Abstract
Multidrug resistance protein 1a (MDR1a) potentiated methylenedioxymethamphetamine (MDMA)-induced decreases of dopamine (DA) and dopamine transport protein in mouse brain one week after MDMA administration. In the present study, we examined if mdr1a wild-type (mdr1a +/+) and knock-out (mdr1a −/−) mice differentially handle the acute effects of MDMA on the nigrostriatal DA system 0–24 h following a single drug injection. 3-way ANOVA revealed significant 2-way interactions of strain X time (F5,152= 32.4, p< 0.001) and strain X dose (F3,152= 25.8, p< 0.001) on 3,4-dihydroxyphenylacetic acid (DOPAC)/DA ratios in mdr1a +/+ and −/− mice. 0.3–3 h after 10 mg/kg MDMA, DOPAC/DA ratios were increased in mdr1a +/+ mice, but decreased 0.3–1 h after MDMA in mdr1a −/− mice. 24 h after 10 mg/kg MDMA, DOPAC/DA ratios were increased 600% in mdr1a +/+ mice compared to saline-treated control mice, while in mdr1a −/− mice DOPAC/DA ratios were unchanged. Striatal MDMA and its metabolite, methylenedioxyamphetamine, concentrations by gas chromatography-mass spectrometry were similar in both strains 0.3 to 4 h after MDMA, discounting the role of MDR1a-facilitated MDMA transport in observed inter-strain differences. Increased DOPAC/DA turnover in mdr1a +/+ mice following MDMA is consistent with the previous report that MDMA neurotoxicity is increased in mdr1a +/+ mice. Increased DA turnover via monoamine oxidase in mdr1a +/+ vs −/− mice might increase exposure to neurotoxic reactive oxygen species.
Keywords: Multi-drug resistance protein 1a (MDR1a), methylenedioxymethamphetamine (MDMA), ecstasy, dopamine, striatum, transgenic mouse
Introduction
Multidrug resistance protein 1a (MDR1a, also known as p-glycoprotein) is a transport protein with wide substrate specificity belonging to the ATP-binding cassette protein family (Sharom, 2008; Zhou, 2008). MDR1a originally was identified as playing a key role in conferring a multidrug resistant phenotype in tumor cells that developed resistance to multiple chemotherapeutic agents. MDR1a plays a role in drug excretion being located in the apical membrane of cells in the intestine, kidney and liver (Sharom, 2008). MDR1a also is highly expressed in brain endothelium that comprises the blood-brain barrier (BBB), where it functions to limit access of drugs to the central nervous system (Ho and Kim, 2005; Sharom, 2008; Zhou, 2008). MDR1a-mediated transport of drugs across the BBB is uni-directional since it is an ATP-dependent process (Ho and Kim, 2005; Sharom, 2008; Zhou, 2008). Altered MDR1a expression or activity influences brain penetration and activity of centrally acting drugs that are substrates for MDR1a (Linnet and Ejsing, 2008; Sharom, 2008; Zhou, 2008).
Methylenedioxymethamphetamine (MDMA, ecstasy) is a commonly abused sympathomimetic amine stimulant that produces increased sociability, extroversion and perceptual disturbances (de la Torre et al., 2004; Morton, 2005). Clinical research suggests long-term memory and learning impairment in human subjects reporting heavy MDMA use (Gouzoulis-Mayfrank and Daumann, 2006; Karlsen et al., 2008), which is thought to be due to damaged serotonergic nerve terminals (Thomasius et al., 2006). In mice, similar to methamphetamine, MDMA produces dopamine (DA) release by disrupting vesicular DA storage causing elevated cytosolic DA concentrations (Riddle et al., 2005). Cytosolic DA is then released from the neuron by reverse transport and/or channel-like activity of the dopamine transporter protein (DAT) (Kahlig et al., 2005; Riddle et al., 2005). Many of MDMA’s neurotoxic effects in mice result from altered intracellular DA distribution leading to reactive oxygen species production (Hrometz et al., 2004; Brown et al., 2006; Riddle et al., 2006; Fleckenstein et al., 2007).
Preliminary studies comparing MDMA toxicity in mice lacking MDR1a protein (mdr1a −/−) versus wild-type mice expressing MDR1a protein (mdr1a +/+) suggest that MDR1a plays a role in MDMA’s neurotoxicity (Mann et al., 1997). In rats and monkeys, MDMA is selectively neurotoxic to serotonergic terminals; however, in mice, MDMA depletion of dopaminergic terminals predominates (O’Shea et al., 2002; Green et al., 2003; Colado et al., 2004; Quinton and Yamamoto, 2006; Baumann et al., 2007; Capela et al., 2009). MDR1a appears to facilitate entry of MDMA into brain, with mice lacking MDR1a protein being more resistant to MDMA-induced reductions in DA and DAT expression in brain (Mann et al., 1997). The finding that MDR1a did not limit MDMA-induced toxicity in the brain was unexpected and inconsistent with conventional thinking regarding the role of MDR1a in the BBB. These results suggest that MDR1a could be considered as a potential target for MDMA abuse treatment, since a candidate drug inhibiting MDR1a might limit MDMA’s acute and neurotoxic effects.
Previous studies evaluated MDMA effects one week post-dose and did not investigate acute effects (Mann et al., 1997). In the current experiment, we tested the hypothesis that MDR1a potentiated MDMA acute effects on striatal monoamines within 24 h of dosing. Unfortunately, MDMA and metabolites were not measured in previous neurotoxicity studies to directly evaluate whether MDR1a-facilitated transfer of MDMA and metabolites across the BBB plays a role in increased neurotoxicity that occurred in brains of mdr1a +/+ mice (Mann et al., 1997). The role of MDR1a-facilitated transfer of MDMA in the greater neurotoxicity observed in striatum of mdr1a +/+ vs. mdr1a −/− mice remains unclear. Upreti and Eddington administered single 5 mg/kg intra-peritoneal MDMA doses to mdr1a +/+ and −/− mice and measured whole brain MDMA and methylenedioxamphetamine (MDA) concentrations 0.5 and 4 h after dosing; there were no significant differences in MDMA and MDA whole brain concentrations between strains (Upreti and Eddington, 2008). However, these studies did not evaluate MDMA pharmacodynamic effects. MDMA’s effects and neurotoxicity are localized to specific brain regions, namely striatum and cortex (Gudelsky and Yamamoto, 2008). Therefore, it is important to characterize drug distribution within these discrete brain regions. We also measured MDMA and metabolite concentrations in contralateral striatal specimens collected from mdr1a +/+ and mdr1a −/− mice during the acute monoaminergic studies to test for inter-strain differences.
Materials and methods
Mice
10–12 week old male FVB (mdr1a +/+) and mdr1a −/− mice were purchased from Taconic Inc, Germantown, NY, USA. Mice were housed individually in a temperature and humidity controlled environment with a 12 h light: dark cycle and had access to food and water ad libitum.
Chemicals
Racemic MDMA-hydrochloride administered to mice was purchased from Lipomed Inc, Cambridge, MA, USA. MDMA-hydrochloride was diluted in sterile saline and the administered dosage expressed as the salt. DA hydrochloride, 3,4-dihydroxyphenylacetic acid (DOPAC), 4-hydroxy-3-methoxy-phenylacetic acid (HVA), serotonin (5-HT) creatinine sulfate complex and 5-hydroxyindole-3-acetic acid (5-HIAA) were obtained from Sigma-Aldrich, St. Louis, MO, USA. Racemic mixtures of MDMA, MDA (1 mg/mL in methanol) and internal standards MDMA-d5, and MDA-d5 (100 μg/mL) were from Cerilliant Corporation (Round Rock TX, USA). Racemic 3-hydroxy-4-methoxy-methamphetamine (HMMA), and 3-hydroxy-4-methoxy-amphetamine (HMA) (1 mg/mL in methanol) were obtained from Lipomed Inc. Heptafluorobutyric acid anhydride and triethylamine were purchased from Pierce Chemical Co. (Rockford, IL, USA). All solvents and buffer salts, HPLC- and reagent-grade, respectively were obtained from JT Baker (Phillipsburg, NJ, USA).
Experimental design
mdr1a +/+ and mdr1a −/− mice were administered MDMA-hydrochloride or sterile saline intraperitoneally (i.p.) and were euthanized via cervical dislocation 0.3, 1, 2, 3, 4 or 24 h after MDMA administration. Brains were removed, dissected on an ice-cold steel plate, immediately frozen on dry ice and stored at −80°C. All animal use and care procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care and Use Committee.
Analysis for monoamines
Monoamine concentrations in striatal specimens were determined by high-performance liquid chromatography with electrochemical detection (Deng et al., 2007). Nine calibrators containing 0.08 to 7.25 ng/mL DA, DOPAC, HVA, 5-HT and 5-HIAA were prepared in 0.1 M perchloric acid. Frozen tissue was weighed, thawed and sonicated in 200 μL 0.1 M perchloric acid. Samples were centrifuged for 14 min at 13,000 g at 4°C. 9 μL of the supernatant was injected onto a Waters Symmetry C18 column (3.5 μm, 4.6 × 150 mm; Waters Corp, Milford, MA, USA). The HPLC system consisted of a Waters 717 plus autosampler and 1525 binary pump (Waters Corp) connected to a Coulochem III electrochemical detector (ESA Inc, Chelmsford, MA, USA). The flow rate was 1 mL/min and the column heater was maintained at 34°C. Degassed, filtered mobile phase consisted of 0.01 M sodium dihydrogenphosphate, 0.01 M citric acid, 2 mM sodium ethylenediaminetetraacetic acid (EDTA), 1 mM sodium octylsulfate, 10% methanol adjusted to pH 3.5. Waters Breeze system was utilized for data collection and analysis. Contents of DA, DOPAC, HVA, 5-HT, and 5HIAA were calculated as pg/mg of tissue weight.
Analysis for MDMA and metabolites
MDMA, MDA, HMMA and HMA in striatum were quantified by gas chromatography mass spectrometry (Scheidweiler et al., 2008). Each striatum specimen was weighed, thawed and homogenized in ice-cold 0.05 M trichloroacetic acid-0.025 M thiourea. An aliquot containing the equivalent of 7.5 mg tissue was removed and diluted to 800 μL with ice-cold 0.05 M trichloroacetic acid-0.025 M thiourea. Supernatants were collected after centrifugation. 100 μL of 12 M hydrochloric acid was added and samples were incubated at 100°C for 45 min to hydrolyze glucuronidated and sulfated metabolites. Diluted specimens were extracted and derivatized as described in Scheidweiler et al. (Scheidweiler et al., 2008).
Analysis was performed on an Agilent 6890 gas chromatograph (Agilent Technologies, Wilmington, DE, USA) with mass selective detector (Agilent 5975) operated in electron impact mode. See Scheidweiler et al. for instrument parameters (Scheidweiler et al., 2008). To extend the linear dynamic range, calibrators, controls and specimens were injected in pulsed splitless and pulsed split (1:5) modes. Two calibration curves were constructed for MDMA (i.e. low= splitless injection and high= 1:5 split injection) to achieve extended linear dynamic range. Extraction efficiencies of MDMA, MDA, HMMA and HMA were between 96.3 and 103.0%. Linear ranges for MDMA, MDA and HMMA were 0.1 to 10 and 0.2 to 10 ng/mg for HMA. The linear range for the MDMA high curve constructed with split injections of calibrators was 10 to 200 ng/mg. Inter-assay analytical recovery (bias) for MDMA, MDA, HMMA and HMA in brain were between 95.9 to 106.9% of target concentrations (n=14). Inter-assay imprecision ranged from 2.5 to 4.7% coefficient of variation for all four analytes (n=14).
Statistics
Effects of strain, time and dose on monoamines in striatum were investigated with univariate 3-way, 2 × 6 × 3, between-subjects analysis of variance (ANOVA) conducted using the General Linear Model in SPSS version 13.0 for Windows. If ANOVA revealed significant 2-way interactions of strain and time or strain and dose employing significance of p<0.05 then follow-up t-tests with Bonferroni-adjusted p-values equivalent to p<0.05 were conducted. A similar 3-way, 2 × 6 × 3, between-subjects ANOVA strategy was employed to analyze effects of strain, time and dose on MDMA and MDA striatal concentrations.
Results
Striatal concentrations of DA, DOPAC, HVA, 5-HT, 5-HIAA and DOPAC/DA ratios in control mice euthanized 1 h after saline injection were not significantly different in mdr1a +/+ vs. mdr1a −/− mice (Table 1).
Table I.
Monoamine concentrations in striatum of wild-type and mdr1a knock-out mice one h after intra-peritoneal saline administration1
| Analyte | Mean ± S.D. (pg/mg, n=5) | |
|---|---|---|
| mdr1a +/+ | mdr1a −/− | |
| Dopamine | 9696.7 ± 918.9 | 9930.9 ± 1276.8 |
| DOPAC | 1834.6 ± 295.3 | 1749.2 ± 218.1 |
| Homovanillic acid | 1044.0 ± 175.3 | 1161.5 ± 153.9 |
| DOPAC/Dopamine2 | 0.19 ± 0.03 | 0.18 ± 0.03 |
| Serotonin | 669.4 ± 54.0 | 628.3 ± 78.2 |
| 5-HIAA | 652.5 ± 208.0 | 593.5 ± 170.4 |
There were no statistical differences of monoamine concentrations between saline-treated mdr1a +/+ and mdr1a −/− mice.
DOPAC/Dopamine ratio is unit-less.
Effects of MDMA on striatal DA system of mdr1a +/+ and −/− mice
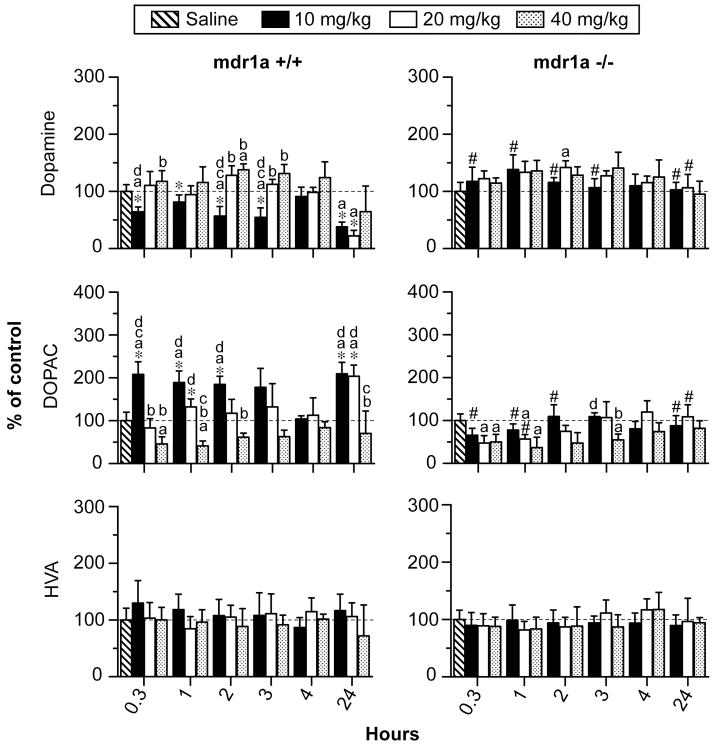
There was a significant 2-way interaction of strain X time on DA concentrations (F5,152= 9.2, p< 0.001; Figure 1). Following 10 mg/kg MDMA, DA concentrations decreased in mdr1a +/+ mice while DA was unaltered in mdr1a −/− mice. Post-hoc comparisons indicated that DA concentrations 0.3 to 3 h after 10 mg/kg MDMA were significantly lower in wild-type than in mdr1a −/− mice. For the first 4 h after 20 and 40 mg/kg MDMA, DA concentrations were minimally altered in both strains, except for increases in mdr1a −/− mice 2 h after 20 mg/kg MDMA and increases in mdr1a +/+ mice 2 h after 40 mg/kg MDMA. There were not any significant differences between DA concentrations in mdr1a +/+ and mdr1a −/− mice 0.3–4 h after 20 or 40 mg/kg MDMA. DA concentrations were significantly decreased 24 h after 10 and 20 mg/kg MDMA in mdr1a +/+ mice compared to unchanged DA concentrations in mdr1a −/− mice. DA concentrations 24 h after 40 mg/kg MDMA administration did not differ from baseline and were not significantly different between mdr1a +/+ and mdr1a −/− mice. There was a significant 2-way interaction of strain X dose on DA concentrations (F3,152= 23.2, p< 0.001; Figure 1). However, post-hoc comparisons did not reveal dose-related effects of MDMA on DA at any time-point in mdr1a −/− mice. In mdr1a +/+ mice, there were significant dose-related effects on DA. Decreases in DA concentrations 0.3, 2 and 3 h after 10 mg/kg MDMA were significantly different from DA increases after 40 mg/kg MDMA post-administration. Decreases in DA occurring in mdr1a +/+ mice 24 h after 10, 20 and 40 mg/kg MDMA were not significantly dose-related.
Figure 1.
Dopamine, dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in striatum of mdr1a +/+ and mdr1a −/− mice 0.3 to 24 h after 10, 20 or 40 mg/kg MDMA i.p. (n=5 for each time-point). Data are expressed as mean percent of saline control ± 95% confidence intervals. Control mice were sacrificed one h after saline administration i.p. (n=5). Post hoc comparisons for inter-strain differences: *differs from corresponding dose and time point in mdr1a −/− mice and # differs from corresponding dose and time point in mdr1a +/+ mice (p<0.0006). Post hoc comparisons for intra-strain differences: adiffers from saline control; bdiffers from 10mg/kg; cdiffers from 20mg/kg; and ddiffers from 40mg/kg MDMA (p<0.0006).
There was a significant 2-way interaction of strain X time on DOPAC striatal concentrations (F5,152= 9.0, p< 0.001; Figure 1). Following 10 mg/kg MDMA, DOPAC concentrations increased in mdr1a +/+ mice while being unaltered in mdr1a −/− mice. Post-hoc comparisons revealed that DOPAC concentrations 0.3, 1, and 2 h after 10 mg/kg MDMA were higher in mdr1a +/+ than in mdr1a −/− mice. DOPAC concentrations in mdr1a −/− mice for the first 4 h after 20 mg/kg MDMA were decreased or minimally altered while being unaltered in mdr1a +/+ mice. The only significant difference between strains after 20 mg/kg MDMA occurred 1 h post-dose with higher DOPAC concentrations in mdr1a +/+ than in mdr1a −/− mice. DOPAC concentrations were decreased in both strains for the first 4 h after 40 mg/kg MDMA, but there were not any significant inter-strain differences. 24 h after 10 and 20 mg/kg MDMA dosing, DOPAC concentrations were increased 200% relative to saline-treated controls in mdr1a +/+ mice and were significantly different from unchanged DOPAC concentrations in mdr1a −/− mice. Like DA concentrations 24 h after 40 mg/kg MDMA, DOPAC concentrations were unchanged in mdr1a +/+ and mdr1a −/− mice. There was a significant 2-way interaction of strain X dose on DOPAC striatal concentrations (F3,152= 54.8, p< 0.001; Figure 1). Dose-related effects of MDMA on DOPAC were minimal in mdr1a −/− mice. In mdr1a +/+ mice, DOPAC concentrations were significantly higher following 10 as compared to 40 mg/kg MDMA 0.3, 1, 2, 3 and 24 h after MDMA administration.
Concentrations of HVA were minimally altered after MDMA administration and the 2-way interaction for strain X time was not significant (F5,152= 1.9, p> 0.1, Figure 1). Similarly, the 2-way interaction of strain X dose was not significant for HVA (F3,152= 2.2, p> 0.07).
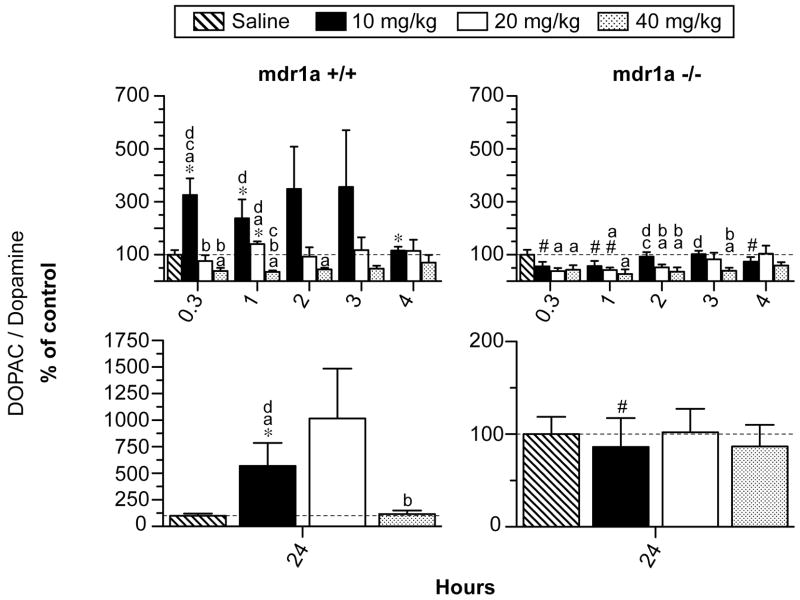
There was a significant 2-way interaction of strain X time on DOPAC/DA ratios in striatal tissue from mdr1a +/+ and mdr1a −/− mice (F5,152= 32.4, p< 0.001; Figure 2). Post-hoc comparisons revealed DOPAC/DA ratios were higher in mdr1a +/+ than in mdr1a −/− mice 0.3, 1, 4 and 24 h after 10 mg/kg MDMA administration. Strain-related differences in DOPAC/DA ratios were not as substantial after 20 mg/kg MDMA. DOPAC/DA ratios were equivalent in mdr1a +/+ and mdr1a −/− mice at all time-points after 40 mg/kg MDMA. There was a significant interaction of strain X dose on DOPAC/DA ratios (F3,152= 25.8, p< 0.001; Figure 2). Dose-related effects of MDMA on DOPAC/DA ratios were less substantial in mdr1a −/− than were observed in mdr1a +/+ mice. In mdr1a +/+ mice, DOPAC/DA concentrations were significantly higher following 10 than 40 mg/kg MDMA 0.3, 1 and 24 h after MDMA administration.
Figure 2.
Upper panels: Dihydroxyphenylacetic acid (DOPAC)/dopamine ratio in striatum of mdr1a +/+ and mdr1a −/− mice 0.3 to 4 h after 10, 20 or 40 mg/kg MDMA i.p. (n=5 for each time-point). Lower panels: DOPAC/dopamine ratio in striatum of mdr1a +/+ and mdr1a −/− mice 24 h after 10, 20 or 40 mg/kg MDMA i.p. (n=5 for each time-point). Data are expressed as mean percent of saline control ± 95% confidence intervals. Control mice were sacrificed one h after saline administration i.p. (n=5). Post hoc comparisons for inter-strain differences: *differs from corresponding dose and time point in mdr1a −/− mice and # differs from corresponding dose and time point in mdr1a +/+ mice (p<0.0006). Post hoc comparisons for intra-strain differences: adiffers from saline control; bdiffers from 10mg/kg; cdiffers from 20mg/kg; and ddiffers from 40mg/kg MDMA (p<0.0006).
Effects of MDMA on striatal 5-HT system of mdr1a +/+ and −/− mice
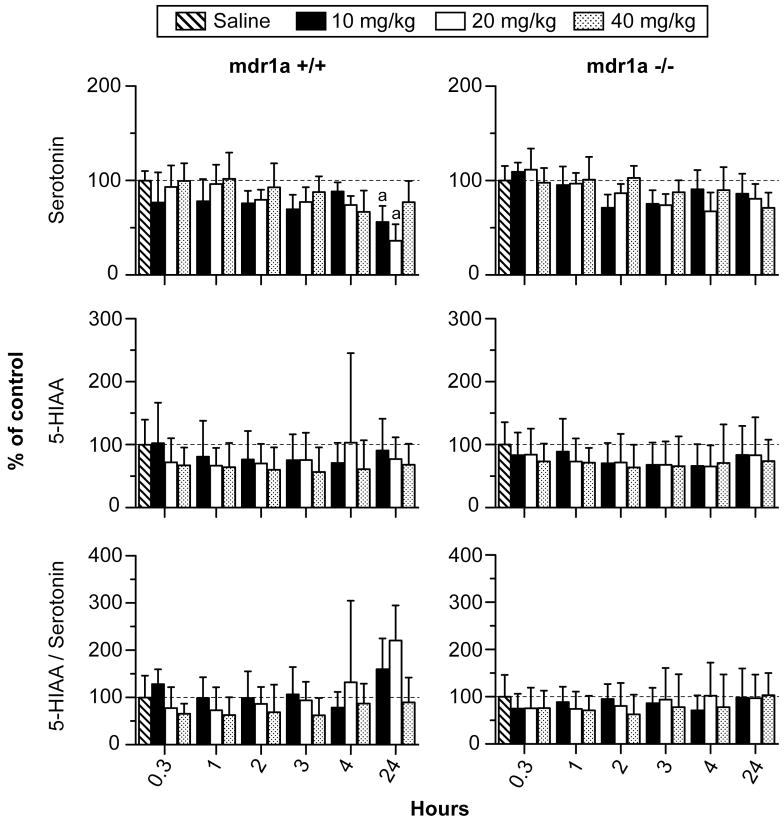
There was a significant 2-way interaction of strain X time on 5-HT concentrations (F5,152= 2.5, p< 0.05; Figure 3). 5-HT was minimally altered for the first 4 h after 10, 20 and 40 mg/kg MDMA in both strains without any significant inter-strain differences. 5-HT concentrations were decreased in mdr1a +/+ mice 24 h after 10 and 20 mg/kg MDMA but were not significantly different from unaltered 5-HT concentrations in mdr1a −/− mice. The 2-way interaction of strain X dose on 5-HT was not significant (F3,152= 1.4, p=0.26).
Figure 3.
Serotonin, 5-hydroxyindole-3-acetic acid (5-HIAA) and 5-HIAA/serotonin ratio in striatum of mdr1a +/+ and mdr1a −/− mice 0.3 to 24 h after 10, 20 or 40 mg/kg MDMA i.p. (n=5 for each time-point). Data are expressed as mean percent of saline control ± 95% confidence intervals. Control mice were sacrificed one h after saline administration i.p. (n=5). Post hoc comparisons for inter-strain differences: *differs from corresponding dose and time point in mdr1a −/− mice and # differs from corresponding dose and time point in mdr1a +/+ mice (p<0.0006). Post hoc comparisons for intra-strain differences: adiffers from saline control; bdiffers from 10mg/kg; cdiffers from 20mg/kg; and ddiffers from 40mg/kg MDMA (p<0.0006).
Concentrations of 5-HIAA were minimally altered after MDMA administration and the 2-way interaction for strain X time was not significant (F5,152= 0.2; p> 1.0, Figure 3). Similarly, the 2-way interaction of strain X dose was not significant for 5-HIAA (F3,152= 0.4, p> 0.70).
MDR1a does not increase MDMA and MDA striatal concentrations
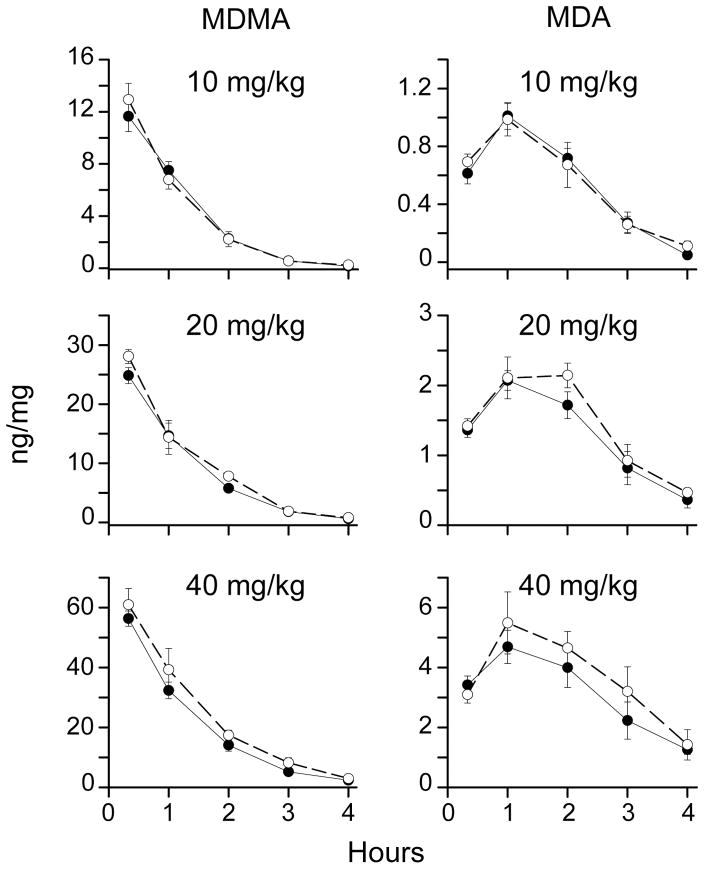
MDMA striatal concentrations decreased over time after MDMA administration and were less than 0.1 ng/mg 24 h after 10, 20 and 40 mg/kg MDMA in mdr1a +/+ and −/− mice (Figure 4). The main effect for dose was significant (F2,144 =888.0, p<0.001) demonstrating dose-related MDMA concentrations in striatum of both mouse strains after 10, 20 and 40 mg/kg MDMA. The interaction of strain X time was not significant (F5,144= 2.1, p= 0.07) and did not support a hypothesis that MDMA concentrations would be higher in mdr1a +/+ than in mdr1a −/− mice. There was a significant interaction of strain X dose (F2,144= 7.5, p< 0.001), however, the pattern did not support a hypothesis that increasing MDMA dose would produce higher MDMA concentrations in striatum with the effect more pronounced in mdr1a +/+ than in mdr1a −/− mice.
Figure 4.
Mean concentrations (± 95% confidence interval) of methylenedioxymethamphetamine (MDMA) and its metabolite methylenedioxyamphetamine (MDA) in mouse striatum after 10, 20 or 40 mg/kg MDMA, i.p. (n=5 for each timepoint). Filled circles depict mdr1a +/+ mice and open circles depict mdr1a −/− mice.
Striatal concentrations of MDA, a minor active metabolite of MDMA, peaked 1 h after dosing and subsequently decreased to less than 0.1 ng/mg 24 h after 10, 20 and 40 mg/kg MDMA (Figure 3). The main effect for dose was significant (F2,144 =597.6, p<0.001) indicating MDA concentrations were dose-related in both mouse strains. The strain X time interaction was not significant (F5,144= 1.7, p=0.2), disproving the hypothesis that MDA concentrations would be higher in mdr1a +/+ than in mdr1a −/− mice. The interaction of strain X dose was significant for MDA (F2,144= 3.7, p< 0.05), but the pattern did not fit the hypothesis that increasing MDMA dose would produce higher MDA striatal concentrations with the effect more pronounced in mdr1a +/+ than in mdr1a −/− mice.
Striatal concentrations of hydroxylated MDMA metabolites, HMA and HMMA, were less than 0.2 ng/mg at all time-points after 10, 20 and 40 mg/kg MDMA administration to mdr1a −/− and mdr1a +/+ mice (data not shown). Striatal specimens collected from control mice administered saline did not contain detectable concentrations of MDMA, MDA, HMMA or HMA and were not included in the ANOVA analysis.
Discussion
These are the first data investigating the role of MDR1a in the acute effects of MDMA in the nigrostriatal DA system. We found that DOPAC/DA ratios, which reflect DA turnover, were increased at 0.3, 3 and 24 h after 10 mg/kg MDMA in mdr1a +/+ mice, while these ratios in mdr1a −/− mice were not statistically different from saline controls. In mdr1a +/+ mice, minimal changes and decreases in DOPAC/DA ratios were observed 0.3–4 h after 20 and 40 mg/kg MDMA, respectively. Decreases in DOPAC/DA ratios were observed in mdr1a −/− mice 0.3–3 h after 20 and 40 mg/kg MDMA. The largest effects on the DA system were 600 and 1000% increases in DOPAC/DA ratios in mdr1a +/+ mice 24 h after 10 and 20 mg/kg MDMA, while these ratios were unchanged in mdr1a −/− mice 24 h after 10, 20 and 40 mg/kg MDMA. There were no significant effects on HVA, 5-HT or 5-HIAA in either mdr1a +/+ or −/− mice. There also were no significant inter-strain differences between MDMA and MDA striatal concentrations after 10, 20 or 40 mg/kg MDMA administration. Therefore, altered MDR1a-mediated transport of MDMA does not account for the observed differences of MDMA’s effects on DOPAC/DA ratios between mdr1a +/+ and −/− mice. A novel MDR1a mechanism or compensatory changes in protein expression/activity in mdr1a −/− mice could account for the observed inter-strain differences on DA turnover.
The large increases in DOPAC/DA turnover 24 h after MDMA administration suggest up-regulation of MAO to compensate for MDMA dopaminergic stimulation. The effects on DA turnover 24 h post-dose in mdr1a +/+ mice exhibited an inverted U dose-response; 40 mg/kg MDMA did not produce a significant effect on DA turnover whereas we observed 600 and 1000% increases after 10 and 20 mg/kg MDMA, respectively. The reason for the inverted U dose-response is unknown but might be related to activation of a presently unknown negative feedback mechanism that might involve striatonigral pathways that serve to suppress DA release from dopaminergic neurons (Wu et al., 2000; Javitt et al., 2005).
Acute MDMA-induced increases in DA in mouse brain tissue are consistent with previously reported observations 0–6 h after MDMA administration to mice (Steele et al., 1989). Elevated concentrations of DA in brain tissue following MDMA administration could result from stimulated DA release/re-uptake (Riddle et al., 2006; Fleckenstein et al., 2007), inhibition of vesicular monoamine transporter (Hansen et al., 2002; Partilla et al., 2006) and/or inhibition of DA metabolism to DOPAC via monoamine oxidase (MAO) (Leonardi and Azmitia, 1994; Fornai et al., 2001; Hrometz et al., 2004). MDMA causes decreased plasmalemmal DA uptake via DAT and decreased vesicular monoamine transporter-2 mediated DA uptake into vesicles (Hansen et al., 2002). The role of MAO on MDMA’s effects has been established with MAO-B deficient mice demonstrating more pronounced striatal DA deficits one week after MDMA administration than wild-type mice (Fornai et al., 2001). Increased striatal concentrations of DA occurring after MDMA administration are alarming considering re-distribution of DA from the reducing environment within synaptic vesicles to cytosolic oxidizing environments that can produce toxic DA-associated oxygen radicals within neurons and ultimately produce terminal destruction (Fleckenstein and Hanson, 2003). DA release could have caused increased hyperthermia, which also plays a role in MDMA toxicity (Capela et al., 2009).
MDMA modestly increased striatal DA concentrations for the first 4 h after MDMA administration in both mdr1a +/+ and mdr1a −/− mice after 20 and 40 mg/kg MDMA. In mdr1a +/+ mice administered 10 mg/kg MDMA, we observed decreased DA and increased DOPAC, indicating increased DOPAC/DA turnover in these mice (Figures 1 and 2). 20 and 40 mg/kg MDMA administration to mdr1a +/+ mice decreased DOPAC/DA turnover, indicating inhibition of MAO-mediated conversion of DA to DOPAC. We also observed decreased DOPAC/DA turnover after 10, 20 and 40 mg/kg MDMA to mdr1a −/− mice. The dose-response relationship for inhibition of DA metabolism was not as striking in mdr1a −/− as in mdr1a +/+ mice. The only significant dose-response differences in DOPAC/DA turnover occurred 2 and 3 h after 10, 20 and 40 mg/kg MDMA in mdr1a −/− mice (Figure 2). This is the first report to detail dose-related inhibition of DA metabolism to DOPAC following MDMA administration to mice. Considering the MDR1a literature, we can speculate on the mechanism of observed inter-strain differences for dose-related MDMA inhibition of DA metabolism. Sanchez-Carbayo et al. observed increased MAO-A in MDR1-transfected human osteosarcoma cells (Sanchez-Carbayo et al., 2003). Therefore, we hypothesize that mdr1a +/+ mice may have higher MAO expression than mdr1a −/− mice. We would predict that increased MAO expression in mdr1a +/+ mice would protect them from MDMA’s inhibitory effects on MAO-mediated metabolism of DA to DOPAC that occurred 0–3 h after MDMA administration. Increased MAO expression in mdr1a +/+ mice could explain why we observed increased DOPAC/DA turnover after 10 mg/kg MDMA, but found decreased DOPAC/DA turnover in mdr1a −/− mice expressing lower MAO levels after 10 mg/kg MDMA.
Excess free radicals play an important role in cell death following amphetamine exposure (Cadet et al., 2007). Production of hydroxyl (Huang et al., 1997) and superoxide (Krasnova et al., 2001) radicals during DA metabolism caused a loss of monoaminergic nerve terminals after amphetamine administration. Quinone formation from elevated intracellular DA concentrations is a factor in amphetamine toxicity (Cadet et al., 2007). Quinone redox cycling generates superoxide radicals and hydrogen peroxide following methamphetamine administration (Stokes et al., 1999; Miyazaki et al., 2006). Increased DA turnover that occurred 24 h after MDMA administration in mdr1a +/+ but not mdr1a −/− mice is an important observation, since hydrogen peroxide and hydroxyl radicals are produced during MAO-mediated metabolism of DA to DOPAC (Hrometz et al., 2004; Brown et al., 2006; Riddle et al., 2006; Fleckenstein et al., 2007). Additionally, 3,4-dihydroxyphenylacetaldehyde (DOPAL) is formed via MAO-mediated deamination of DA, prior to conversion to DOPAC (Burke et al., 2004; Eisenhofer et al., 2004). DOPAL is highly toxic in vivo and in vitro; however, we were unable to investigate the effect on DOPAL concentrations, as DOPAL standards are not commercially available (Burke et al., 2004; Eisenhofer et al., 2004) Increased DA striatal concentrations and increased DOPAC/DA turnover via MAO following MDMA administration are consistent with our previous report of increased neurotoxicity in mdr1a +/+ mice administered MDMA (Mann et al., 1997). Further studies are required to characterize the role of MAO in the disparate effects of MDMA in mdr1a +/+ and −/− mice.
We did not observe any significant effect of MDMA on HVA, 5-HT or 5-HIAA. Catechol-O-methyltransferase (COMT) metabolizes DOPAC to HVA (Eisenhofer et al., 2004); however, dopaminergic neurons in striatum do not highly express COMT (Matsumoto et al., 2003). Neuronal uptake via DAT is thought to be the key mechanism for termination of DA action in striatum (Giros et al., 1996). The limited amount of COMT-mediated conversion of DOPAC to HVA in striatum probably accounts for the unaltered HVA striatal concentrations observed. In contrast to other species, MDMA effects in mice are primarily DA-associated with little effect on the 5-HT system, consistent with the minimal effects on this system observed in our results (Green et al., 2003; Colado et al., 2004; Quinton and Yamamoto, 2006).
MDMA and MDA striatal concentrations were not elevated in mdr1a +/+ mice, indicating that there was no MDR1a-facilitated transport of MDMA and no potentiation of acute and chronic, neurotoxic effects from MDMA due to this mechanism. Our data demonstrate that MDMA and MDA striatal concentrations were similar in mdr1a −/− and mdr1a +/+ mice suggesting that MDMA and MDA are poor MDR1a substrates.
It is possible that 10, 20 and 40 mg/kg MDMA doses exceed MDR1a transport capacity yielding similar MDMA and MDA striatal concentrations in mdr1a +/+ and mdr1a −/− mice. Admittedly, the doses administered to mice during this study exceed 0.5–2 mg/kg MDMA, which estimate typical human oral recreational doses (Hall and Henry, 2006), however a 10 mg/kg dose may simulate heavy MDMA use. It also should be noted that similar MDMA brain concentrations were reported in mdr1a +/+ and mdr1a −/− mice 0.5 and 4 h after administration of 5 mg/kg MDMA, a lower dose than we evaluated in the current studies (Upreti and Eddington, 2008). Furthermore, a number of in vitro studies demonstrated weak or non-existent MDR1a-mediated MDMA transport (Ketabi-Kiyanvash et al., 2003; Bertelsen et al., 2006; Crowe and Diep, 2008; Upreti and Eddington, 2008). Thus, the existing literature and the results presented here regarding the interaction of MDR1a and MDMA indicate that MDMA does not efficiently undergo MDR1a-mediated transport. However, in both our previous report (Mann et al., 1997) and again in this study, we observe inter-strain differences between MDMA-induced alterations of DA concentrations in mdr1a +/+ and −/− mice.
MDMA displays non-linear pharmacokinetics (PK) in human, squirrel monkey and rat MDMA dose escalation studies, such that maximum MDMA plasma concentrations and area under the curves (AUC) are higher than would be predicted by the PK profile of lower MDMA doses (Chu et al., 1996; de la Torre et al., 2000; Kolbrich et al., 2008; Mueller et al., 2008; Baumann et al., 2009). MDMA auto-inhibition of CYP2D6 is the proposed mechanism for non-linear MDMA PK in humans (Heydari et al., 2004). MDMA non-linear PK yielding prolonged exposure to MDMA and metabolites could potentiate MDMA toxicity. Non-linear PK may be occurring at doses administered during this study, however our observations of equivalent brain concentrations of MDMA in mdr1a +/+ and −/− mice indicate that non-linear PK does not account for the observed inter-strain differences in DA turnover.
The key findings of this study were that effects on DA turnover were different following 10 mg/kg MDMA, although MDMA striatal concentrations were equivalent in mdr1a +/+ and −/− mice. DA turnover was increased in mdr1a +/+, but unchanged in mdr1a −/− mice, 0.3–24 h after 10 mg/kg MDMA. Although our data do not support the hypothesis that MDR1a-mediated efflux altered distribution of MDMA in the brain, this does not rule out a role for MDR1a in the observed altered dopaminergic responses in mdr1a +/+ and −/− mice. We propose that either MDR1a is functioning through a novel non-efflux mediated mechanism or else compensatory changes in protein expression/activity in mdr1a −/− mice account for these effects. Further studies are necessary to reveal the mechanism accounting for the differences in MDMA’s actions in mdr1a +/+ and −/− mice. This study highlights disparities of MDMA’s actions in mdr1a +/+ and mdr1a −/− mice and indicates that modulation of MDR1a might be a factor in MDMA’s effects and neurotoxicity.
Acknowledgments
We would like to thank David Epstein and Jennifer Bossert from the National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health, Baltimore, MD for assistance with statistical analysis. This research was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health.
References
- Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol. 1999;12:1150–1157. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Wang X, Rothman RB. 3,4-Methylenedioxymethamphetamine (MDMA) neurotoxicity in rats: a reappraisal of past and present findings. Psychopharmacology (Berl) 2007;189:407–424. doi: 10.1007/s00213-006-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of Dose and Route of Administration on Pharmacokinetics of ({+/−})-3,4-Methylenedioxymethamphetamine (MDMA) in the Rat. Drug Metab Dispos. 2009 doi: 10.1124/dmd.109.028506. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertelsen KM, Greenblatt DJ, von Moltke LL. Apparent active transport of MDMA is not mediated by P-glycoprotein: a comparison with MDCK and Caco-2 monolayers. Biopharm Drug Dispos. 2006;27:219–227. doi: 10.1002/bdd.501. [DOI] [PubMed] [Google Scholar]
- Brown JM, Gouty S, Iyer V, Rosenberger J, Cox BM. Differential protection against MPTP or methamphetamine toxicity in dopamine neurons by deletion of ppN/OFQ expression. J Neurochem. 2006;98:495–505. doi: 10.1111/j.1471-4159.2006.03902.x. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Li SW, Chung HD, Ruggiero DA, Kristal BS, Johnson EM, Lampe P, Kumar VB, Franko M, Williams EA, Zahm DS. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25:101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F. Molecular and cellular mechanisms of ecstasy-induced neurotoxicity: an overview. Mol Neurobiol. 2009;39:210–271. doi: 10.1007/s12035-009-8064-1. [DOI] [PubMed] [Google Scholar]
- Chu T, Kumagai Y, DiStefano EW, Cho AK. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem Pharmacol. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- Colado MI, O’Shea E, Green AR. Acute and long-term effects of MDMA on cerebral dopamine biochemistry and function. Psychopharmacology (Berl) 2004;173:249–263. doi: 10.1007/s00213-004-1788-8. [DOI] [PubMed] [Google Scholar]
- Crowe A, Diep S. pH dependent efflux of methamphetamine derivatives and their reversal through human Caco-2 cell monolayers. Eur J Pharmacol. 2008;592:7–12. doi: 10.1016/j.ejphar.2008.06.090. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Navarro M, Pacifici R, Zuccaro P, Pichini S. Clinical pharmacokinetics of amfetamine and related substances: monitoring in conventional and non-conventional matrices. Clin Pharmacokinet. 2004;43:157–185. doi: 10.2165/00003088-200443030-00002. [DOI] [PubMed] [Google Scholar]
- de la Torre R, Farre M, Ortuno J, Mas M, Brenneisen R, Roset PN, Segura J, Cami J. Non-linear pharmacokinetics of MDMA (‘ecstasy’) in humans. Br J Clin Pharmacol. 2000;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Erives GV, Lau SS, Monks TJ. Accumulation of neurotoxic thioether metabolites of 3,4-(+/−)-methylenedioxymethamphetamine in rat brain. J Pharmacol Exp Ther. 2008;324:284–291. doi: 10.1124/jpet.107.128785. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol. 2003;479:283–289. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fornai F, Giorgi FS, Gesi M, Chen K, Alessri MG, Shih JC. Biochemical effects of the monoamine neurotoxins DSP-4 and MDMA in specific brain regions of MAO-B-deficient mice. Synapse. 2001;39:213–221. doi: 10.1002/1098-2396(20010301)39:3<213::AID-SYN1002>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. Neurotoxicity of methylenedioxyamphetamines (MDMA; ecstasy) in humans: how strong is the evidence for persistent brain damage? Addiction. 2006;101:348–361. doi: 10.1111/j.1360-0443.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylenedioxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’ (MDMA) and related compounds: overview of pathophysiology and clinical management. Br J Anaesth. 2006;96:678–685. doi: 10.1093/bja/ael078. [DOI] [PubMed] [Google Scholar]
- Hansen JP, Riddle EL, Sandoval V, Brown JM, Gibb JW, Hanson GR, Fleckenstein AE. Methylenedioxymethamphetamine decreases plasmalemmal and vesicular dopamine transport: mechanisms and implications for neurotoxicity. J Pharmacol Exp Ther. 2002;300:1093–1100. doi: 10.1124/jpet.300.3.1093. [DOI] [PubMed] [Google Scholar]
- Heydari A, Yeo KR, Lennard MS, Ellis SW, Tucker GT, Rostami-Hodjegan A. Mechanism-based inactivation of CYP2D6 by methylenedioxymethamphetamine. Drug Metab Dispos. 2004;32:1213–1217. doi: 10.1124/dmd.104.001180. [DOI] [PubMed] [Google Scholar]
- Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Hrometz SL, Brown AW, Nichols DE, Sprague JE. 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated production of hydrogen peroxide in an in vitro model: the role of dopamine, the serotonin-reuptake transporter, and monoamine oxidase-B. Neurosci Lett. 2004;367:56–59. doi: 10.1016/j.neulet.2004.05.075. [DOI] [PubMed] [Google Scholar]
- Huang NK, Wan FJ, Tseng CJ, Tung CS. Amphetamine induces hydroxyl radical formation in the striatum of rats. Life Sci. 1997;61:2219–2229. doi: 10.1016/s0024-3205(97)00924-7. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Hashim A, Sershen H. Modulation of striatal dopamine release by glycine transport inhibitors. Neuropsychopharmacology. 2005;30:649–656. doi: 10.1038/sj.npp.1300589. [DOI] [PubMed] [Google Scholar]
- Jones DC, Lau SS, Monks TJ. Thioether metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine inhibit human serotonin transporter (hSERT) function and simultaneously stimulate dopamine uptake into hSERT-expressing SK-N-MC cells. J Pharmacol Exp Ther. 2004;311:298–306. doi: 10.1124/jpet.104.069260. [DOI] [PubMed] [Google Scholar]
- Jones DC, Duvauchelle C, Ikegami A, Olsen CM, Lau SS, de la Torre R, Monks TJ. Serotonergic neurotoxic metabolites of ecstasy identified in rat brain. J Pharmacol Exp Ther. 2005;313:422–431. doi: 10.1124/jpet.104.077628. [DOI] [PubMed] [Google Scholar]
- Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A. 2005;102:3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen SN, Spigset O, Slordal L. The dark side of ecstasy: neuropsychiatric symptoms after exposure to 3,4-methylenedioxymethamphetamine. Basic Clin Pharmacol Toxicol. 2008;102:15–24. doi: 10.1111/j.1742-7843.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Ketabi-Kiyanvash N, Weiss J, Haefeli WE, Mikus G. P-glycoprotein modulation by the designer drugs methylenedioxymethamphetamine, methylenedioxyethylamphetamine and paramethoxyamphetamine. Addict Biol. 2003;8:413–418. doi: 10.1080/13556210310001646475. [DOI] [PubMed] [Google Scholar]
- Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Plasma pharmacokinetics of 3,4-methylenedioxymethamphetamine after controlled oral administration to young adults. Ther Drug Monit. 2008;30:320–332. doi: 10.1097/FTD.0b013e3181684fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Jayanthi S, Oyler J, Moran TH, Huestis MA, Cadet JL. Amphetamine-induced toxicity in dopamine terminals in CD-1 and C57BL/6J mice: complex roles for oxygen-based species and temperature regulation. Neuroscience. 2001;107:265–274. doi: 10.1016/s0306-4522(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Leonardi ET, Azmitia EC. MDMA (ecstasy) inhibition of MAO type A and type B: comparisons with fenfluramine and fluoxetine (Prozac) Neuropsychopharmacology. 1994;10:231–238. doi: 10.1038/npp.1994.26. [DOI] [PubMed] [Google Scholar]
- Linnet K, Ejsing TB. A review on the impact of P-glycoprotein on the penetration of drugs into the brain. Focus on psychotropic drugs. Eur Neuropsychopharmacol. 2008;18:157–169. doi: 10.1016/j.euroneuro.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Mann H, Ladenheim B, Hirata H, Moran TH, Cadet JL. Differential toxic effects of methamphetamine (METH) and methylenedioxymethamphetamine (MDMA) in multidrug-resistant (mdr1a) knockout mice. Brain Res. 1997;769:340–346. doi: 10.1016/s0006-8993(97)00754-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR. Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience. 2003;116:127–137. doi: 10.1016/s0306-4522(02)00556-0. [DOI] [PubMed] [Google Scholar]
- Miller RT, Lau SS, Monks TJ. Effects of intracerebroventricular administration of 5-(glutathion-S-yl)-alpha-methyldopamine on brain dopamine, serotonin, and norepinephrine concentrations in male Sprague-Dawley rats. Chem Res Toxicol. 1996;9:457–465. doi: 10.1021/tx9501546. [DOI] [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Fukuda M, Kitaichi K, Miyoshi K, Ogawa N. Methamphetamine-induced dopaminergic neurotoxicity is regulated by quinone-formation-related molecules. Faseb J. 2006;20:571–573. doi: 10.1096/fj.05-4996fje. [DOI] [PubMed] [Google Scholar]
- Morton J. Ecstasy: pharmacology and neurotoxicity. Curr Opin Pharmacol. 2005;5:79–86. doi: 10.1016/j.coph.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Mueller M, Peters F, Maurer H, McCann U, Ricaurte GA. Non-linear Pharmacokinetics of MDMA (“Ecstasy”) and its Major Metabolites in Squirrel Monkeys at Plasma Concentrations of MDMA that Develop After Typical Psychoactive Doses. J Pharmacol Exp Ther. 2008;327:38–44. doi: 10.1124/jpet.108.141366. [DOI] [PubMed] [Google Scholar]
- O’Shea E, Easton N, Fry JR, Green AR, Marsden CA. Protection against 3,4-methylenedioxymethamphetamine-induced neurodegeneration produced by glutathione depletion in rats is mediated by attenuation of hyperthermia. J Neurochem. 2002;81:686–695. doi: 10.1046/j.1471-4159.2002.00844.x. [DOI] [PubMed] [Google Scholar]
- Partilla JS, Dempsey AG, Nagpal AS, Blough BE, Baumann MH, Rothman RB. Interaction of amphetamines and related compounds at the vesicular monoamine transporter. J Pharmacol Exp Ther. 2006;319:237–246. doi: 10.1124/jpet.106.103622. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. Aaps J. 2006;8:E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Role of monoamine transporters in mediating psychostimulant effects. Aaps J. 2005;7:E847–851. doi: 10.1208/aapsj070481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. Aaps J. 2006;8:E413–418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Carbayo M, Belbin TJ, Scotlandi K, Prystowsky M, Baldini N, Childs G, Cordon-Cardo C. Expression profiling of osteosarcoma cells transfected with MDR1 and NEO genes: regulation of cell adhesion, apoptosis, and tumor suppression-related genes. Laboratory investigation; a journal of technical methods and pathology. 2003;83:507–517. doi: 10.1097/01.lab.0000064702.63200.94. [DOI] [PubMed] [Google Scholar]
- Scheidweiler KB, Barnes AJ, Huestis MA. A validated gas chromatographic-electron impact ionization mass spectrometric method for methamphetamine, methylenedioxymethamphetamine (MDMA), and metabolites in mouse plasma and brain. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;876:266–276. doi: 10.1016/j.jchromb.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. MDMA transiently alters biogenic amines and metabolites in mouse brain and heart. Pharmacol Biochem Behav. 1989;34:223–227. doi: 10.1016/0091-3057(89)90303-1. [DOI] [PubMed] [Google Scholar]
- Stokes AH, Hastings TG, Vrana KE. Cytotoxic and genotoxic potential of dopamine. J Neurosci Res. 1999;55:659–665. doi: 10.1002/(SICI)1097-4547(19990315)55:6<659::AID-JNR1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20:211–225. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Upreti VV, Eddington ND. Fluoxetine pretreatment effects pharmacokinetics of 3,4-methylenedioxymethamphetamine (MDMA, ECSTASY) in rat. J Pharm Sci. 2008;97:1593–1605. doi: 10.1002/jps.21045. [DOI] [PubMed] [Google Scholar]
- Wu Y, Pearl SM, Zigmond MJ, Michael AC. Inhibitory glutamatergic regulation of evoked dopamine release in striatum. Neuroscience. 2000;96:65–72. doi: 10.1016/s0306-4522(99)00539-4. [DOI] [PubMed] [Google Scholar]
- Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]