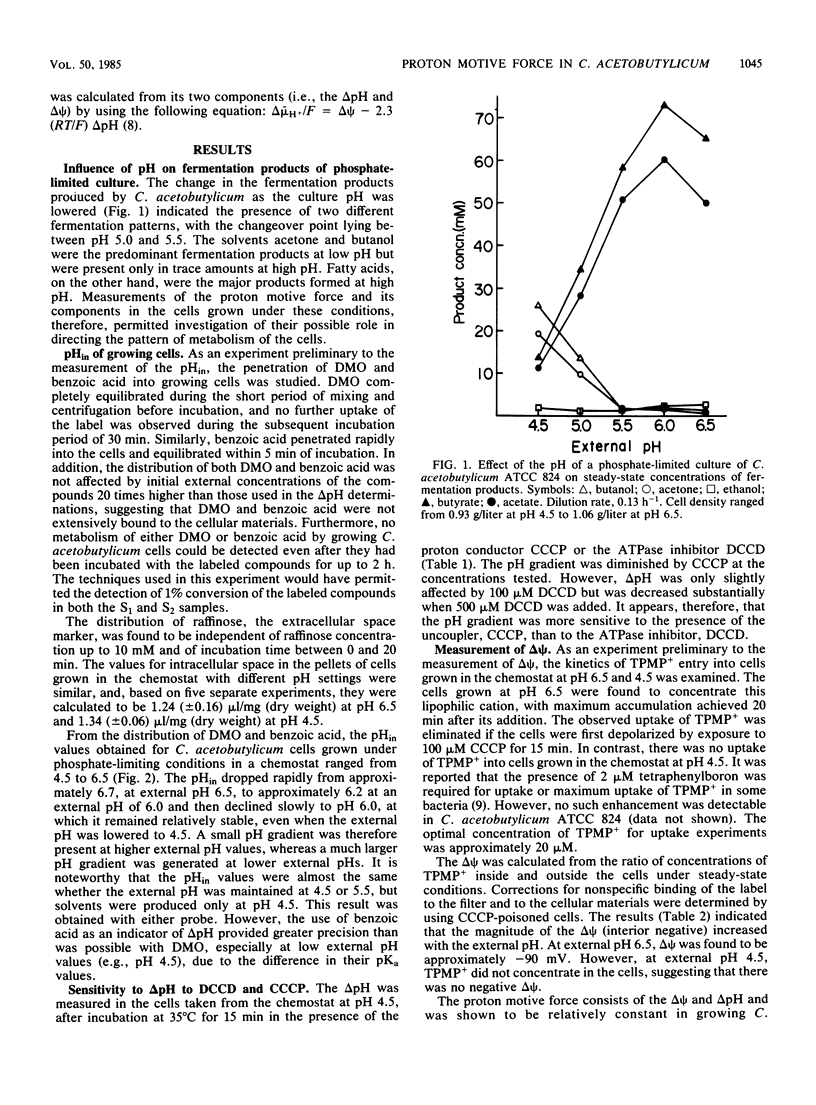
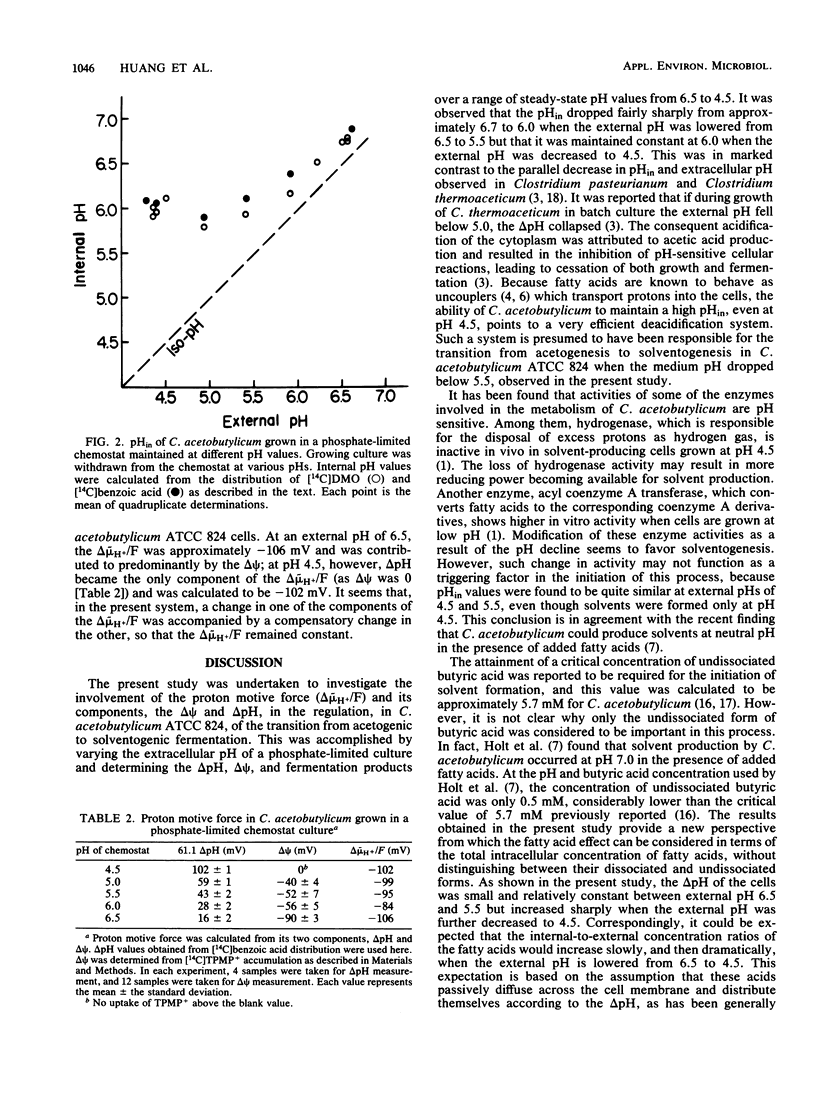
Abstract
The proton motive force and its electrical and chemical components were determined in Clostridium acetobutylicum, grown in a phosphate-limited chemostat, using [14C]dimethyloxazolidinedione and [14C]benzoic acid as transmembrane pH gradient (delta pH) probes and [14C]triphenylmethylphosphonium as a membrane potential (delta psi) indicator. The cells maintained an internal-alkaline pH gradient of approximately 0.2 at pH 6.5 and 1.5 at pH 4.5. The delta pH was essentially constant between pH 6.5 and 5.5 but increased considerably at lower extracellular pH values down to 4.5. Hence, the intracellular pH fell from 6.7 to 6.0 as the external pH was lowered from 6.5 to 5.5 but did not decrease further when the external pH was decreased to 4.5. The transmembrane electrical potential decreased as the external pH decreased. At pH 6.5, delta psi was approximately -90 mV, whereas no negative delta psi was detectable at pH 4.5. The proton motive force was calculated to be -106 mV at pH 6.5 and -102 mV at pH 4.5. The ability to maintain a high internal pH at a low extracellular pH suggests that C. acetobutylicum has an efficient deacidification mechanism which expresses itself through the production of neutral solvents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. A., Stephens G. M., Morris J. G. Production of Solvents by Clostridium acetobutylicum Cultures Maintained at Neutral pH. Appl Environ Microbiol. 1984 Dec;48(6):1166–1170. doi: 10.1128/aem.48.6.1166-1170.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. The transmembrane electrical potential and intracellular pH in methanogenic bacteria. Can J Microbiol. 1981 Jul;27(7):720–728. doi: 10.1139/m81-110. [DOI] [PubMed] [Google Scholar]
- Kashket E. R. Active transport of thallous ions by Streptococcus lactis. J Biol Chem. 1979 Sep 10;254(17):8129–8131. [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Stoichiometry of the H+-ATPase of growing and resting, aerobic Escherichia coli. Biochemistry. 1982 Oct 26;21(22):5534–5538. doi: 10.1021/bi00265a024. [DOI] [PubMed] [Google Scholar]
- Kell D. B., Peck M. W., Rodger G., Morris J. G. On the permeability to weak acids and bases of the cytoplasmic membrane of Clostridium pasteurianum. Biochem Biophys Res Commun. 1981 Mar 16;99(1):81–88. doi: 10.1016/0006-291x(81)91715-0. [DOI] [PubMed] [Google Scholar]
- Mitchell W. J., Booth I. R., Hamilton W. A. Quantitative analysis of proton-linked transport systems. Glutamate transport in Staphylococcus aureus. Biochem J. 1979 Nov 15;184(2):441–449. doi: 10.1042/bj1840441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS D. The acetone-butanol fermentation. Prog Ind Microbiol. 1961;3:71–90. [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]


