Abstract
Purpose
To evaluate the feasibility of spectral domain optical coherence tomography (SD-OCT) macular scanning as a means of studying the afferent visual system in nystagmus patients.
Methods
Nystagmus patients who underwent SD-OCT, clinical evaluation, and eye movement recordings were recruited for this prospective, single-center, noncomparative study. Three SD-OCT macular three-dimensional cube scans per eye (200 × 200 × 1024 samplings in a 6 × 6 mm region) were obtained for qualitative retinal morphology analysis.
Results
Nineteen patients (6-68 years; average, 19 years) were analyzed. Of these, 17 patients had infantile nystagmus syndrome, and 2 had fusion maldevelopment nystagmus; 17 patients (89%) had associated sensory system abnormalities, including 9 (47%) with albinism. Macular images were successfully obtained in all but 1 patient (95%). Of the 8 successfully imaged oculocutaneous patients, 7 patients demonstrated “fovea plana,” and all demonstrated abnormal morphology.
Conclusion
SD-OCT reliably provides detailed structural imaging of the fovea in nystagmus patients.
Evaluation of the retina in patients with nystagmus can occasionally be difficult because of the constant motion of the eye. Eye motion has impeded the implementation of time domain optical coherence tomography (TD-OCT, Stratus OCT; Carl Zeiss Meditec, Dublin, CA) for diagnostic purposes in the nystagmus population. In addition to eye motion, compromised vision and the shortened attention span of children often compound the challenge of quality TD-OCT macular image acquisition. With the advent of spectral domain optical coherence tomography (SD-OCT, Cirrus OCT; Carl Zeiss Meditec, Dublin, CA), high-resolution 3-dimensional macular imaging is possible at greater than 50 times the speed of TD-OCT, thereby offering new hope for imaging in nystagmus.1-5
The use of SD-OCT for macular imaging has been reported in patients with albinism and nystagmus. It has demonstrated several features in this population, including the persistence of an abnormal, reflective nerve fiber layer band; persistence of multiple inner retinal layers; loss of the normal thickened photoreceptor nerve layer; and increased reflectivity of the choroid due to decreased pigmentation.6 Chong and colleagues6 hypothesized that the most external retinal layers—specifically the external limiting membrane, photoreceptor inner segment layer, and photoreceptor outer segment layer—appeared normal. These foveal morphologic findings echo the description by Marmor and colleagues,7 of an absent foveal pit, or fovea plana, and agree with histopathological studies8-11 and less-detailed TD-OCT reported findings of either absent or rudimentary foveal pits in oculocutaneous albinism.12-17
In this study we evaluated the feasibility of SD-OCT macular scanning as a means of studying the afferent visual systems of nystagmus patients. To our knowledge, this is the largest study of the use of SD-OCT to image the macula in patients with nystagmus.
Patients and Materials
Informed consent for all subjects or parents of subjects was obtained and all procedures were performed in accordance with the declaration of Helsinki. All testing was approved by the Institutional Review Board of the University of Pittsburgh and conformed to the requirements of the United States Health Insurance Portability and Accountability Act.
All subjects in this single-center, prospective study underwent clinical examination by a pediatric ophthalmologist (R.W.H) and eye-movement recordings. A series of 3 SD-OCT (Carl Zeiss Meditec, Dublin, CA) macular 3-dimensional cube scans were obtained at the UPMC Eye Center on any patient of at least 6 years of age able to sit at the OCT machine.
Clinical examination included documentation of best-corrected binocular distance visual acuity by linear Snellen letters (or, in our youngest patient, single, surrounded HOTV optotypes), systemic diagnoses (including oculocutaneous albinism, prematurity, and developmental delay), nystagmus waveform, the presence of iris transillumination defects on undilated slit lamp biomicroscopy, fundus examination with indirect ophthalmoscope, and either a 20 diopter (D), 28 D, or 78 D condensing lens. The presence of potential sensory abnormalities, including congenital cataracts, foveal hypoplasia, optic nerve dysplasia, and suspected retinal dystrophy, was recorded.
All subjects had eye movement recordings performed by an experienced laboratory researcher.18 Standard MicrosoftR and Mat-labR and specially designed and created software such as Eyelink Co., Applied Science Laboratories, Visual and EXperimentation (VEX), and Real-time EXperimentation (REX) packages were used to control the presentation of stimuli and the acquisition, display, and storage of data. Horizontal and vertical eye movement recordings were made by the use of an EyelinkR IR reflection method or remote video eye movement system with a bandwidth from 0 to 1000 Hz. Signal calibration at the beginning of each recording session used the end of the fast phase of the nystagmus cycle while having the patient fixate on small target lights located on a screen 1 meter away. Data were sampled at 500 to 1000 Hz. Calibration was accomplished monocularly by the useof pictures of 3° size or stationary 1° targets presented on a screen at a distance of 1 meter from the patient. Eye movement during patient fixation between targets at 0, ±5, ±10, ±15, and ±20° with the right eye, left eye, and both eyes was recorded. Next, eye movement during convergence was recorded. Finally, fixation at 0° with both eyes was recorded for 10 minutes to rule out asymmetric periodic alternating nystagmus. The type of nystagmus was then interpreted (RWH) as infantile nystagmus syndromefusion maldevelopment nystagmus syndrome or mixed waveform, and the frequency, amplitude, and direction were calculated and recorded.
All study participants underwent a series of 3-dimensional SD-OCT macular scans (n = 3) in each eye covering a 6 × 6 mm region in the posterior pole imaged during the course of 1.5 seconds. The macular scans encompassed 200 horizontal B-scans each comprising 200 A-scans (200 × 200 × 1024 samplings). The SD-OCT light source is a superluminescent diode with a central wavelength of 840 nm and axial resolution of 5 μm. Software developed by one of the authors (HI) was used to construct 2-dimensional movie playbacks of the 6 × 6 mm imaged area for orientation of real-time B-scan image acquisition over a scanning laser ophthalmoscope fundus background (Video 1, available at jaapos.org). Scanning laser ophthalmoscope images also were used as fundus photographs for verification of clinical fundus examination. The presence or absence of a foveal depression on the scanning laser ophthalmoscope image was compared with both the foveal architecture captured on the 2-dimensional movie playback and that described by clinical examination. The success rate of macular image acquisition was simply defined as the ability to obtain a recognizable SD-OCT and scanning laser ophthalmoscope image. Qualitative retinal morphology was described for each subject and a comparison of foveal architecture was performed between clinical fundus examination of the macula and the SD-OCT image, as well as between the scanning laser ophthalmoscope photography and the SD-OCT image.
Results
Study Population
Nineteen patients (6-68 years; average, 19 years) were recruited for this study between June and October 2008. On eye movement recording, 17 subjects demonstrated an increasing velocity slow phase waveform consistent with infantile nystagmus syndrome, and 2 subjects had decreasing slope velocity waveforms consistent with fusional maldevelopment nystagmus syndrome, also known as latent nystagmus. Seventeen patients (89%) displayed sensory abnormalities, including 9 (47%) with OCA (e-Supplement 1, available at jaapos.org).
Afferent Qualitative Results
SD-OCT was able to successfully obtain high-resolution macular images in all but one subject (95%), a 9-year-old patient with albinism (Patient 19). Among the 18 successfully imaged maculae, there was a high positive correlation between clinical examination and SD-OCT macular findings (r = 0.943, Pearson's correlation coefficient), with both exceptions consisting of a normal foveal architecture by clinical examination and a slight depression with persistence of some inner retinal layers by SD-OCT (Patients 1 and 2, e-Supplement 1). The correlation between the foveal architecture on SD-OCT and scanning laser ophthalmoscope fundus images was even greater (r = 0.961, Pearson's correlation coefficient), with Patient 13 showing no evidence of a foveal depression on SD-OCT (or clinical examination) and a rudimentary foveal reflex on photography (e-Supplement 1). In these cases of discrepancy, the authors consider the possibility that, because of excessive eye motion, the clinical examination was insufficient and possibly the SD-OCT scanning failed to detect the fovea.
Of 8 OCA patients with successful imaging, 7 (88%) lacked a foveal pit by clinical exam, SD-OCT imaging, and photography. The remaining OCA subject (Patient 6) displayed a slight foveal depression with a suggested “pinching upward,” or widening, of the nerve fiber layer at the fovea. However, in all of the OCA patients, the innermost retinal layers, from the outer plexiform layer to the internal limiting membrane continued to persist across the fovea (Figure 1A and 1B). The 2 patients without sensory system abnormalities appeared to have normal foveal architecture on SD-OCT (Figure 1C). Also, the 2 patients with fusion maldevelopment nystagmus syndrome displayed normal macular morphology on SD-OCT. All but 1 infantile nystagmus syndrome patient had identifiable sensory defects on both clinical and SD-OCT examinations. Of the 2 fusion maldevelopment nystagmus patients, 1 had a sensory system abnormality thought to be a retinal dystrophy (Patient 9); however, this diagnosis remains to be proven by electroretinography. The foveal architecture of the one individual with primary foveal hypoplasia (Patient 10) did not differ from that of the foveal hypoplasia in the OCA population.
FIG 1.
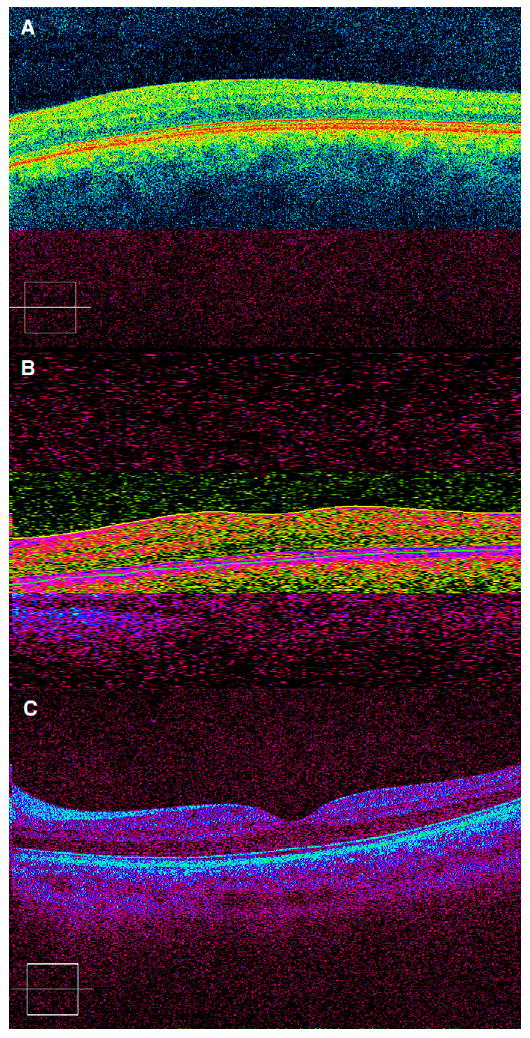
Spectral domain optical coherence tomography image demonstrating (A) foveal plana in oculocutaneous albinism (Patient 4), (B) slight foveal differentiation in oculocutaneous albinism (Patient 6), and (C) normal foveal morphology in a patient with infantile nystagmus syndrome and no identifiable sensory abnormality (Patient 3).
Discussion
Our study reveals that the faster speed of the spectral domain OCT allows a high success rate of capturing high-resolution macular images in nystagmus patients. Our observations of the retinal morphology in the nystagmus population reveal that all cases of clinical foveal hypoplasia demonstrate a persistence of the outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer, and nerve fiber layer. Our results agree with the description by Chong and colleagues6 of the involved retinal layers responsible for the lack of foveal differentiation in albinism: we did not observe thickening of the photoreceptor layer in any patient with foveal hypoplasia described by some of the time domain OCT reports, such as McGuire and colleagues.13
Although the definitive neurophysiological pathology leading to the ocular oscillations in infancy is unknown, we have hypothesized that a main cause is a developmental disruption in calibration of the ocular motor system. This may be the result of a genetic cause or as a result of a lack of communication between anomalous developing afferent and efferent systems. For example, in foveal hypoplasia or congenital cataracts, the development of the sensory system lags behind the motor system. The presence of foveal differentiation in the 2 fusion maldevelopment nystagmus syndrome patients suggests anomalous afferent–efferent developmental communication to be more posterior, perhaps in the structures responsible for binocular vision. Alternatively, the communication anomaly leading to fusion maldevelopment nystagmus syndrome may be time related, suggesting a later developmental disruption in the sensory–motor connection.
Protocols, similar to the TruTrak™ eye-tracking software available on the Spectralis SD-OCT (Heidelberg Engineering, Vista, CA), are currently underway to align images with scanning laser ophthalmoscope fundus landmarks, such as blood vessels, to remove eye motion artifact caused by microsaccades. Applying this software to the extreme case of eye motion, nystagmus, and incorporating it into a technology that is already available to clinicians would be an exciting and powerful application of the SD-OCT. Because the nystagmus population can also fall victim to common ocular diseases, such as diabetes, glaucoma, and macular degeneration, the use of SD-OCT, especially with software that may reduce eye motion and help recover 3-dimensional spatial integrity, would be an important diagnostic and management tool.
One limitation of the study is that not all subjects had confirmatory blood work, genetic testing, or electroretinography confirming a suspected associated sensory system diagnosis. Another limitation of this study is that because of the eye motion artifact and the lack of foveal differentiation for visual confirmation of fixation location, a possibility exists for the SD-OCT to miss imaging slices through the fovea. The fact that we found a high correlation between clinical examination and OCT as well as fundus photography and OCT makes this possibility unlikely. Despite these limitations, the potential to capture more information in a faster, noninvasive manner makes SD-OCT an appealing technology for both the patient and the physician.
Literature Search
PubMed was searched for the years 1950-present for the following terms: nystagmus and optical coherence tomography and albinism and optical coherence tomography. No foreign databases were searched.
Acknowledgments
We thank Dongsheng Yang, PhD, for his help with the ocular motility recordings.
Footnotes
Presented at the 35th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, San Francisco, California, April 17-21, 2009.
References
- 1.Wojtkowski M, Bajraszewski T, Gorczynska I, et al. Ophthalmic imaging by spectral optical coherence tomography. Am J Ophthalmol. 2004;138:412–19. doi: 10.1016/j.ajo.2004.04.049. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113:2054.e1–14. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao S, Knighton RW, Huang X, Gregori G, Puliafito CA. Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography. Optics Express. 2005;13:444–52. doi: 10.1364/opex.13.000444. [DOI] [PubMed] [Google Scholar]
- 4.Wojtkowski M, Srinivasan VJ, Fujimoto JG, et al. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112:1734–46. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko TH, Fujimot JG, Schuman JS, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology. 2005;112:1922. doi: 10.1016/j.ophtha.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong GT, Farsiu S, Freedman SF, et al. Abnormal foveal morphology in ocular albinism imaged with spectral-domain optical coherence tomography. Arch Ophthalmol. 2009;127:37–44. doi: 10.1001/archophthalmol.2008.550. [DOI] [PubMed] [Google Scholar]
- 7.Marmor MF, Choi SS, Zawadzki RJ, Werner JS. Visual insignificance of the foveal pit: Reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol. 2008;126:907–13. doi: 10.1001/archopht.126.7.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mietz H, Green WR, Wolff SM, Abundo GP. Foveal hypoplasia in complete oculocutaneous albinism: A histopathologic study. Retina. 1992;12:254–60. doi: 10.1097/00006982-199212030-00011. [DOI] [PubMed] [Google Scholar]
- 9.Naumann GO, Lerche W, Schroeder W. Foveolar aplasia in tyrosinase-positive oculocutaneous albinism (author's transl) [in German] Albrecht von Graefes Arch Klin Exp Ophthalmol. 1976;200:39–50. doi: 10.1007/BF00411431. [DOI] [PubMed] [Google Scholar]
- 10.Fulton AB, Albert DM, Craft JL. Human albinism: Light and electron microscopy study. Arch Ophthalmol. 1978;96:305–10. doi: 10.1001/archopht.1978.03910050173014. [DOI] [PubMed] [Google Scholar]
- 11.Zhou M, Gradstein L, Gonzales JA, Tsilou ET, Gahl WA, Chan CC. Ocular pathologic features of Hermansky-Pudlak syndrome type 1 in an adult. Arch Ophthalmol. 2006;124:1048–51. doi: 10.1001/archopht.124.7.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo JH, Yu YS, Kim JH, Choung HK, Heo JW, Kim SJ. Correlation of visual acuity with foveal hypoplasia grading by optical coherence tomography in albinism. Ophthalmology. 2007;114:1547–51. doi: 10.1016/j.ophtha.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 13.McGuire DE, Weinreb RN, Goldbaum MH. Foveal hypoplasia demonstrated in vivo with optical coherence tomography. Am J Ophthalmol. 2003;135:112–4. doi: 10.1016/s0002-9394(02)01923-2. [DOI] [PubMed] [Google Scholar]
- 14.Meyer CH, Lapolice DJ, Freedman SF. Foveal hypoplasia in oculocutaneous albinism demonstrated by optical coherence tomography. Am J Ophthalmol. 2002;133:409–10. doi: 10.1016/s0002-9394(01)01326-5. [DOI] [PubMed] [Google Scholar]
- 15.Meyer CH, Lapolice DJ, Freedman SF. Foveal hypoplasia demonstrated in vivo with optical coherence tomography. Am J Ophthalmol. 2003;136:397–8. doi: 10.1016/s0002-9394(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 16.Harvey PS, King RA, Summers CG. Spectrum of foveal development in albinism detected with optical coherence tomography. J AAPOS. 2006;10:237–42. doi: 10.1016/j.jaapos.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Recchia FM, Carvalho-Recchia CA, Trese MT. Optical coherence tomography in the diagnosis of foveal hypoplasia. Arch Ophthalmol. 2002;120:1587–8. [PubMed] [Google Scholar]
- 18.Hertle RW, Yang D. Clinical and electrophysiological effects of extraocular muscle surgery on patients with infantile nystagmus syndrome (INS) Semin Ophthalmol. 2006;21:103–10. doi: 10.1080/08820530600614249. [DOI] [PubMed] [Google Scholar]


