Abstract
Surfactant protein A (SP-A) inhibits hemagglutination (HA) activity and infectivity of Influenza A viruses (IAV). As we have showed before in different assays, SP-A2 gene products are more active than SP-A1. Here, we hypothesized that SP-A1 and SP-A2 mammalian CHO-cell-expressed proteins also differentially modulate HA inhibition of IAV. We found that both SP-A1 and SP-A2 equally displayed α(2,3)-linked sialic acids, and had similar activity against a strain (PR-8) that preferentially binds to α(2,3)-linked sialic acids. Based on these Findings, we speculate that in human lung SP-A1 and SP-A2 will not be different in their activity against IAV that preferably bind to α(2,3)-linked sialic acids (like avian strains).
Keywords: Surfactant protein A variants, Collectins, IAV
Introduction
Influenza A viruses (IAV) initiate infection by attachment to host cells via multivalent interactions of its haemagglutinins with sialyloligosaccharide moieties of cellular glyco-conjugates [1]. Soluble macromolecules containing sialic acids (SA) from animal sera and mucosal fluids can act as decoy receptors and competitively inhibit virus-mediated hemagglutination and infection [1]. Most commonly, IAV receptors contain α(2,3)-linked SA and α(2,6)-linked SA. Of interest, avian IAV strains have a binding preference for α(2,3)-linked sialic acids [2, 3]. IAV have two envelope glycoproteins, haemagglutinin (HA) and neuraminidase that are involved in viral interactions with cells and/or other viral particles. HA is heavily glycosylated in most IAV strains and is responsible for viral binding to cell-surface sialoglycoproteins, subsequently leading to viral adsorption [4, 5]. SP-A has been shown to inhibit haemagglutination activity and infectivity of IAV [6]. This activity may involve Ca2+-independent binding of IAV to sialylated carbohydrates associated with the carbohydrate recognition domain of SP-A [7]. Mice lacking SP-A exhibit more severe infection with IAV [8]. In contrast to mice that have one SP-A gene, humans have two functional genes, SP-A1 and SP-A2. SP-A1 and SP-A2 gene products have sequence differences that may affect SP-A conformation and, in turn, SP-A function. Indeed, SP-A1 and SP-A2 were found to differ in their ability to stimulate phagocytosis [9, 10], bind carbohydrates [11], inhibit surfactant secretion [12], and stimulate production of TNF-α by macrophage-like THP-1 cells [13–15], as well as in their aggregation properties and oligomerization patterns [12, 16, 17]. In addition, SP-A1 and SP-A2 mRNAs are differentially present in the respiratory tract sites [18, 19]. In all functional assays SP-A2 was found to be more active than SP-A1. Because of the above functional and structural differences and the fact that different individuals may have different amounts of SP-A1 and SP-A2 in their lungs, as shown in a recent study [20], it became important to establish if SP-A1 and SP-A2 also differ in their activity against IAV.
Materials and methods
SP-A1 and SP-A2 variants were expressed from stably transfected Chinese hamster ovary (CHO)-K1 mammalian cells and purified as described previously [15]. For this study, SP-A variants expressed in T-REx-CHO (Tre) inducible mammalian cell system (Invitrogen, Carlsband, CA) were used as well. As a negative control, SP-A variants were produced using the baculovirus-mediated insect expression system, which does not express sialylated proteins, as described previously [14]. As a positive control, human SP-A that was purified [21] from the bronchoalveolar lavage fluid obtained from a patient with alveolar proteinosis (AP BAL hSP-A), as described previously [22], was used.
IAV was grown and purified as described previously [23]. The A/Puerto Rico/8/34(H1N1) (PR-8) strain was provided by Dr J. Abramson (Department of Pediatrics, Bowman Gray School of Medicine, Wake Forest University, Winston Salem, NC). The A/Phillipines/82(H3N2) strain, called “Phil82” later in the paper, was provided by Dr E. M. Anders (Department of Microbiology, University of Melbourne, Melbourne, Australia). HA titers of IAV were determined by titration of virus samples in PBS with thoroughly PBS-washed human type O, Rh (−) red blood cells, as described [23]. HA inhibition was measured by serially diluting SP-A preparations in 96-well round-bottom plates (Serocluster U-Vinyl plates; Costar, Cambridge, MA) using PBS as diluent. After adding IAV solution, giving a final concentration of 40 HA Units per ml, the IAV/SP-A mixture was pre-incubated for 15 min, followed by addition of human erythrocyte suspension in PBS. The entire procedure was performed at room temperature. The minimal concentration of SP-A required to fully inhibit HA caused by the virus was determined by reading the plates after 2 h. HA inhibition was detected as the formation of a pellet of red blood cells.
Presence and linkage patterns of terminally linked SAs on SP-A preparations were analyzed by reducing SDS-PAGE and Western blotting, followed by detection with digoxigenin-labeled lectins according to manufacturer’s instruction (DIG Glycan Differentiation Kit, Roche Diagnostics GmbH). A control gel was included as reference (Coomassie stain). The MAA and SNA lectins were used to detect α(2,3)-linked and α(2,6)-linked sialic acids, respectively. Control glycoproteins were included to assess lectin binding efficiency and specificity. Quantitative comparisons were obtained by densitometric analysis of blots.
Results
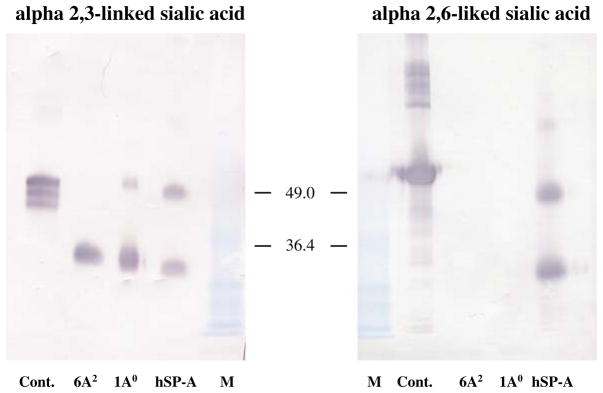
To study, if SP-A1 and SP-A2 gene products differentially inhibit the HA activity of IAV, we tested SP-A variants expressed in two systems—mammalian (CHO) cells (non-inducible and inducible), and insect cells. The density of sialic acids on SP-As was tested by densitometry of glycan blots. A representative glycan blot is shown in Fig. 1. The density of specific sialic acids linkages found on SP-A preparations correlated with SP-A activity. There were no α(2,6)-linked sialic acids found in CHO-cell-expressed SPA variants, and no activity against the Phil82 strain (used as a negative control) that binds to α(2,6)-linked sialic acids was found for recombinant proteins (Table 1), respectively. CHO cell-expressed SP-A1 and SP-A2 had similar HA inhibitory activity to hSP-A against PR-8 (Table 1). Within each set of preparations (I or II, Table 1) there was no significant difference between SP-A1 and SP-A2 in either α(2,3)-linked sialic acids content or in their ability to inhibit HA of IAV. Recombinant preparations II (Tre-system) had lower concentrations of α(2,3)-linked sialic acids and greater SP-A concentrations were required to inhibit PR-8 activity. Neither natural hSP-A nor CHO-cell-produced SP-A variants had any detectable high mannose oligosaccharides based on glycan blotting (not shown). Glycan blots were also performed with insect cell-derived SP-A1 and SP-A2 (used as a negative control), and neither of them showed any sialic acid linkages, or any measurable hemagglutinin inhibiting activity against either IAV (Table 1).
Fig. 1.
Sialylation of SP-A1 and SP-A2 variants expressed in mammalian (CHO) cells. Sialic acid linkage analysis of SP-A variants was performed as described in “Results”. Presence and linkage patterns of terminally linked sialic acids on various SP-A preparations were analyzed by SDS-PAGE and Western blotting, followed by detection with digoxigenin-labeled lectins and anti-digoxigenin-alkaline phosphatase system. To assess lectin binding efficiency and specificity control (cont.) glycoproteins (fetuin for α(2,3)-linkage and transferrin for α(2,6)-linkage) were included
Table 1.
Inhibition of hemagglutination activity of IAV by recombinant SP-A variants expressed either in mammalian or insect cells and by natural human SP-A; association with patterns of SP-A sialylation
| SP-A preparation | I (CHO, pEEs14) |
II (CHO, Tre) |
III (insect) |
hSP-A | |||
|---|---|---|---|---|---|---|---|
| 1A0 | 6A2 | 1A0 | 6A2 | 1A0 | 6A2 | ||
| HA inhibition PR-8 straina | 4.1 ± 0.5 | 4.2 ± 1.1 | 14.1 ± 2.4 | 11.2 ± 1.0 | 0 | 0 | 4.4 ± 1.1 |
| HA inhibition Phil82 strainb | 23.4 ± 1.6 | 17.1 ± 4.7 | >25.0 | 21.8 ± 3.2 | 0 | 0 | 6.2 ± 1.5 |
| Density of α(2,3) sialic acids | 706 ± 151 | 419 ± 77 | 198 ± 52 | 152 ±61 | 0 | 0 | 449 ± 136 |
| Density of α(2,6) sialic acids | 0 | 0 | 0 | 0 | 0 | 0 | 381 ± 44 |
Values shown are mean ± SEM concentrations of SP-A (μg/ml) required for inhibition of 40 hemagglutinin units of IAV. Significantly greater concentrations of the CHO cell expressed recombinant preparations II (Tre-system) were required to inhibit HA activity of PR-8 than for CHO cell expressed recombinant preparations I (pEE14) or natural hSP-A (P < 0.05). Insect cell expressed recombinant SP-A variants (III) lacked sialic acids and did not inhibit HA of IAV.
PR-8 strain binds specifically to α(2,3)-linked sialic acids.
Phil82 strain binds specifically to α(2,6)-linked sialic acids. Significant differences (P < 0.05) between inhibitory concentrations of CHO cell expressed SP-A variants for two Influenza A viruses (PR-8 and Phil82) strains were found. Density of α(2,6) or α(2,3)-linked sialic acids on SP-A was measured by densitometric analysis of glycan blots. Recombinant SP-A had no appreciable α(2,6) sialic acids, whereas natural hSP-A did. Three experiments were performed for HA inhibition of PR-8 (except for 6A2 preparation I where n = 2) and assessment of sialylation levels. Two experiments were performed for inhibition of Phil82
Discussion
The activities of SP-A1 and SP-A2 have been shown previously to differ in a variety of assays. Here, we showed for the first time that CHO-cell-produced-SP-A variants had similar activity to that of natural hSP-A with regards to their ability to inhibit HA of IAV specific to α(2,3)-linked sialic acids. However, no significant difference between SP-A1 and SP-A2 activities was observed.
The PR-8 strain of IAV used in this study, resembles avian IAV strains that bind specifically to sialic acids in an α(2,3)-linkage [2, 3]. Of further interest, as we showed before, surfactant protein D and mannose binding lectin lack activity against PR-8 [24]. Hence, the activity of SPA against this strain highlights the potential importance of SP-A in innate host defense against potential pandemic strains of IAV specific to α(2,3)-linked sialic acids [25–27]. Because different individuals may have different proportions of SP-A1/SP-A2 in their lung, and SP-A1 and SP-A2 have differences in their sequence, oligomerization and function, as it was shown by different assays, studying the effect of SP-A1 and SP-A2 on HA inhibition of IAV was a point of interest. It has been noted that CHO cells are only capable of synthesizing α(2,3)-linked sialic acids due to lack of a functional copy of the gene encoding the α(2,6)-sialyltransferase [28]. Thus, SP-A1 and SP-A2 variants expressed in CHO-cells are an appropriate model to study the effect of SP-A on the IAV strains that specifically bind to α(2,3)-linked sialic acids, like avian strains. The baculovirus-derived insect cell-expressed SP-A variants lacked sialic acid and IAV inhibiting activity, and these variants were used as a negative control. Differences between SP-A1 and SP-A2 in their sequence and in oligomerization patterns, may affect, in turn, the interaction of SP-A proteins with IAV. The present data indicate that functional activity of SP-A1 and SP-A2 with respect to IAV may depend principally on the type and extent of sialylation, and not on sequence or oligomerization differences between SP-A1 and SP-A2. The latter do not play a significant role in the anti-IAV function of SP-A. This is the first time where no differences between SP-A1 and SP-A2 have been observed. In all published reports where other than the anti-viral activity of SP-A was studied, differences were observed with SP-A2 being more active than SP-A1.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (RO1 ES009882-04A1) and HL69031 (KLH). The authors thank Dr Martin van Eijk for initial pilot experiments with glycan blots, and Karen Chorney for technical assistance.
Contributor Information
Anatoly N. Mikerov, Department of Cellular and Molecular Physiology, H166, The Pennsylvania State University College of Medicine, 500 University Drive, Hershey, PA 17033, USA
Mitch White, Department of Medicine, Section of Hematology/Oncology, Boston University School of Medicine, Boston, MA USA.
Kevan Hartshorn, Department of Medicine, Section of Hematology/Oncology, Boston University School of Medicine, Boston, MA USA.
Guirong Wang, Department of Cellular and Molecular Physiology, H166, The Pennsylvania State University College of Medicine, 500 University Drive, Hershey, PA 17033, USA.
Joanna Floros, Email: jfloros@psu.edu, Department of Cellular and Molecular Physiology, H166, The Pennsylvania State University College of Medicine, 500 University Drive, Hershey, PA 17033, USA. Department of Pediatrics, The Pennsylvania State University College of Medicine, Hershey, PA 17033, USA. Department of Obstetrics and Gynecology, The Pennsylvania State University College of Medicine, Hershey, PA 17033, USA.
References
- 1.Matrosovich M, Klenk HD. Natural and synthetic sialic acid-containing inhibitors of Influenza virus receptor binding. Rev Med Virol. 2003;13:85–97. doi: 10.1002/rmv.372. [DOI] [PubMed] [Google Scholar]
- 2.Inkster MD, Hinshaw VS, Schulze IT. The hemagglutinins of duck and human H1 Influenza viruses differ in sequence conservation and in glycosylation. J Virol. 1993;67:7436–7443. doi: 10.1128/jvi.67.12.7436-7443.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Eijk M, White MR, Batenburg JJ, Vaandrager AB, van Golde LM, Haagsman HP, Hartshorn KL. Interactions of Influenza A virus with sialic acids present on porcine surfactant protein D. Am J Respir Cell Mol Biol. 2004;30:871–879. doi: 10.1165/rcmb.2003-0355OC. [DOI] [PubMed] [Google Scholar]
- 4.Skehel JJ, Stevens DJ, Daniels RS, Douglas AR, Knossow M, Wilson IA, Wiley DC. A carbohydrate side chain on hemagglutinins of Hong Kong Influenza viruses inhibits recognition by a monoclonal antibody. Proc Natl Acad Sci USA. 1984;81:1779–1783. doi: 10.1073/pnas.81.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the Influenza virus A/PR/8/34 hemagglutinin (H1 subtype) Cell. 1982;31:417–427. doi: 10.1016/0092-8674(82)90135-0. [DOI] [PubMed] [Google Scholar]
- 6.Hartshorn KL, Crouch EC, White MR, Eggleton P, Tauber AI, Chang D, Sastry K. Evidence for a protective role of pulmonary surfactant protein D (SP-D) against Influenza A viruses. J Clin Invest. 1994;94:311–319. doi: 10.1172/JCI117323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benne CA, Kraaijeveld CA, van Strijp JA, Brouwer E, Harmsen M, Verhoef J, van Golde LM, van Iwaarden JF. Interactions of surfactant protein A with Influenza A viruses: binding and neutralization. J Infect Dis. 1995;171:335–341. doi: 10.1093/infdis/171.2.335. [DOI] [PubMed] [Google Scholar]
- 8.LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary Influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- 9.Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;288:L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, Floros J. SP-A2 variants expressed in CHO-cells stimulate phagocytosis of Pseudomonas aeruginosa more than SP-A1. Infect Immun. 2007;75(3):1403–1412. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberley RE, Snyder JM. Recombinant human SP-A1 and SP-A2 proteins have different carbohydrate-binding characteristics. Am J Physiol Lung Cell Mol Physiol. 2003;284:L871–L881. doi: 10.1152/ajplung.00241.2002. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- 13.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000;278:L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 15.Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect. 2002;110:79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Verdugo I, Wang G, Floros J, Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry. 2002;41:14041–14053. doi: 10.1021/bi026540l. [DOI] [PubMed] [Google Scholar]
- 17.Selman M, Lin HM, Montano M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo S, Guo X, Umstead TM, Lang CM, Pardo A, Phelps DS, Floros J. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary Fibrosis. Hum Genet. 2003;113:542–550. doi: 10.1007/s00439-003-1015-4. [DOI] [PubMed] [Google Scholar]
- 18.Saitoh H, Okayama H, Shimura S, Fushimi T, Masuda T, Shirato K. Surfactant protein A2 gene expression by human airway submucosal gland cells. Am J Respir Cell Mol Biol. 1998;19:202–209. doi: 10.1165/ajrcmb.19.2.3239. [DOI] [PubMed] [Google Scholar]
- 19.Khubchandani KR, Goss KL, Engelhardt JF, Snyder JM. In situ hybridization of SP-A mRNA in adult human conducting airways. Pediatr Pathol Mol Med. 2001;20:349–366. [PubMed] [Google Scholar]
- 20.Tagaram HR, Wang G, Umstead TM, Mikerov AN, Thomas NJ, Graff GR, Hess JC, Thomassen MJ, Kavuru MS, Phelps DS, Floros J. Characterization of a human surfactant protein A1 (Sp-A1) gene-specific antibody; Sp-A1 content variation among individuals of varying age and pulmonary health. Am J Physiol Lung Cell Mol Physiol. 2006 doi: 10.1152/ajplung.00249.2006. in press. [DOI] [PubMed] [Google Scholar]
- 21.Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ. The major lung surfactant protein, SP 28–36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987;262:13877–13880. [PubMed] [Google Scholar]
- 22.Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. Am J Physiol Lung Cell Mol Physiol. 2002;283:L94–L102. doi: 10.1152/ajplung.00434.2001. [DOI] [PubMed] [Google Scholar]
- 23.Hartshorn KL, Collamer M, Auerbach M, Myers JB, Pavlotsky N, Tauber AI. Effects of Influenza A virus on human neutrophil calcium metabolism. J Immunol. 1988;141:1295–1301. [PubMed] [Google Scholar]
- 24.Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. Mechanisms of anti-Influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol. 1997;273:L1156–L1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- 25.Kandel R, Hartshorn KL. Novel strategies for prevention and treatment of Influenza. Expert Opin Ther Targets. 2005;9:1–22. doi: 10.1517/14728222.9.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Trampuz A, Prabhu RM, Smith TF, Baddour LM. Avian Influenza: a new pandemic threat? Mayo Clin Proc. 2004;79:523–530. doi: 10.4065/79.4.523. quiz 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webby RJ, Webster RG. Are we ready for pandemic influenza? Science. 2003;302:1519–1522. doi: 10.1126/science.1090350. [DOI] [PubMed] [Google Scholar]
- 28.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, Bar-Ilan A, Bloushtain N, Lev M, Joseph A, Kedar E, Porgador A, Mandelboim O. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]