Abstract
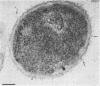
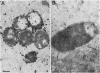
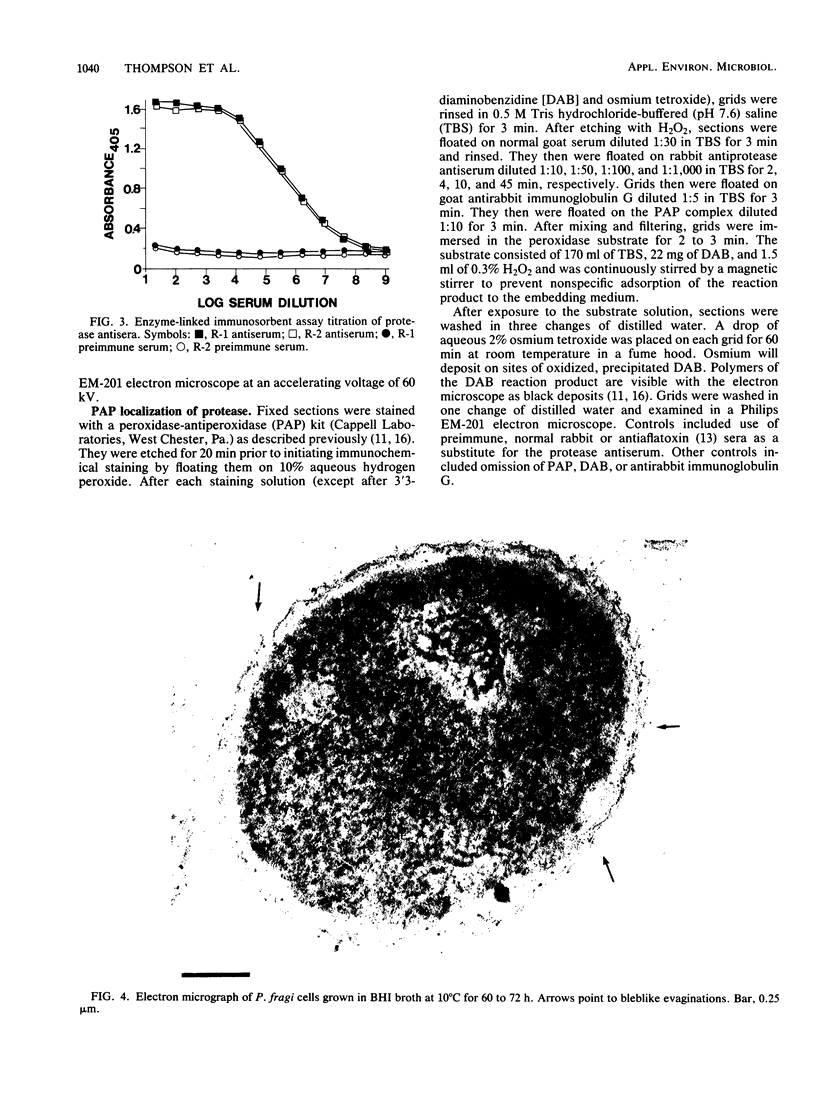
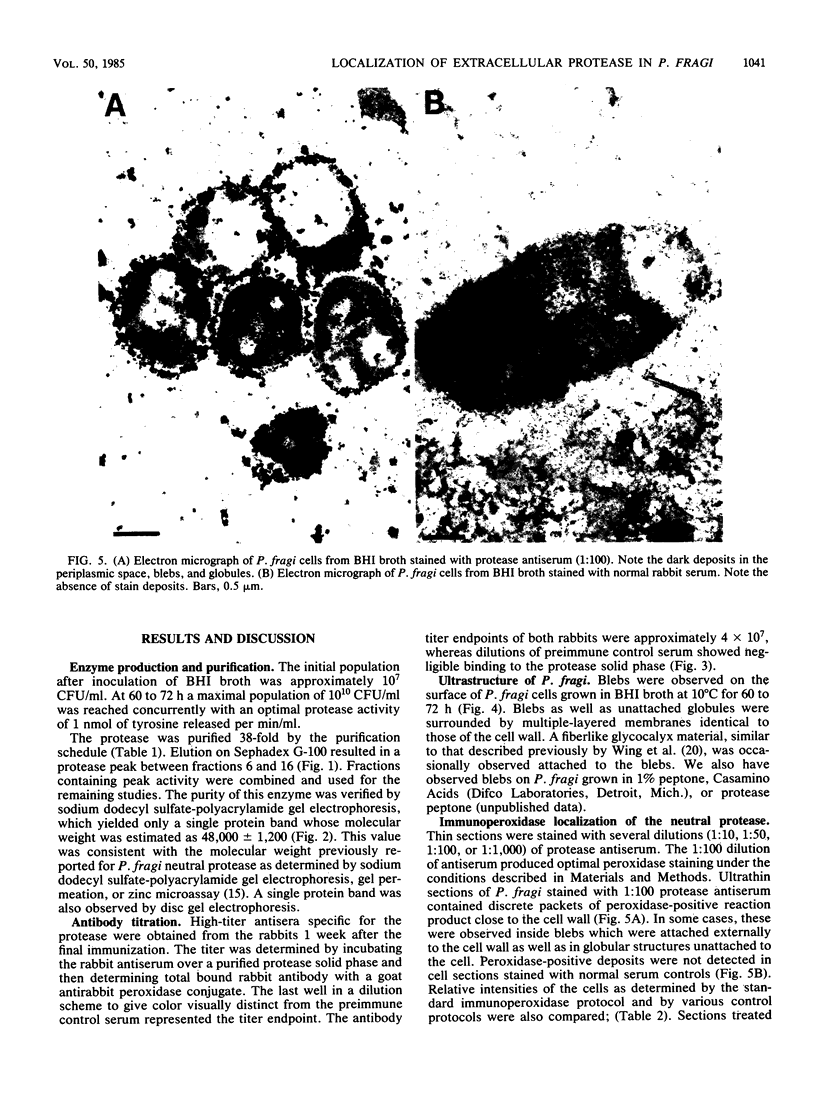
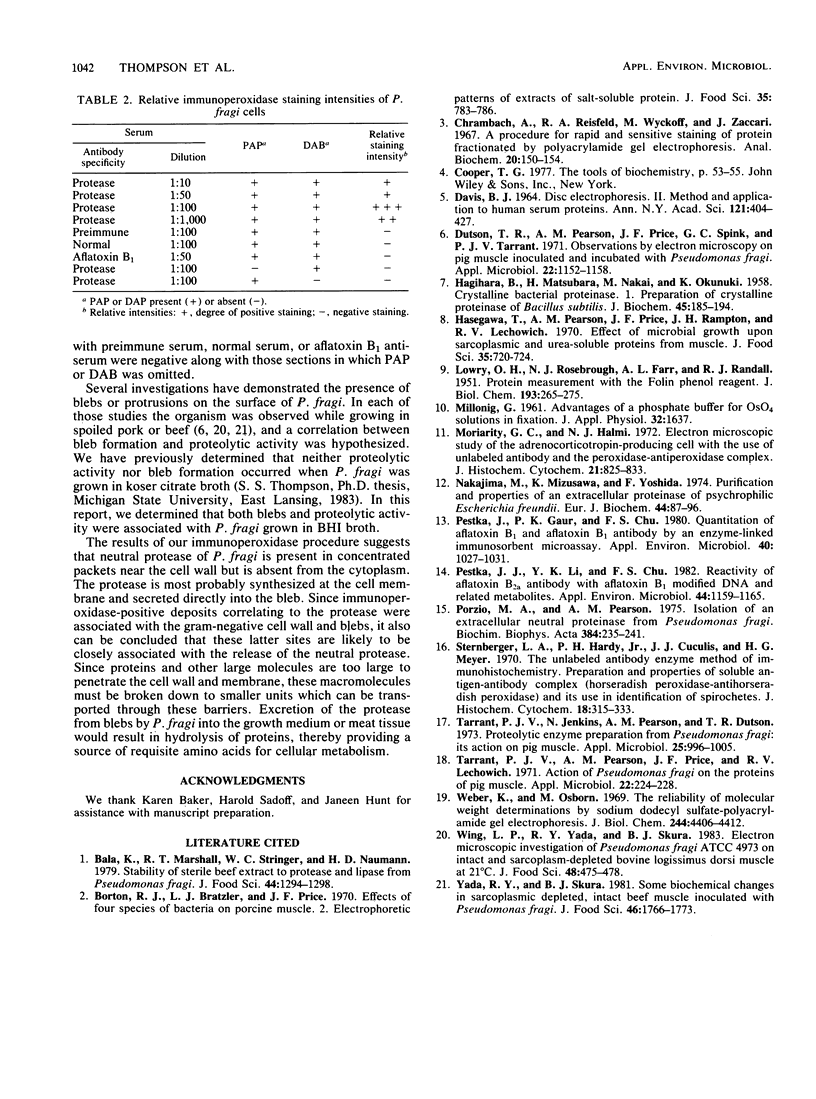
An extracellular protease, which previously has been found to correlate with the appearance of bleblike evaginations on the cell wall of Pseudomonas fragi ATCC 4973, was purified 38-fold by ammonium sulfate precipitation and Sephadex chromatography to yield a single band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Polyclonal rabbit antiserum raised against the purified enzyme had an enzyme-linked immunosorbent assay titer of 4 X 10(7). The peroxidase antiperoxidase method was used to localize the neutral protease in P. fragi at the ultrastructural level. Electron microscopy of cell sections of this organism revealed that high concentrations of positive immunoperoxidase reaction product were located near the cell wall, whereas control sections stained with preimmune or heterologous serum did not show similar deposits to be present. These results are consistent with the hypothesis that blebs appearing on P. fragi contain high concentrations of neutral protease.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bala R. M., Bhaumick B. Purification of a basic somatomedin, from human plasma Cohn fraction IV-1, with physicochemical and radioimmunoassay similarity to somatomedin-C and insulin-like growth factor. Can J Biochem. 1979 Nov;57(11):1289–1298. doi: 10.1139/o79-172. [DOI] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dutson T. R., Pearson A. M., Price J. F., Spink G. C., Tarrant P. J. Observations by electron microscopy on pig muscle inoculated and incubated with Pseudomonas fragi. Appl Microbiol. 1971 Dec;22(6):1152–1158. doi: 10.1128/am.22.6.1152-1158.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nakajima M., Mizusawa K., Yoshida F. Purification and properties of an extracellular proteinase of psychrophilic Escherichia freundii. Eur J Biochem. 1974 May 2;44(1):87–96. doi: 10.1111/j.1432-1033.1974.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Pestka J. J., Gaur P. K., Chu F. S. Quantitation of aflatoxin B1 and aflatoxin B1 antibody by an enzyme-linked immunosorbent microassay. Appl Environ Microbiol. 1980 Dec;40(6):1027–1031. doi: 10.1128/aem.40.6.1027-1031.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J., Li Y. K., Chu F. S. Reactivity of aflatoxin B2a antibody with aflatoxin B1-modified DNA and related metabolites. Appl Environ Microbiol. 1982 Nov;44(5):1159–1165. doi: 10.1128/aem.44.5.1159-1165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porzio M. A., Pearson A. M. Isolation of an extracellular neutral proteinase from Pseudomonas fragi. Biochim Biophys Acta. 1975 Mar 28;384(1):235–241. doi: 10.1016/0005-2744(75)90112-6. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tarrant P. J., Jenkins N., Pearson A. M., Dutson T. R. Proteolytic enzyme preparation from Pseudomonas fragi: its action on pig muscle. Appl Microbiol. 1973 Jun;25(6):996–1005. doi: 10.1128/am.25.6.996-1005.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant P. J., Pearson A. M., Price J. F., Lechowich R. V. Action of Pseudomonas fragi on the proteins of pig muscle. Appl Microbiol. 1971 Aug;22(2):224–228. doi: 10.1128/am.22.2.224-228.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]