Abstract
Cyclin D1 belongs to the family of proteins that regulates progression through the G1-S phase of the cell cycle through binding to cyclin dependent kinase 4 to phosphorylate the retinoblastoma protein and release E2F transcription factors for progression through cell cycle. Several cancers, including breast, colon and prostate over-express the cyclin D1 gene. However, the correlation between cyclin D1 over-expression with E2F target gene regulation or cyclin dependent kinase-dependent cyclin D1 activity with tumor development have not been identified. This suggests that the role of cyclin D1 in oncogenesis may be independent of its function as a cell cycle regulator. One such function is the role of cyclin D1 in cell adhesion and motility. Filamin A, a member of the actin-binding filamin protein family, regulates signaling events involved in cell motility and invasion. Filamin A has also been associated with a variety of cancers including lung, prostate, melanoma, human bladder cancer, and neuroblastoma. We hypothesized that elevated cyclin D1 facilitates motility in the invasive MDA-MB-231 breast cancer cell line. We show that MDA-MB-231 motility is affected by disturbing cyclin D1 levels or cyclin D1-cdk4/6 kinase activity. Using mass spectrometry, we found that cyclin D1 and Filamin A co-immunoprecipitate and that lower levels of cyclin D1 are associated with decreased phosphorylation of FLNa at serine 2152 and 1459. We also identify many proteins related to cytoskeletal function, biomolecular synthesis, organelle biogenesis, and calcium regulation whose levels of expression change concomitant with decreased cell motility induced by decreased cyclin D1 and cyclin D1-cdk4/6 activity.
Introduction
The canonical function of cyclin D1 is to promote progression through the G1-S phase of the cell cycle through binding to cyclin dependent kinase 4 (cdk4) to phosphorylate and inactivate the retinoblastoma protein and release E2F transcription factors. Several human cancers, including breast, colon and prostate, and hematopoietic malignancies, over-express the cyclin D1 gene (1–3). However, there is no correlation between cyclin D1 over-expression and regulation of E2F target genes by microarray analysis nor between cdk-dependent cyclin D1 activity and tumor development, suggesting that the role of cyclin D1 in oncogenesis is at least partially independent of its function as a cell cycle regulator (4, 5). Cyclin D1 has recently been associated with cell adhesion and motility in primary bone macrophages (6). Studies in cyclin D1−/− mouse embryo fibroblasts revealed that cyclin D1 inhibits Rho-activated kinase II and thrombospondin 1 to promote cell migration (7). The cdk inhibitor p16INK4a has also been shown to inhibit the migration of erythroleukemia and endothelial cells (8, 9). Indeed, p16INK4a co-localized in the ruffles and lamellipodia of migrating endothelial cells together with cyclin D1, cdk4/6, and the αvβ3-integrin machinery.
Filamin A (FLNa), a member of the non-muscle actin-binding protein family, is a widely expressed molecular scaffold protein that regulates signaling events involved in cell motility and invasion by interacting with integrins, transmembrane receptor complexes, adaptor molecules, and second messengers (10, 11). FLNa has recently been shown to bind cyclin B1/cdk1 in a yeast two-hybrid system using recombinant glutathione S-transferase cyclin B1 protein as bait and a 10.5 day old embryonic mouse library as prey (12). Using truncated recombinant FLNa and cyclin B1 protein fragments, the regions of interaction between FLNa and cyclin B1 was shown to be located within amino acids 1–40 of cyclin B1 and the FLNa amino-terminal region in repeat 9. In addition to cyclin B1, filamins have been reported to bind with over thirty proteins and because many filamin-interacting proteins are membrane receptors for cell signaling molecules, filamins may be involved in coordinating a variety of signal transduction pathways (13). For example, FLNa has been shown to be a substrate for calcium-calmodulin-dependent kinase II, interacting with F-actin to promote migration of human neck squamous cell carcinoma cells (14). FLNa has also been shown to interact with prostate specific antigen and regulate androgen receptor (15, 16). In addition, FLNa has been shown to be a key element in TGF-β signaling through its association with SMADs(17) and in A549 lung carcinoma cells undergoing Epithelial-Mesenchymal Transition(18). FLNa has also been associated with a variety of cancers including prostate, melanoma, human bladder cancer, and neuroblastoma (10, 19, 20).
We hypothesized that elevated cyclin D1 facilitates motility in the highly invasive and metastatic MDA-MB-231 breast cancer cell line. Although there are many proteins that have been shown to affect migration and invasion in this cell line, our focus on these molecules is due to the fact that many of the known proteins affect mitogenic signals which effect levels of cyclin D1 and there are several known kinases effecting FLNa and the increasing evidence that FLNa plays a key role in many processes such as Epithelial-Mesenchymal Transition. In our studies, we found that the cell motility of MDA-MB-231 cells can be affected by altering cyclin D1 levels or cyclin D1-cdk4/6 kinase activity. Using matrix assisted laser desorption ionization mass spectrometry (MALDI MS), we found cyclin D1 co-immunoprecipitates with the actin cytoskeleton protein FLNa and that the phosphorylation state of FLNa was concomitantly affected when either cyclin D1 levels or cyclin D1-cdk4/6 kinase activity were altered. We also found that lower levels of cyclin D1 are associated with decreased phosphorylation of FLNa at serine 2152 and 1459. We also analyzed the effects of decreasing cyclin D1 and cyclin D1-cdk4/6 activity on the global phosphoproteome. Our analyses revealed changes in protein expression in many proteins related to cytoskeletal function, biomolecular synthesis, organelle biogenesis, and calcium regulation concomitant with decreased cell motility induced by decreased cyclin D1 and cyclin D1-cdk4/6 activity.
Materials and Methods
Cell culture
Human breast carcinoma MDA-MB-231 cells (ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing penicillin and streptomycin (100 mg of each/liter) and supplemented with 10% fetal bovine serum (FBS) at 37°C in 5.0% CO2. The Stable Isotopic Labeling by Amino Acids in Cell Culture (SILAC™) Flex DMEM Media (Invitrogen, Carlsbad, CA) was prepared as per manufacturer’s recommendation using (heavy amino acids 13C6 15N4 Arg and 13C6 Lys)and these cells were used in SILAC-based mass spectrometry experiments (21).
Cell invasion/migration assay
The cell invasion assay was conducted using BD Biocoat Matrigel 24-well invasion chambers with filters coated with extracellular matrix on the upper surface (BD Biosciences, Bedford, MA). The experiments were done according to the manufacturer’s protocol. Experiments were done in triplicate (mean ± standard error). 2.5 × 104 cells were added to the upper chamber and allowed to invade for 24 hours. The experiments were done according to the manufacturer’s protocol.
Wound healing assay
MDA-MB-231 cells were grown to 70% confluence in 6-well plates. Linear scratches were made with a micropipette tip across the diameter of the well, and dislodged cells were rinsed with PBS. Cell culture medium was replaced with fresh DMEM containing 10% FBS. The cells were allowed to grow and the width of the wound was monitored at the specified times for the degree of wound healing.
Fluorescent Immunocytochemistry
Cells were prepared as in the wound healing assay and probed with mouse anti-cyclin D1(DCS-6, 1:50, Cell Signaling Technology, Danvers, MA) and anti-FLNa (1:100, Santa Cruz Biotechnology, Santa Cruz, CA) primary antibodies in 1% bovine serum albumin (BSA) at 4°C overnight. Then cells were incubated with goat anti-mouse IgG conjugated to Alexa 488 and goat anti-rabbit IgG conjugated to Alexa 647 (1:2000, Invitrogen Corporation, Carlsbad, CA) 1% BSA for 1 hour. Cells were stained with 100 ng/ml 4', 6’-diamidino-2-phenylindole hydrochloride (DAPI) in PBS for two minutes to visualize the nuclei. Images were captured digitally using a Zeiss LSM 510 META confocal microscope.
Lysis buffers, immunoprecipitation and Western blot
Whole cell lysates for Western blots were prepared in a modified RIPA buffer (25 mM Tris–HCl pH 7.5, 150 mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS) supplemented with 1 mM sodium orthovanadate and protease inhibitor cocktail (Complete EDTA-free protease inhibitor cocktail from Roche Applied Science, Indianapolis, IN). For the immunoprecipitation studies, whole cell lysates were prepared in COPRE lysis buffer (20 mM HEPES, pH 7.9, 50 mM sodium chloride, 0.1% NP-40, 10% glycerol, 1 mM dithiothreitol). 500 µg of COPRE lysates were incubated with 1µg of antibodies for an hour at 4°C before adding 40 µl of protein G magnetic beads (Invitrogen Corporation, Carlsbad, CA) for an overnight incubation at 4°C. The following antibodies were used for Western blotting: anti-cyclin D1 (Ab-3) polyclonal antibody (Lab Vision/Neomarker, Fremont, CA); anti-cyclin D1 (DCS-6) monoclonal antibody, anti-CDK4 (C-22) and anti-actin (C-11) polyclonal antibodies, anti-FLNa and anti-phospho-FLNa (Ser2152) polyclonal antibodies (Cell Signaling Technology, Danvers, MA); and ImmunoPure horseradish peroxidase conjugated goat anti-rabbit antibodies (Pierce Biotechnology, Rockford, IL). Primary antibodies were used at 1:1000 dilution and secondary HRP-conjugated antibodies were used at 0.05ug/ml.
Perturbation of the cyclin D1-cdk complex by p16Ink4 peptides
Peptides corresponding to amino acids 84 to 103 of human p16INK4a protein with a C-terminal sequence of 16 amino acids encoding the Antennapedia homeodomain (Penetratin) were synthesized. Peptide 20 (DAAREGFLATLVVLHRAGARRQIKIWFQNRRMKWKK) with the substitution of aspartic acid-92 with alanine has a lower 50% inhibitory concentration (IC50) to inhibit cyclin D1-cdk4/6 phosphorylation of a glutathione S-transferase (GST)-pRb protein in vitro and to arrest cell cycle progression in G1 than the corresponding peptide containing the wild-type sequence and Peptide 21 (DAAREGFLDTLAALHRAGARRQIKIWFQNRRMKWKK) carrying the substitution of valine-95 with alanine and valine-96 with alanine has an increased IC50 in vitro and has lost ~60% of the cell cycle inhibitory capacity (22, 23). These peptides were added to the cell culture medium at a concentration of 20 µM.
Perturbation of the cyclin D1-cdk complex by cyclin D1 siRNA
Cyclin D1 expression was inhibited using validated Stealth™ siRNAs (Invitrogen Corporation, Carlsbad, CA). Two different inhibiting RNA used in our studies and were found to have different efficiencies of cyclin D1 knockdown. They are CCND1(51) (AUGGUUUCCACUUCGCAGCACAGGA) and CCND1(52) (UUAGAGGCCACGAACAUGCAAGUGG). Invitrogen’s pre-designed negative control siRNAs were also used. Transfections were done with Lipofectamine 2000 as per manufacturer’s recommendation, 200 pmole of siRNA and 5 µl of Lipofectamine 2000 for each well of a 6 well plate (Invitrogen Corporation, Carlsbad, CA).
Proteomics
The identification of phosphoproteins was accomplished using two complementary methods. For both methods, cells were first grown in SILAC medium as described. In the first method, after treatment with the inhibitory peptides, phosphoproteins were isolated using the Qiagen PhosphoProtein Purification kit as per the manufacturer’s instructions. In the second method, phosphopeptides were isolated using PhosSelect (Sigma Aldrich) as per the manufacturer’s instructions. Proteins separated on gels were identified using LC-MALDI on a 4800 Proteomics Analyzer (Applied Biosystems, Inc) and the phosphopeptides were analyzed usng ESI-MS on a Proteome X workstation (Thermo-Fisher). Peptide identification was performed on an in-house Mascot Server for the LC-MALDI spectra and on Sequest for the ESI-MS spectra. Additional information can be found in the supplemental material.
Bioinformatics Analysis
Function information of identified phosphoproteins was obtained from the Swiss-Prot database. To determine if any types of proteins are over-represented, enrichment analysis of their gene ontology (GO) terms was performed. To find statistically over-represented GO categories among phosphoproteins identified in this study, we used the BiNGO plugin for Cytoscape (24). The required dataset files were created as described in the BiNGO User Guide. The enrichment analysis was done using the "HyperGeometric test" with correction for multiple hypothesis testing using the following parameters: GO_Biological_Process ontology, annotation for H. sapiens. GO terms that were significant with p-value <0.05 were determined to be over-represented.
Results
Decreased cyclin D1 and cyclin D1-cdk4/6 kinase activity reduces the invasion and migration potential of MDA-MB-231 breast cancer cells
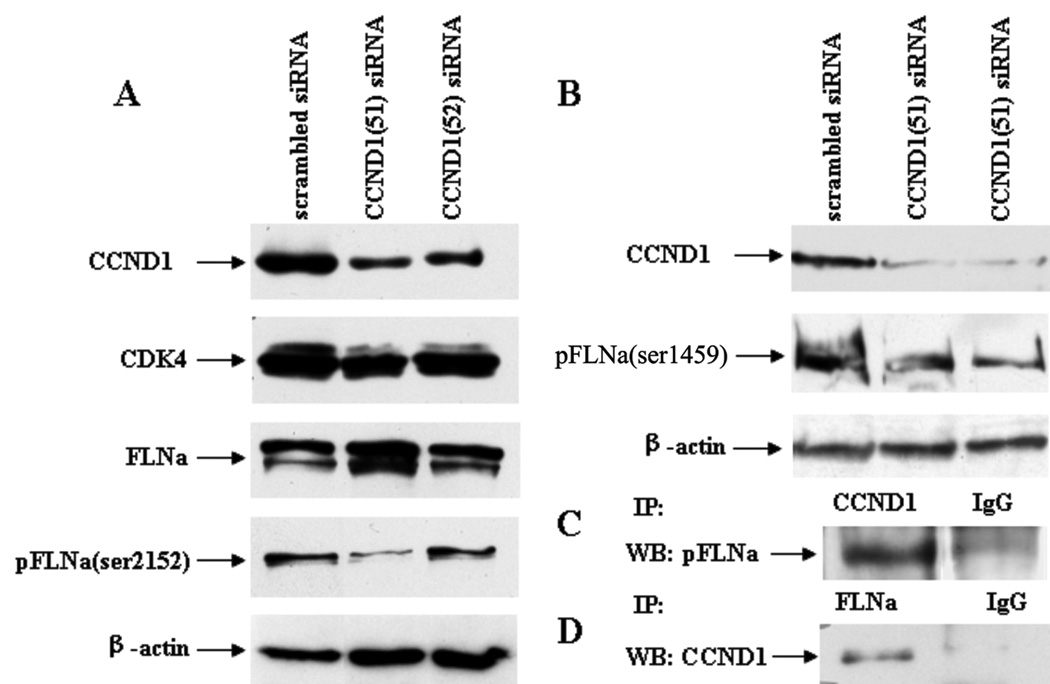
Previous studies have shown that cyclin D1−/− mouse embryo fibroblasts display increased cellular adherence, defective motility, and impaired wound response compared to those with restored cyclin D1 levels (7). In order to determine if cyclin D1 also helps control motility in the highly migratory MDA-MB-231 breast cancer cells, cyclin D1 mRNA and protein expression was inhibited using two available siRNA, CCND1(51) and CCND1(52). A control siRNA with random sequence was used as negative control. Western blot data shows that both cyclin D1-specific siRNAs inhibit cyclin D1 protein expression, albeit at different levels. CCND1(52) inhibits cyclin D1 by approximately 50% and CCND1(51) inhibits cyclin D1 by greater than 90% compared to cells transfected with control siRNA (Figure 1A).
Figure 1.
MDA-MB-231 cells were transfected with scrambled, CCND1(51), and/or CCND1(52) siRNA. The amount of cyclin D1 protein was lower after transfection with the cyclin D1-specific siRNA compared to scrambled siRNA. The amount of CDK4 and FLNa was not affected while the amount of pFLNa (ser-2152) (A) and pFLNa(ser-1459) (B) decreased in cells transfected with the cyclin D1-specific siRNA with greater effect with CCND1(51).
Filamin A (FLNa) is a binding partner of cyclin D1. (C) Anti-cyclin D1 immunoprecipitations were performed using MDA-MB-231 protein lysate. Phosphorylated FLNa was detected by Western blot of the immunoprecipitated fraction. (D) Anti-FLNa immunoprecipitations were performed using MDA-MB-231 protein lysate. Cyclin D1 was detected by Western blot of the immunoprecipitated fraction.
Using these siRNAs, the role of cyclin D1 on MDA-MB-231 cell migration was assessed using the wound healing assay. In Table 1, we report the average width of the wound for the cells treated with the two cyclin D1-specific siRNAs and for the control siRNA relative to the initial wound width 12 hours after scratching. Cells treated with the cyclin D1-specific siRNAs had significantly wider wounds compared to cells treated with control siRNA (p<0.001).
Table 1.
Summary of wound healing and invasion assays using MDA-MB-231 cells transfected with cyclin D1-specific siRNA or p16Ink4a peptides.
| Treatment | Width of wounds after 12 hours (% of initial ± SD) |
Number of invading cells after 24 hours(avg±SD) |
|---|---|---|
| Scr siRNA (M) | 0.12 ± 0.08 | 38.4 ± 11.6 |
| CCND1(51) | 0.72 ± 0.22* | 0.15 ± 0.48* |
| CCDN1(52) | 0.42 ± 0.16* | 0.2 ± 0.52* |
| Width of wounds after 24 hours (% of initial ± SD) |
||
| Wt | 0.04 ± 0.02 | |
| p20 | 0.53 ± 0.07* | |
| p21 | 0.38 ± 0.04* | |
We next examined the role of cyclin D1 in the ability of MDA-MB-231 cells to invade in Matrigel-coated modified Boyden Invasion Chambers. Transfection with either of the cyclin D1-specific CCND1(51) or CCND1(52) siRNAs almost completely abolished the ability of MDA-MB-231 cells to cross the membrane. Very few cells transfected with either cyclin D1-specific siRNA crossed the membrane (< 1 cell per field of view) when assayed 12 hours after transfection (Table 1). In contrast, in cells transfected with control scrambled siRNA, an average of 38.4 cells were seen per field of view.
To determine if cell migration is also dependent on cdk4/6 activity, we inhibited kinase activity by introducing two peptides derived from p16INK4a into the culture media of MDA-MB-231 cells (22, 23). The p16 INK4 family of proteins inhibits cdk4 and cd6 kinase activities through direct interaction with the kinase subunit only. The p16 INK4a p21 peptide contains two alanine substitutions at valine 95 and 96 of the p16 INK4a protein and the p16 INK4a p20 peptide contains a substitution of aspartic acid-92 to alanine. These peptides have been shown to be taken up from the tissue culture medium (22). In these studies, cyclin D1-cdk4/6 kinase activity was first blocked by incubating cells with 20µM of p20 or p21 p16INK4a peptide for 24 hours. The monolayer was then scratched to create a wound, the monolayer washed and incubated with peptides for an additional 24 hours. The wound was completely healed in the untreated cells after 24 hours, while in the cells treated with either p20 or p21 peptide, there is incomplete wound healing and the difference for both p20- and p21-treated cells and wild-type was statistically significant with a p-value < 4.0×10−5 (Table 1). The p20 peptide has been shown to be a stronger kinase inhibitor than p21 (22). Consistent with this, p20 was more effective than p21 at inhibiting the wound healing activity of these cells.
Filamin A (FLNa) binds to cyclin D1 in vitro
Although the mechanism by which cyclin D1 influences cellular migration is not well understood, several studies using cells from cyclin D1-deficient mice have been reported (6, 7, 22). In order to identify proteins that interact directly with cyclin D1, immunoprecipitation experiments were conducted using MCF-7 cells transfected with FLAG-tagged cyclin D1. The immunoprecipitated proteins were first separated on a SDS-PAGE gel and stained with Coomassie R250. Bands were excised, digested with trypsin and the proteins identified by MALDI tandem mass spectrometry. A novel binding partner, the actin cytoskeleton protein filamin A (FLNa) was identified in a band that migrated above the 220 kDa molecular weight marker, consistent with the molecular weight of 280 kDa of FLNa. FLNa has been shown to be critical for cellular motility as it promotes orthogonal branching of actin filaments and links actin filaments to membrane glycoproteins and various transmembrane proteins.
Because of the role of FLNa in cell motility and also because FLNa is a known phosphoprotein, we hypothesized that our observation of cyclin D1-cdk4’s effect on cell migration is mediated through phosphorylation of FLNa. While there are many potential sites of phosphorylation on FLNa, only two S2152 and S2523 of the 28 have been associated with cytoskeletal reorganization. (http://www.phosphosite.org/proteinAction.do?id=2546&showAllSites=false). Phosphorylation at S2152 has been shown to be required for Pak1-mediated membrane ruffling and for regulation of cellular migration by Ribosomal S6 Kinase (RSK), a key kinase in the Ras-MAPK pathway (25, 26). We therefore checked to see if FLNa phosphorylated at S2152 immunoprecipitates with endogenous cyclin D1 in MDA-MB-231 cells. We found that FLNa phosphorylated at serine 2152 (pFLNa) co-precipitated with cyclin D1 using an antibody against phosphorylated FLNa in a Western blot of the cyclin D1 precipitate (Figure 1C), establishing the interaction of phosphorylated FLNa and with endogenous cyclin D1 in MDA-MB-231 cells. As further proof, we immunoprecipitated endogenous FLNa and then used an antibody against cyclin D1 in a Western blot of the FLNa precipitate and found that cyclin D1 co-precipitated with FLNa, verifying the interaction between FLNa and cyclin D1 (Figure 1D). These results show that cyclin D1 and pFLNa(Ser2152) are binding partners in MDA-MB-231 protein lysates.
Cyclin D1 and FLNa co-localize in MDA-MB-231 cell ruffles
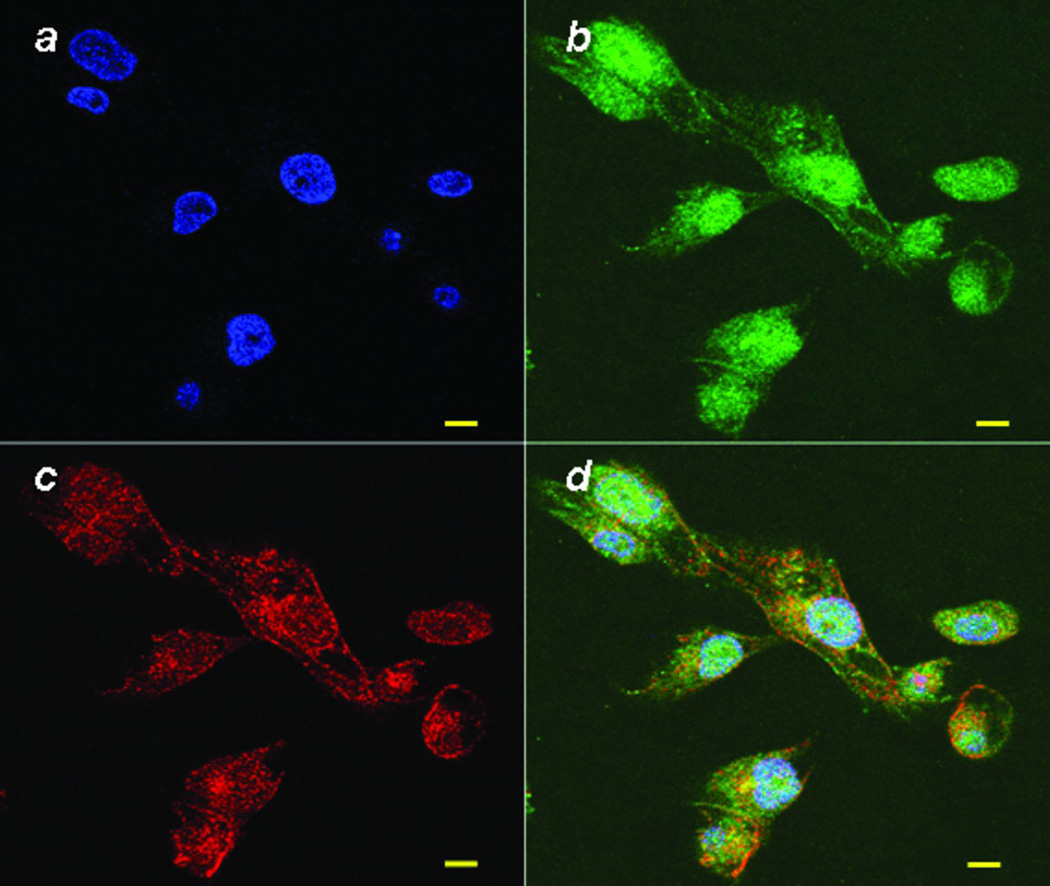
Although cyclin D1 is primarily located in the nucleus, there have been some reports that there are significant levels in the cytoplasm, particularly near the cell membrane. To further investigate if cyclin D1 and FLNa are interacting at the cell membrane and that this co-localization is likely a functional interaction related to migration, we performed immunofluorescence double labeling of cyclin D1 and FLNa. In Figure 2, we show using confocal microscopy that the signal of cyclin D1 was mainly shown in nucleus with lower, but significant signal in the cytoplasm and cell membrane. FLNa was observed to be localized primarily in the cytoplasm and in cell membrane. We observed that cyclin D1 and FLNa proteins co-localize strongly in cell ruffles in those cells that migrated into the gaps created by wounding indicating that the co-localization of cyclin D1 with FLNa is in migrating cells, but not in the cells that are not migrating.
Figure 2.
Cyclin D1 and FLNa co-localize in cell ruffles. MDA-MB-231 cells were stained for cyclin D1 (Green), FLNa (Red) and DNA (blue). Co-localization of cyclin D1 and FLNa is indicated by the yellow color in the merged picture. The scale bar is 10 µm.
Mechanism of Cyclin D1-dependent migration and invasion
We have shown that decreased cyclin D1 protein expression and cyclin D1-cdk4/6 activity decreases the invasion and migration potential of MDA-MB-231 cells. We have also shown that cyclin D1 and FLNa precipitate together in an immunoprecipitation assay. Because cyclin D1 is known as the regulatory subunit of a dimeric holoenzyme that includes cdk4, we next measured the effects of cyclin D1 knockdown on the level of protein expression of cdk4, FLNa, and pFLNa. MDA-MB-231 cells were transfected with either control siRNA or cyclin D1-specific siRNA (CCND1(51) and CCND1(52)) for 48 hours and protein expression was then measured by Western blot (Figure 1A). As expected, cyclin D1 protein expression was lower in cells transfected with cyclin D1-specific siRNA (when compared to the actin loading control it is reduced by 80% and 40% respectively), but neither FLNa nor cdk4 protein expression was affected. However, the level of phosphorylated FLNa was lower in cells transfected with both cyclin D1-specific siRNA. These data support the hypothesis that FLNa phosphorylation is cyclin D1 dependent.
Identification of other cyclin D1/cdk4-dependent phosphoproteins
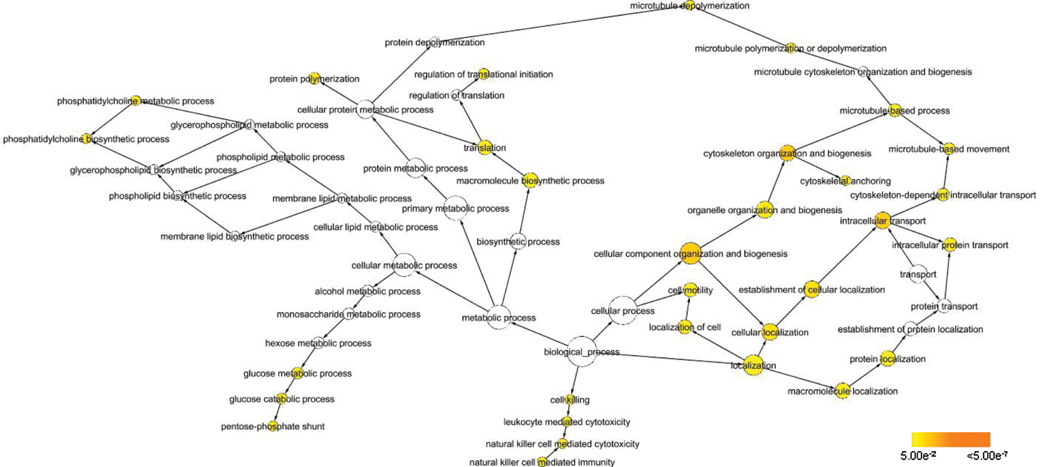
To identify other proteins whose phosphorylation status is dependent on cyclin D1 and cdk4/6, we used the SILAC™ in vivo labeling strategy combined with phosphoprotein enrichment to identify proteins that are differentially expressed 48 hours after introduction of the p16INK4a peptide P20 to inhibit cyclin D1-cdk4/6. We identified approximately 150 phosphoproteins. By comparing the peak intensities of the isotopically-labeled peptides, we were able to determine that 44 were up-regulated and 19 were down-regulated responding with at least a 1.5-fold change in expression level in response to cyclin D1-cdk4/6 inhibition (Table 2). The majority of these phosphoproteins have been previously associated with some type of cancer either in vitro or in vivo. We used Biological Network Gene Ontology tool (BiNGO), a Java-based tool to determine which Gene Ontology (GO) categories are statistically over-represented in the set of identified phosphoproteins (24). Although it is currently not possible to analyze the entire phosphoproteome in a single experiment and the enrichment process may result in an unbalanced enrichment of certain types of proteins, we assume that particular functional groups containing a larger-than-expected number of affected proteins are isolated based on the underlying physiological process and not due to the enrichment process. Figure 3 shows the graphical representation of the results. The colored nodes are those determined to be over-represented with statistical significance. We found that by looking at the closest branch points of the over-represented GO_biological processes, four major categories are represented: 1) cellular organization and biogenesis/localization, which contains cytoskeleton-related processes, 2) metabolic processes, 3) cell motility, and 4) metabolic processes. Table 3 lists the Gene ID residing under each of these categories. Many of these proteins are classified under multiple processes. Specifically, many of the proteins classified under cellular organization and biogenesis/localization are involved with cell motility as well as cytoskeletal-related processes such as actin- and microtubule-based movement and actin binding, suggesting that increased protein synthesis and processes related to organelle and cytoskeletal components are important in the decreased cell migration phenotype resulting from cyclin D1-cdk4/6 repression. Many of the proteins categorized under metabolism are involved in protein synthesis.
Table 2.
List of phosphoproteins whose level of expression was altered by at least 1.5-fold are listed by inhibition of Cyclin D1-cdk4/6 activity using p16INK4a peptides in MDA-MB-231 cells.
| Gene Symbol | Name | GenBank Accession No. |
Fold Change (p16Ink4 peptide vs. no peptide) |
Associatio n with cancer |
|---|---|---|---|---|
| c11orf30 | EMSY(chromosome 11 ORF 30) | Q7Z589 | 2.3 | × |
| CALU | Calumenin precursor(Crocalbin) | O43852 | 2.9 | × |
| EEF1B2 | eukaryotic translation elongation factor 1 beta 2 | P24534 | 2.4 | × |
| EIF5 | eukaryotic translation initiation factor 5 | P55010 | 2.8 | × |
| ENO1 | enolase 1, (alpha) | P06733 | 2.4 | × |
| ERBB2IP | erbb2 interacting protein | Q96RT1 | 2.6 | × |
| FAM120A | family with sequence similarity 120A | Q9NZB2 | 2.4 | |
| GATA4 | GATA binding protein 4 | P43694 | 2.8 | × |
| HSPB1 | heat shock 27kDa protein 1 | P04792 | 2.2 | × |
| ITIH2 | inter-alpha(globulin) inhibitor H2 | P19823 | 2.5 | × |
| ITSN2 | intersectin2(ITSN2, SH3P18) | Q9NZM3 | 2.3 | |
| KRT1 | Keratin 1 (epidermolytic hyperkeratosis, CK1) | P04264 | 2.1 | × |
| KRT10 | keratin 10(epidermolytic hyperkeratosis) | P13645 | 2.2 | × |
| LAMB2 | laminin, beta 2(laminin S) | P55268 | 7.9 | × |
| LASP1 | LIM and SH3 Protein 1 | Q14847 | 2.9 | × |
| LRIT1 | Leucine-rich repeat-containing protein21 precusor(LRC21) | Q9P2V4 | 2.2 | |
| MARCKS | myristoylated alanine-rich protein kinase C substrate | P29966 | 2.2 | × |
| MLL | myloid/lymphoid or mixed lineage leukimia | Q03164 | 2.7 | × |
| MYO7A | myosin VIIA | Q13402 | 2.1 | |
| NOL1 | nucleolar protein 1, 120 kDa | P46087 | 2.5 | × |
| PCYT1A | phosphate cytidylyltranferase 1, choline, alpha | P49585 | 2.3 | |
| PCYT1B | phosphate cytidylyltranferase 1, choline, beta | Q9Y5K3 | 2.1 | |
| PDLIM4 | PDZ and LIM domain 4 | P50479 | 2 | × |
| PKP4 | Plakophilin-4 | Q99569 | 2.5 | × |
| PPFIA2 | Liprin-alpha-2(LIPA2, PTPRF-interacting protein alpha-2) | Q75334 | 2.4 | × |
| PRSS1 | protease, serine, 1(trypsin 1) | Q07477 | 3.7 | × |
| RCN1 | Reticulocalbin-1 precusor | Q15293 | 2.5 | × |
| RHPN2 | rhophilin-like protein | Q8IUC4 | 10.1 | |
| RPL10 | 60S ribosomal protein L10 | P27635 | 2.4 | × |
| RPL13 | ribosomal protein L13 | P26373 | 3.6 | × |
| RPL27A | ribosomal protein L27a | P46776 | 2.2 | × |
| RPS6 | 40S ribosomal protein S6 | P62753 | 2 | × |
| SEC22A | vesicle-trafficking protein SEC22a | Q96IW7 | 3 | |
| SEC61B | protein transport protein Sec61 beta subunit | P60468 | 2.1 | |
| TALDO1 | transaldolase 1 | P37837 | 6.3 | × |
| TPI1 | triosephosphate isomerase 1 | P60174 | 1.7 | |
| TUBA1B | tubulin, alpha_ubiquitous chain | P68363 | 2.1 | |
| TUBA1C | tubulin, alpha 6 | Q9BQE3 | 3.1 | |
| TUBB1 | tubulin, beta 1 | P07436 | 2.3 | × |
| TUBB5 | tubulin, beta 5 | Q7JJU6 | 2.4 | × |
| TUFM | Tu translation elongation factor, mitochondrial (EFTU) | P49411 | 2.4 | |
| VPS13D | vacuolar protein sorting-associated protein 13D | Q5THJ4 | 3.3 | |
| YLPM1 | YLP motif-containing protein 1(YLPM1, ZAP113) | P49750 | 2.6 | |
| ZNF148 | Zinc finger protein 148 | Q9UQR1 | 3.2 | × |
| AFAP1 | actin filament associated protein | Q8N556 | −3.3 | |
| AHI1 | Abelson helper integration site 1 protein homolog(AHI-1 ,Jouberin) | Q8N157 | −2.2 | × |
| AHNAK | AHNAK nucleoprotein isoform 1 | Q09666 | −2.5 | |
| ARHGEF2 | rho/rac guanine nucleotide exchange factor 2 | Q9H023 | −2.7 | × |
| BCLAF1 | BCL2-associated transcription factor 1 | Q9NYF8 | untreated only | |
| C11orf58 | small acidic protein | O00193 | −7.1 | |
| CLIC6 | chloride intracellular channel 6 | Q96NY7 | untreated only | |
| CST4 | Cystatin-S precusor | P01036 | −2.1 | |
| DYNC1LI1 | dynein light chain-A | Q9Y6G9 | −2.2 | |
| EHA2 | ephrin receptor EphA2 | P29317 | untreated only | × |
| ERRFI1 | mitogen-inducible gene 6 protein | Q9UJM3 | −2.6 | × |
| FAM129B | Hypothetical protein LOC64855 | Q5VVW7 | −5.3 | |
| FAM82C | family with sequence similarity 82, member C | Q96TC7 | untreated only | |
| FLNA | filamin 1(actin binding protein-280) | P21333 | −1.5 | × |
| G3BP1 | Ras-GTPase-activiting protein SH3-domain-binding protein | Q13283 | −3.7 | × |
| HN1 | hematological and neurological expressed 1 isoform 1 | Q9UK76 | −6.7 | × |
| HRNR | Hornerin | Q86YZ3 | −2.9 | × |
| MACF1 | microtubule-actin crosslinking factor 1 | Q96PK2 | −2.1 | × |
| NCOA3 | nuclear receptor coactivator 3 | Q9Y6Q9 | −2.1 | × |
| NOP5/NOP58 | nucleolar protein NOP5/NOP58 | Q9Y2X3 | −2.8 | × |
Figure 3.
Pathway output from BiNGO analysis. Colored circles indicate GO_processes determined to be over-represented with statistical significance as depicted in the legend.
Table 3.
Categorization of over-represented phosphoproteins under the major GO_processes identified by BiNGO analysis.
| Cell Motility |
Cellular Component Organization and Biogenesis |
Localization | Metabolic Processes | |||||
|---|---|---|---|---|---|---|---|---|
| FLNA | ARHGEF2 | LASP1 | SH3KBP1 | ARHGEF2 | MYO7A | TUBA1C | ARHGEF2 | RPL27A |
| HSPB1 | C11ORF30 | MAACF1 | STMN1 | ERBB2IP | NOP5/NOP58 | TUBB1 | EEF1B2 | RPS6 |
| LAMB2 | EIF5 | MLL | SYTL4 | FLNA | NUP153 | TUBB5 | EIF5 | STMN1 |
| MACF1 | EIF5B | MYO7A | TLN1 | G3BP1 | SEC22A | TXNDC1 | EIF5B | TALDO1 |
| MARCKS | ENO1 | NOP5/NOP58 | TUBA1B | HSPB1 | SEC61B | VPS13C | ENO1 | TPI1 |
| TLN1 | ERBB2IP | NUUP153 | TUBA1C | ITSN2 | SH3KBP1 | VPS13D | HSPB1 | TUBA1B |
| TUBB1 | FLNA | PLEC1 | TUBB1 | LAMB2 | STMN1 | PCYT1A | TUBA1C | |
| TUBB5 | HSPB1 | SEC22A | TUBB5 | LASP1 | SYTL4 | PCYT1B | TUBB1 | |
| ITSN2 | SEC61B | TXNDC1 | MACF1 | TLN1 | PGM2L1 | TUBB5 | ||
| LAMB2 | 9-Sep | MARCKS | TUBA1B | RPL10 | TUFM | |||
| RPL13 | ||||||||
A majority of these proteins have a known role either directly in affecting cell motility or in a related role such as cell adhesion and cytoskeletal relationship. Many of these proteins also play roles in transcription, translation and Ca2+-binding, suggesting that these mechanisms are important in the increased cell motility phenotype induced by p16INK4a.
In addition to looking at the global changes in phosphorylated proteins, we looked for specific changes in FLNA phosphorylation. We identified decreased levels of phosphorylated FLNa at ser1459 upon cyclin D1-cdk4/6 repression (Figure 1 Supplemental shows the spectrum of the singly and doubly charged daughter ions of phosphorylated FLNa peptide (m/z 650.2, in the doubly charged state)). Serine 1459 has been previously identified as a site of FLNa phosphorylation but has never been linked to a specific kinase (27). Incubation with p16INK4a or upon cyclin D1 knockdown using siRNA caused a decrease of approximately 50% of both pFLNa(ser2152) as determined via Western blot and pFLNa(ser1459) in the mass spectrometry experiments. To validate this result, we obtained an antibody specific to pFLNa(ser1459) and as shown in Figure 1B, treatment of the cells with siRNA against cyclin D1 causes an approximate 50% decrease in the amount of pFLNA(ser1459).
Discussion
We demonstrated that cyclin D1 siRNA and inhibition of the cyclin D1-cdk4/6 kinase complex through peptide treatment resulted in decreased motility and an impaired wound healing response in the invasive MDA-MB-231 breast cancer cell line. We also show that cyclin D1 binds to the actin binding protein filamin A (FLNa) and that the amount of phosphorylated FLNa(ser2152) and FLNa(ser1459) decreases concomitant with the decreased migration resulting from cyclin D1 siRNA transfection. We found that cyclin D1 and FLNa co-immunoprecipitate as well as co-localize in migrating cells which suggests that the interaction is likely functional as the interaction occurs at the cell membrane where phosphorylated FLNa can recruit other molecules that are required for cytoskeleton reorganization(11). These findings identify a migration-related function of cyclin D1 in breast cancer cells and also provide new information regarding the mechanism for affecting motility in these cells.
Recent studies suggest a role for cyclin D1 in cellular migration (6, 28). The motility of bone marrow macrophages isolated from cyclin D1-deficient mice display decreased motility (6). It was also shown that cyclin D1−/− mouse embryo fibroblasts exhibited increased adherence and decreased cellular motility compared to wild-type cells (7). Further evidence shows that these cyclin D1-related phenotypic changes were regulated through a member of the Rho family of small GTPases which is known to play an important role in the regulation of cell motility. In these cells, the activity of Rho-activated kinase (ROCKII) was increased. Thrombospondin 1 (TSP-1), a matrix glycoprotein that inhibits cellular metastasis was also shown to be regulated by cyclin D1 in these cells. The cdk inhibitor p27KIP has also been determined to be required for cyclin D1 regulation of cellular migration mouse embryo fibroblasts (28). In T47D breast cancer cells, cyclin D1 was shown to act similarly. Fewer numbers of T47D cells transfected with cyclin D1 siRNA were able to across the membrane in the Boyden chamber migration assay compared to control cells (29).
In our studies, we find that decreased cyclin D1 levels in MDA-MB-231 cells also lead to decreased wound healing capacity as well as decreased invasion potential. We also provide evidence that cyclin D1 and pFLNA co-immunoprecipitate and cyclin D1 and FLNA co-localize in MDA-MB-231. The amounts of two phosphorylated forms of FLNa, pFLNa(ser1459) and pFLNa(ser2152) are dependent on the level of cyclin D1 protein. To our knowledge, this is the first report of a mediator of phosphorylation at ser1459. FLNA has been shown to interact with the carboxy-terminal pleckstrin homology domain of ROCK and that this complex localizes at protrusive cell membranes(30). FLNa’s known association with other small GTPases regulates actin remodeling, formation of filopodia and membrane ruffles which taken together with binding of FLNa to cyclin D1 suggests the mechanism for increased activity of ROCKII.
At least 28 sites of phosphorylation, including ser2152 and ser1459, have been identified in human FLNa (www.phosphosite.org). With regard to ser2152, FLNa has been reported to be a substrate for the serine/threonine kinase p21-activated kinase (Pak1) identified by yeast two-hybrid screening using the N-terminal 1–270 amino acids of Pak1 as bait (31). In fibroblasts, FLNa has been identified as a substrate for one of member of the Ras-mitogen-activated kinase pathway, p90 ribosomal S6 protein kinase-2 (25, 26). Ser1459 as a site of phosphorylation of FLNa has been identified in Hela cell lysate that was enriched for phosphoproteins; however, the associated kinase was not identified (27, 32). Herein we have found that inhibition of cyclin D1-cdk4/6 activity resulted in the reduction of pFLNa(ser1459) via mass spectrometry experiments and validated by Western blotting. A recent report by Lee et Al. showed that cyclin B1-cdk1 can interact with and partially regulate the function of FLNa by phosphorylating ser1436 in vitro and decrease its ability to cross-link actin in vitro. However, more experiments must be conducted to determine whether cyclin D1/cdk4 kinase directly phosphorylates serine 1459 or if it is part of a larger signaling pathway. An alternative hypothesis is that cyclin D1/cdk4 and FLNa are part of a complex and that phosphorylation of FLNa is accomplished by another molecule. FLNa could also be a potential scaffold for some unknown transmembrane receptors or signaling molecules.
We have identified approximately 65 phosphorylated proteins whose level of expression was significantly different in cells transfected with the p16INK4a peptide inhibitor of cyclin D1-cdk4/6 activity (Table 2). The majority of these phosphoproteins have been reported to play a role in some type of cancer either in vitro or in vivo. BiNGO was used to determine that localization, metabolism, cell motility, and cellular component organization/biogenesis Gene Ontology (GO) categories are statistically over-represented in our set of identified phosphoproteins. Many of the proteins categorized under metabolism are involved in protein synthesis. Regulation of translation is critical for proper protein expression. Deregulation of protein translation is associated with abnormal gene expression leading to altered physiology including cell growth and possibly cancer (33). Eukaryotic initiation factor 4E (elF-4E) is one of several initiation factors responsible for the regulation of translation in eukaryotes and elevated levels of elF-4E is associated with many solid tumors including breast, prostate, and cervix (33). In this study we identify several other proteins related to protein translation that may play a role in increased cell motility. Signaling pathways identified as deregulated in some cancers are also represented in our data set.
In addition to FLNa, the other proteins associated specifically with cell motility are tubulin beta 1 and beta 5 (TUBB1 and TUBB5), microtubule-acting crosslinking factor 1 (MACF1), heat shock 27kDa protein 1, (HSPB1), myristolyated alanine-rich protein kinase C substrate (MARCKS), talin 1 (TLN1), and laminin beta 2 (LAMB2). Except for TUBB1 and TUBB5 which, with actin and intermediate filaments comprise the cytoskeleton, and laminin beta 2, a component of the extracellular matrix, all of the proteins in this category are actin binding proteins. These data suggest that FLNa is only one of several other actin binding proteins involved in the cyclin D1-driven cell motility in MDA-MB-231 cells.
Taken together, our results show for the first time a direct interaction (and involvement) of cyclin D1 with the cytoskeletal protein FLNa, which is necessary for cell remodeling – a feature critical to cancer cell motility, and hence migration and extravasation, to other sites. Consistent with our results there are reports that FLNa and in particular its phosphorylated form plays an important role in breast cancer cell migration. FLNa phosphorylated at Ser2152 has been identified as a target of caveloin-1 in IGF-I stimulated migration of MCF-7 breast cancer cells (34) and FLNa has been identified in the plasma of patients with metastatic breast cancer(35).
Supplementary Material
Acknowledgements
This work was supported in part by grants from the Pennsylvania Department of Health Breast and Cervical Cancer Section #05-07-09 (J.N.Q., A.A.Q.), a grant from the Breast Cancer Research Foundation (A.A.Q. and J.N.Q.), and grants from the NIH R01CA70896, R01CA75503, R01CA86072, R01CA107382 (R.G.P.). The Kimmel Cancer Center was supported by the NIH Cancer Center Core grant P30CA56036 (R.G.P). This project is funded in part from the Dr. Ralph and Marian C. Falk Medical Research Trust and a grant from the Pennsylvania Department of Health (R.G.P.). The Department specifically disclaims responsibility for any analysis, interpretations or conclusions.
References
- 1.Massague J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 2.Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-cardo C. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clinical Cancer Research. 2000;6:1891–1895. [PubMed] [Google Scholar]
- 3.Jares P, Rey MJ, Fernandez PL, et al. Cyclin D1 and retinoblastoma gene expression in human breast carcinoma: correlation with tumour proliferation and oestrogen receptor status. J Pathol. 1997;182:160–166. doi: 10.1002/(SICI)1096-9896(199706)182:2<160::AID-PATH814>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol. 2005;23:4215–4224. doi: 10.1200/JCO.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 5.Coqueret O. Linking cyclins to transcriptional control. Gene. 2002;299:35–55. doi: 10.1016/s0378-1119(02)01055-7. [DOI] [PubMed] [Google Scholar]
- 6.Neumeister P, Pixley FJ, Xiong Y, et al. Cyclin D1 governs adhesion and motility of Macrophages. Mol Biol Cell. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Wang C, Jiao X, et al. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao WJ, Liang Y, Chen K, et al. Changes of biophysical behavior of k562 cells for p16 gene transfer. Clin Hemorheol Microcirc. 2002;27:177–183. [PubMed] [Google Scholar]
- 9.Alhaja E, Adan J, Pagan R, et al. Anti-migratory and anti-angiogenic effect of p16: a novel localization at membrane ruffles and lamellipodia in endothelial cells. Angiogenesis. 2004;7:323–333. doi: 10.1007/s10456-005-0368-9. [DOI] [PubMed] [Google Scholar]
- 10.Zhu TN, He HJ, Kole S, et al. Filamin A-mediated down-regulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J Biol Chem. 2007;282:14816–14826. doi: 10.1074/jbc.M611430200. [DOI] [PubMed] [Google Scholar]
- 11.Stossel TP, Condeelis J, Cooley L, et al. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2001;2:138–145. doi: 10.1038/35052082. [DOI] [PubMed] [Google Scholar]
- 12.Cukier IH, Li Y, Lee JM. Cyclin B1/Cdk1 binds and phosphorylates Filamin A and regulates its ability to cross-link actin. FEBS Lett. 2007;581:1661–1672. doi: 10.1016/j.febslet.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- 14.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluron-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phosphlipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–14040. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 15.Lin JF, Xu J, Tian HY, et al. Identification of candidate prostate cancer biomarkers in prostate needle biopsy specimens using proteomic analysis. Int J Cancer. 2007;121:2596–2605. doi: 10.1002/ijc.23016. [DOI] [PubMed] [Google Scholar]
- 16.Loy CJ, Sims KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci U S A. 2003;100:4562–4567. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasaki A, Masuda Y, Ohta Y, Ikeda K, Watanabe K. Filamin associates with Smads and regulates transforming growth factor-beta signaling. The Journal of biological chemistry. 2001;276:17871–17877. doi: 10.1074/jbc.M008422200. [DOI] [PubMed] [Google Scholar]
- 18.Keshamouni VG, Michailidis G, Grasso CS, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. Journal of proteome research. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 19.Bachmann AS, Howard JP, Vogel CW. Actin-binding protein filamin A is displayed on the surface of human neuroblastoma cells. Cancer Science. 2006;97:1359–1365. doi: 10.1111/j.1349-7006.2006.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin MF, Puig-de-Morales M, Bursac P, Mellema M, Millet E, Fredberg JJ. Filamin-a and rheological properties of cultured melanoma cells. Biophys J. 2006;90:2199–2205. doi: 10.1529/biophysj.105.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong SE, Blagoev B, Kratchmarova I, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 22.Fahraeus R, Lane DP. The p16(INK4a) tumour suppressor protein inhibits alphavbeta3 integrin-mediated cell spreading on vitronectin by blocking PKC-dependent localization of alphavbeta3 to focal contacts. EMBO J. 1999;18:2106–2118. doi: 10.1093/emboj/18.8.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahraeus R, Paramio JM, Ball KL, Lain S, Lane DP. Inhibition of pRb phosphorylation and cell-cycle progression by a 20-residue peptide derived from p16CDKN2/INK4A. Curr Biol. 1996;6:84–91. doi: 10.1016/s0960-9822(02)00425-6. [DOI] [PubMed] [Google Scholar]
- 24.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 25.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta Y, Hartwig JH. Phosphorylation of actin-binding protein 280 by growth factors is mediated by p90 ribosomal protein S6 kinase. J Biol Chem. 1996;271:11858–11864. doi: 10.1074/jbc.271.20.11858. [DOI] [PubMed] [Google Scholar]
- 27.Beausoleil SA, Jedrychowski M, Schwartz D, et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Jiao X, Wang C, et al. Cyclin D1 induction of cellular migration requires p27(KIP1) Cancer Res. 2006;66:9986–9994. doi: 10.1158/0008-5472.CAN-06-1596. [DOI] [PubMed] [Google Scholar]
- 29.Ostrander JH, Daniel AR, Lofgren K, Kleer CG, Lange CA. Breast tumor kinase (protein tyrosine kinase 6) regulates heregulin-induced activation of ERK5 and p38 MAP kinases in breast cancer cells. Cancer Res. 2007;67:4199–4209. doi: 10.1158/0008-5472.CAN-06-3409. [DOI] [PubMed] [Google Scholar]
- 30.Ueda K, Ohta Y, Hosoya H. The carboxy-terminal pleckstrin homology domain of ROCK interacts with filamin-A. Biochemical and biophysical research communications. 2003;301:886–890. doi: 10.1016/s0006-291x(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 31.Vadlamudi RK, Li F, Adam L, et al. Filamin is essential in actin cytoskeletal assembly mediated by p21-activated kinase 1. Nat Cell Biol. 2002;4:681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- 32.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 33.Thumma SC, Kratzke RA. Translational control: a target for cancer therapy. Cancer Lett. 2007;258:1–8. doi: 10.1016/j.canlet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Ravid D, Chuderland D, Landsman L, Lavie Y, Reich R, Liscovitch M. Filamin A is a novel caveolin-1-dependent target in IGF-I-stimulated cancer cell migration. Experimental cell research. 2008;314:2762–2773. doi: 10.1016/j.yexcr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Alper O, Stetler-Stevenson WG, Harris LN, et al. Novel anti-filamin-A antibody detects a secreted variant of filamin-A in plasma from patients with breast carcinoma and high-grade astrocytoma. Cancer science. 2009;100:1748–1756. doi: 10.1111/j.1349-7006.2009.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.