts the equilibrium position for particles of different dielectric responses, without drawing them entirely into either electrode chamber array. The amount by which the equilibrium position is shifted depends on the applied voltages but mainly on the relative force response at high and low frequency that is the opacity value characteristic of each particle type.
Figure 1.
Sorting by MFDEP. (a) Schematic of the cell sorting device and its electric connections. The device consists mainly of two parts; Pt electrodes and the fluidic network. The three fluidic inlets merge to form the central channel, with the electrode arrays in both sides; after the active element, the channel splits again, allowing to collect the purified samples in different outlets. (b) Magnified view of the central channel with the electrode arrays. We generally apply a low frequency field to both electrode arrays, focusing the particle stream toward the symmetry axis of the channel (Ffocusing). To differentiate the particles, a third signal is added to one of the electrode arrays, designated as Fsorting. This leads to a different total force being exerted on different particles and they get focused to different equilibrium lines. The channel widening after the sorting array allows us to slow down the flow for image acquirement.
This strategy can be understood from the typical cellular dielectric function. At low frequency, the membrane blocks current flow and the cell appears as a high impedance object. This leads to the particle being less polarizable than the medium, and as a consequence we have a repulsive DEP force Ffocus acting from both sides. This defines a potential well along the channel midline. When flowing down the sorting structure, the cells therefore get focused. In the high frequency (megahertz) range, the magnitude and sign of the force depend on the interplay of the dielectric properties of the membrane and cytoplasm. The high frequency signal therefore shifts the DEP equilibrium line by varying amounts according to particle types. Since the cells still get focused toward their respective minimum total DEP potential, applying a steady fluid flow down the main channel leads to a continuous sorting. This is why the high frequency force is termed Fsorting in Fig. 1.
In more general terms, the sorting structure translates the differences in the dielectric response, as quantified by the opacity, into a difference in lateral position. As long as the total DEP force from both liquid electrode arrays remains repulsive, the sorting is equilibrium based since the particles spontaneously converge toward a stable final equilibrium position across the channel.
The particle positions after the sorting element are related to the ratio of the forces acting from the two electrode arrays in a quantitative manner,
| (1) |
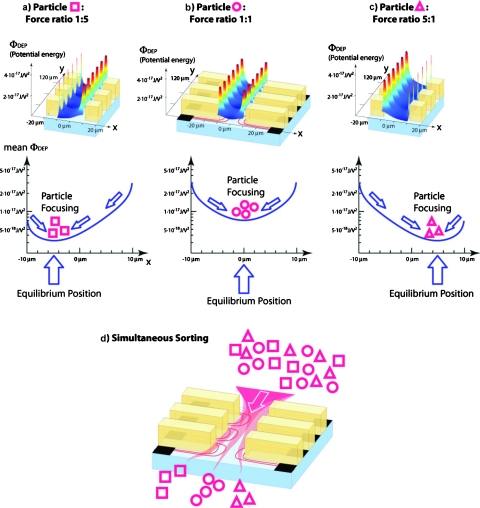
where ⟨F1(0)⟩ and ⟨F2(0)⟩ are the mean DEP forces experienced by a given particle type when flowing down the channel midline, yeq is the final equilibrium position, and ws is the physical width of the sorting channel. g(yeq) is obtained from the calculation of the electric fields in the sorting element.16 The exponential approximation given in Eq. 1 is specific to the standard geometry used for the fabrication of the sorting chips, where channel height, main channel width, width of the electrode chambers, and width of the separating insulator between the electrode chambers and the recess depth of the metal electrodes with respect to the main channel are all equal. When equal DEP forces act from the two electrode arrays lining the main channel, as in Fig. 2b, the particles get focused toward the channel midline, characterized by yeq=0. In terms of Eq. 1, we have ⟨F1(0)⟩=⟨F2(0)⟩ and so g(0)=1. For the situation shown in Fig. 2a, ⟨F1(0)⟩∕⟨F2(0)⟩=0.2 and solving Eq. 1, using ws=20 μm gives yeq≈5.0 μm. For a force ratio of 5 [corresponding to Fig. 2c], yeq≈5.0 μm. Here, we have used Eq. 1 to predict the equilibrium position from a supposedly known force ratio. In practice, we observe the particle positions after the sorting element by taking microscope images and reconstruct the force ratio by means of Eq. 1.
Figure 2.
Equilibrium-based DEP sorting. Different particle types, symbolized here by squares, circles, and triangles, respectively, experience different forces for a given set of electric fields acting from the electrode arrays lining the sorting channel. The force ratio describes the relative efficiency of the electric fields acting from either side of the sorting element to push them toward the opposite side. In the example shown, the force ratio for the square particles is 5 [Fig. 2a], for the circle particles it is 1 [Fig. 2b], and for the triangle particles, it is 0.2 [Fig. 2c]. Accordingly, the square particles are focused toward a position close to the left channel wall, the circle particles toward the channel midline, whereas the minimum of the potential well for the triangle particles is located toward the right channel wall. If a mixture of the three particle types is flowed down the sorting channel, each particle type is centered toward its own equilibrium positions, and hence, the mixture gets sorted [Fig. 2d].
Opacity meter
The force ratio is equal to the opacity if two signals at two different frequencies, but identical voltages, are applied to the two liquid electrode arrays. However, in practice, this is rarely the case. We typically use three different signals to drive the microelectrode arrays in the sorting element. The force ratio in Eq. 1 is then not only a function of the opacity but also the voltages and frequencies of all three signals. We apply low frequency signals with voltages Vlf,1 and Vlf,2 on both sides of the channel to repulse cells from the electrode array, toward the channel midline. A high frequency signal Vhf is superimposed on the side of the signal Vlf,1 (cf. Fig. 1), allowing to probe the cell interior and to discriminate between different particle types. Therefore, F1 representing the sum of Flf,1 and Fhf is acting from one side of the electrode array, whereas F2, associated solely with Flf,2, acts from the other side.
The additivity of the DEP forces and the symmetry of the sorting element gives for the forces on the channel midline,
| (2) |
where fCM(lf,1), fCM(lf,2), and fCM(hf) are the Clausius–Mossotti factors at the two focusing frequencies (lf for low frequency) and the high frequency (index hf), respectively. The two low frequency signals Vlf,1 and Vlf,2 are chosen at close frequencies, or, if 90° phase-shifted signals are used, at identical frequencies. We can therefore assume identical Clausius–Mossotti factors for the two low frequency signals. This simplifies Eq. 2,
| (3) |
where Vlf,1, Vlf,2, and Vhf are the voltages used to produce the forces Flf,1, Flf,2, and Fhf, respectively. Finally, we define the dielectrophoretic opacity Ω as
| (4) |
The term opacity is used in analogy to on-chip impedance flow cytometry,4 where opacity designates the ratio of the impedance of a cell at high compared to low frequency. By combining Eq. 3 with Eq. 4, we obtain an explicit relation between the opacity and the particle equilibrium positions,
| (5) |
Equation 5 establishes an explicit link between the horizontal particle equilibrium positions and their dielectric response.
Sorting applications
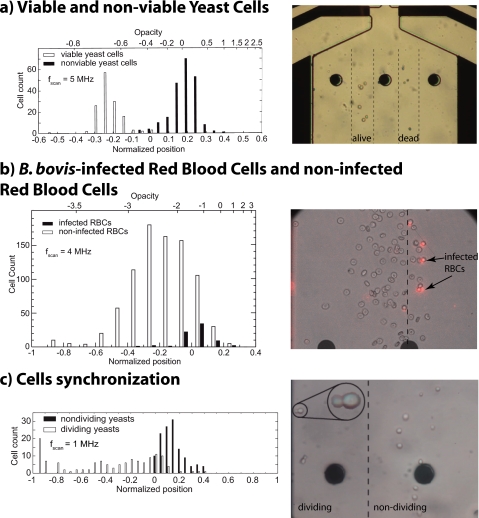
In this section separations of different biological samples are shown and prove the excellent performance of our miniaturized continuous DEP-cell sorter device, establishing the technique as application ready. A summary of the results obtained are shown in Fig. 3 in the form of histograms, where the horizontal axis represents the lateral position and the vertical axis being the cell count. Next to each histogram, a micrograph illustrates the actual sorting. The images were taken at the exit of the electrode array in the widening channel [see Fig. 1b]. It is possible to fully sort viable and non viable yeast cells [Fig. 3a] since live and dead cells have clearly different electrical behaviors. Live cells are characterized by a well isolating membrane, and when suspended in low conductivity buffers, active accumulation of ions inside the cell maintain near-physiological electrolyte conditions inside the cells. In terms of electrical behavior, a strong intracellular conductivity leads to strong pDEP in the megahertz range. Dead cells, on the other hand, are unable to maintain concentration gradients across their membrane. In low conductivity buffers, this means that ions leak out and the cell appears more resistive than the medium at all frequencies because of the presence of diverse isolating parts, such as membrane rests and intracellular organites. In terms of DEP, we observe negative dielectrophoresis (nDEP) at all frequencies. Our device allows us to obtain viable and nonviable yeast cells with a purity approaching 100%.17 Sorting of viable and nonviable yeast cells using DEP has been reported in literature. Markx et al.18, 19 described a semicontinuous cell sorting approach providing high efficiency and sorting rates, but involving a time-consuming protocol based on cyclic switching of fluid flow direction and voltages. Li et al.14 presented a truly continuous cell sorting device using an isomotive force field approach but with low efficiency and sorting rate. Our approach combines high efficiency and sorting rates with the simplicity of truly continuous cell sorting. As shown in Fig. 3b, an enrichment of B. bovis infected red blood cells without resorting to a label was possible with our DEP sorting device. When sorting infected from noninfected red blood cells, we observe a lower pDEP force for the infected cells. Due to the infection, ion loss through the membrane occurs and this gives a lower intracellular conductivity for the infected cells. Also the presence of the parasite itself inside the cell has an influence blocking the current flow. In the case of the B. bovis, we do not achieve a fraction showing 100% infected cells, as visualized by ethidium bromide staining. We do, however, get an enrichment of a factor of 7×, achieving a final infection rate of 50% in the enriched sample.17 This rivals with the best available results in literature on isolation of B. bovis infected cells by label-free techniques.20 The fore-mentioned results show sorting of biological samples based on changes in cell physiology, mostly due to the conductivity gradient between intracellular and extracellular spaces. However, cell morphology has also an influence on the dielectrophoretic properties. A good example where cell physiology and cell morphology are tightly coupled is the cell cycle division. A particular case is the budding yeast, which is a perfect model for the cell division cycle. A suspension of yeast cells undergoing active division was injected in our miniaturized dielectrophoretic sorter and when using a high frequency discriminating signal at 1 MHz, dividing yeasts equilibrate on flow lines substantially shifted toward the high frequency side, indicating earlier or stronger onset of pDEP for these cells [Fig. 3c]. This sorting effect is consistent with known dielectric properties of yeast cells in division.21 Indeed, there is a low frequency additional dispersion for budding yeasts, shifting to lower frequencies still when the neck narrows. This could be the case for yeast cells in late anaphase being the most attracted toward the high frequency side. After neck closure, cytokinesis stage, the low frequency dispersion disappears and the pair behaves like two individual single yeast cells. Therefore, with our dielectrophoretic cell sorter using MFDEP, we are capable of synchronizing a yeast cell culture based on the different polarization mechanisms of cells that are associated to their cell cycle phases.22, 23
Figure 3.
Sorting applications: (a) sorting of viable and nonviable yeast cells; low frequency signals are set at 60 and 90 KHz, respectively, on both electrode arrays to focus all cells toward a common equilibrium position. Adding a high frequency signal set at 5MHz actually performs the sorting of viable and nonviable subpopulations. (b) Enrichment of B. bovis parasitized red blood cells; one side of the electrode array a low frequency signal of 90 KHz is applied and a combination of 60 KHz and 4 MHz signal is acting from the other side. This signal combination gives the best results as an enrichment of infected cells on one side of the channel and a population almost free of infected cells on the other side. (c) Separation of yeast cells in division. The superimposed high frequency signal (1 MHz) on side of the electrode array exerts stronger pDEP on dividing yeasts compared to the nondividing ones. The dividing cells are spread over a large portion of the outlet channel due to large dispersions in size and dielectric properties. However, a subpopulation of the yeasts undergoing division seems more attracted to the high frequency side than the average, probably related to one specific stage of the cell cycle.
ONGOING WORK
Other options that our cell sorter device offers is the possibility of combining cell sorting and cell counting within the same device, by having a pair of coulter counter electrodes at the outlet channels. After cell sorting, cells are counted by means of an integrated coulter counter by monitoring the current variations occurring at each cell passage through an aperture, across which a constant voltage is maintained. The obtained excellent signal-to-noise ratio allows automated counting with very simple signal processing and since impedimetric cell size measurement is not sought, no calibration of individual channel sensitivity is needed. This device allows determining the concentration of cell subpopulations in a sample, with applications such as viability studies and differential cell counting for point-of-care diagnosis.24 Other ongoing work using our dielectrophoretic cell sorter is the separation of antibiotic-generated bacterial subpopulations. In addition to providing important information on the dielectric properties of single cells in the presence or absence of antibiotics, this method will also facilitate detailed studies of the separated bacterial subpopulations using conventional approaches.25
CONCLUSIONS
We have developed a robust, noninvasive, label-free cell separation technique based on MFDEP. The device presented here analyses and sorts cells according to their dielectric properties. By opposing two highly inhomogeneous electric fields at different frequencies, cells with identical dielectric properties get focused toward the same lateral position (or potential well) independent of the flow speed. The combination of multiple frequency signals allows both to focus the cell stream at low frequency, largely independent of the exact dielectric function of the cells, and to probe the cell membrane and cell interior with a high frequency signal to discriminate between different cell types. Finally, the theory relating the equilibrium positions to the force ratio experienced by the cells establishes a direct link between the dielectric response and the equilibrium position. This enables us to extract the dielectric response in the form of an opacity, which is an intrinsic property of the cell. The applications shown are of biological relevance; a clean separation of viable and nonviable cells is achieved. The classical assessment of the cell viability with colorants is time consuming and may alter the cells, whereas the technique presented here works in a label-free way and could potentially be parallelized to achieve high throughput at relatively low cost. The enrichment of the Babesia bovis infected cells can compete with other label-free techniques; furthermore, a label-free synchronization technique for budding yeast, allowing to select different cell division stages in different outlets, could open new perspectives in cell cycle and cell lineage analysis. Our immediate research perspectives comprise viability assessment of bacteria and stem cell sorting. It might be interesting to carry out subsequent sorting steps using different sets of frequencies and amplitude to increase the specificity of the method; it would also be interesting to find dielectric labels that are highly sensitive to the frequency, such as to quantify the presence of many different antigens in a combinatorial fashion. We expect that advantages, such as ease of use, efficiency, and high throughput, would encourage physicians to use our sorting method in daily analysis.
METHODS
Microfluidic device design and operation
The microfluidic device consists of a network of photopatterned channels in SU8 with Ti–Pt electrodes previously structured by a lift-off process. The chip is reversibly sealed by a molded PDMS block containing embedded liquid reservoirs and the full device is mounted into a custom-made fluidic and electrical interface. More details can be found in Refs. 17, 26, 27.
Image acquisition
The microfluidic device was mounted on to an XYZ translation stage in an inverted microscope (Leica DMIL, Leica Microsystems, Wetzlar, GmbH, Germany). The microscope system is equipped with several different magnification objectives and a computer-controlled camera (uEye) is mounted in the microscope for image∕video recording.
Preparation of yeasts
Viability studies: viable yeasts (99% viability according to trypan blue stain) were obtained from a local grocery store (baker’s yeast, Saccharomyces cerevisae). Nonviable yeast cells were obtained by heat treatment (20 min at 90 °C) and colored by methylene blue stain. Cell synchronization studies: The yeast cells are cultured at 20 °C on yeast peptone dextrose (YPD) agar plates (1000 ml of de-ionized water,10 g of bactoyeast extract, 20 g of bactopeptone, 20 g of dextrose, and 20 g of agar). For the experiments, cells in logarithmic growth phase are transferred to YPD liquid media, which is adjusted with de-ionized water to a conductivity of 60 mS∕m.
Preparation of Babesia bovis infected cells
The Mo7 strain of B. bovis was grown in vitro according to the Levy and Ristic method.28, 29 To quantitatively detect the degree of separation, the cells were labeled with 25 μg∕ml of ethidium bromide (Qbiogene, ETBC1001, Basel, Switzerland), a nucleic acid intercalator (red blood cells do not contain DNA except when they are infected).
Suspension medium
For all experiments, the suspension medium conductivity, phosphate buffer saline (Sigma-Aldrich, Buchs, Switzerland) for viability and parasitemia studies and YPD in case of cell synchronization studies, was adjusted to 60 mS∕m. When using red blood cells, the medium was adjusted to physiological osmolarity using saccharose. To reduce cell sticking in the microfluidic channels, we supplemented the medium with 0.1% w∕w bovine serum albumine (Sigma-Aldrich).
References
- Van Dilla M. A., Trujillo T. T., Mullaney P. F., and Coulter J. R., Science 163, 1213 (1969). 10.1126/science.163.3872.1213 [DOI] [PubMed] [Google Scholar]
- Male D. K., Brostoff J., Roitt I. M., and Roth D., Immunology (Elsevier, New York, 2006). [Google Scholar]
- Morgan H. and Green N., AC Electrokinetics: Colloids and Nanoparticles, Research Studies (Press Ltd., Hertfordshire, 2002), pp. 39, 186, 192, 201, 202, and 204. [Google Scholar]
- Gawad S., Cheung K., Seger U., Bertsch A., and Renaud P., Lab Chip 4, 241 (2004). 10.1039/b313761a [DOI] [PubMed] [Google Scholar]
- Seger U., Gawad S., Johann R., Bertsch A., and Renaud P., Lab Chip 4, 148 (2004). 10.1039/b311210a [DOI] [PubMed] [Google Scholar]
- Braschler T., Theytaz J., Zvitov-Marabi R., Van Lintel H., Loche G., Kunze A., Demierre N., Tornay R., Schlund M., and Renaud P., Lab Chip 7, 1111 (2007). 10.1039/b708360b [DOI] [PubMed] [Google Scholar]
- Braschler T., Metref L., Zvitov-Marabi R., van Lintel H., Demierre N., Theytaz J., and Renaud P., Lab Chip 7, 420 (2007). 10.1039/b617673a [DOI] [PubMed] [Google Scholar]
- Goater A. D., Dand Burt J. P. H., and Pethig R., J. Phys. D: Appl. Phys. 30, L65 (1997). 10.1088/0022-3727/30/18/001 [DOI] [Google Scholar]
- Pethig R., Talary M. S., and Lee R. S., IEEE Eng. Med. Biol. Mag. 22, 43 (2003). 10.1109/MEMB.2003.1266046 [DOI] [PubMed] [Google Scholar]
- Markx G. H., Dyda P. A., and Pethig R., J. Biotechnol. 51, 175 (1996). 10.1016/0168-1656(96)01617-3 [DOI] [PubMed] [Google Scholar]
- Vahey M. and Voldman J., Anal. Chem. 80, 3135 (2008). 10.1021/ac7020568 [DOI] [PubMed] [Google Scholar]
- Müller T., Schnelle T., Gradl G., Shirley S. G., and Fuhr G., J. Liq. Chromatogr. Relat. Technol. 23, 47 (2000). 10.1081/JLC-100101435 [DOI] [Google Scholar]
- Wang X. -B., Yang J., Huang Y., Vykoukal J., Becker F., and Gascoyne P., Anal. Chem. 72, 832 (2000). 10.1021/ac990922o [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Dalton C., Crabtree H., Nilsson G., and Kaler K., Lab Chip 7, 239 (2007). 10.1039/b613344d [DOI] [PubMed] [Google Scholar]
- Pohl H., U.S. Patent No. 4 326 934 (1981).
- Demierre N., Braschler T., Linderholm P., Seger U., van Lintel H., and Renaud P., Lab Chip 7, 355 (2007). 10.1039/b612866a [DOI] [PubMed] [Google Scholar]
- Braschler T., Demierre N., Nascimento E., Silva T., Oliva A., and Renaud P., Lab Chip 8, 280 (2008). 10.1039/b710303d [DOI] [PubMed] [Google Scholar]
- Markx G. H., Talary M. S., and Pethig R., J. Biotechnol. 32, 29 (1994). 10.1016/0168-1656(94)90117-1 [DOI] [PubMed] [Google Scholar]
- Markx G. H. and Pethig R., Biotechnol. Bioeng. 45, 337 (1995). 10.1002/bit.260450408 [DOI] [PubMed] [Google Scholar]
- Rodriguez S., Buening G., Vega C., and Carson C., Exp. Parasitol. 61, 236 (1986). 10.1016/0014-4894(86)90157-8 [DOI] [PubMed] [Google Scholar]
- Asami K. and Sekine K., J. Phys. D: Appl. Phys. 40, 1128 (2007). 10.1088/0022-3727/40/4/033 [DOI] [Google Scholar]
- Demierre N., Braschler T., Valero A., and Renaud P., The 12th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 2008.
- Valero A., Braschler T., Rauch A., Demierre N., Barral Y., and Renaud P., “Tracking and Synchronisation of the Yeast Cell Cycle using Dielectrophoretic Opacity,” Nature Methods (in press). [DOI] [PubMed]
- Mernier G., Piacentini N., Tornay R., Buffi N., and Renaud P., “Cell viability assessment by flow cytometry using yeast as cell model,” Sens. Actuators B (to be published).
- Elitas M., Valero A., Braschler T., Dhar N., McKinney J., and Renaud P., The 13th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 2009.
- Demierre N., “Continuous-flow separation of cell in a lab-on-a-chip using “liquid electrodes” and multiple frequency dielectrophoresis,” Ph.D. thesis, Ecole Polytechnique Federal de Lausanne, 2009. [Google Scholar]
- Braschler T., “Controlled entrapment of cells in hydrogels on chip and cell sorting by dielectrophoresis using liquid electrodes,” Ph.D. thesis, Ecole Polytechnique Federal de Lausanne, 2009. [Google Scholar]
- Levy M. and Ristic M., Science 207, 1218 (1980). 10.1126/science.7355284 [DOI] [PubMed] [Google Scholar]
- Nascimento E., Nogueira N., Silva T., Braschler T., Demierre N., Renaud P., and Oliva A., Bioelectrochemistry 73, 123 (2008). 10.1016/j.bioelechem.2008.04.018 [DOI] [PubMed] [Google Scholar]