Abstract
Polybrominated diphenyl ethers (PBDEs) have been measured in the home environment and in humans, but studies linking environmental levels to body burdens are limited. This study examines the relationship between PBDE concentrations in house dust and serum from adults residing in these homes. We measured PBDE concentrations in house dust from 50 homes and in serum of male-female couples from 12 of the homes. Detection rates, dust-serum and within-matrix correlations varied by PBDE congener. There was a strong correlation (r = 0.65–0.89, p < 0.05) between dust and serum concentrations of several predominant PBDE congeners (BDE 47, 99 and 100). Dust and serum levels of BDE 153 were not correlated (r < 0.01). The correlation of dust and serum levels of BDE 209 could not be evaluated due to low detection rates of BDE 209 in serum. Serum concentrations of the sum of BDE 47, 99, and 100 were also strongly correlated within couples (r = 0.85, p = 0.0005). This study provides evidence that house dust is a primary exposure pathway of PBDEs and supports the use of dust PBDE concentrations as a marker for exposure to PBDE congeners other than BDE 153.
INTRODUCTION
Polybrominated biphenyl ethers (PBDEs) are a group of flame retardant chemicals that have been added to numerous consumer products, such as home electronics (e.g. televisions, computers), textiles (e.g. carpeting, drapery) and items containing polyurethane foam (e.g. mattresses, upholstered furniture) to meet fire safety standards and to slow burning in case of fire. There are 10 different homologue groups of PBDEs (mono- to deca-), that consist of 209 possible congeners, or combinations of the number and position of bromine atoms on the diphenyl ether backbone. These different compounds have different chemical, exposure and toxicological properties (1). Commercial formulations of PBDEs consist of a mixture of congeners and are mainly described as Penta-, Octa- and Deca- BDE.
Due to concerns over the persistence of PBDEs in the environment and bioaccumulation in wildlife and particularly in human milk, the European Union banned the use of Penta- and Octa-BDEs in 2004 (2). The sole U.S. manufacturer phased out production of Penta- and Octa-BDEs in 2004. The major manufacturers and importer of DecaBDE in the U.S. have agreed to phase out production for most uses by 2012 (3). There is currently no U.S. federal regulation on the use of PBDEs, but several states have issued their own restrictions (4). BDE 209, which is the major component of the Deca commercial mixture still in use, has the shortest half-life in the body, as it is more readily transformed or eliminated than the lower-brominated congeners (5). However, BDE 209 in the environment can break down into the lower-brominated congeners that are more bioaccumulative (6, 7). Although Penta- and Octa-BDEs, once in mass production, are now banned in Europe and discontinued in the United States, the general population continues to be exposed to these compounds due to their persistence and continued release into home environments from older products. Importation of products from countries that continue to use certain PBDEs is another potential source of PBDEs in the indoor environment (8).
PBDEs are additive, or not chemically bound, and can leach out or physically degrade into particles and thus may end up in indoor air and house dust (9). Potential routes of exposure to these compounds include ingestion, inhalation and dermal uptake. Ingestion may include dietary exposure, particularly meats and dairy products that have accumulated PBDEs, but PBDE exposure in North America has been estimated to be primarily from ingesting and/or inhaling dust (10, 11, 12, 13). In support of these estimates, a recent U.S. study found a stronger association of PBDE concentrations in house dust with PBDE levels in human breast milk than with reported consumption of meat or dairy products (14). However, that study's analysis did not include BDE 209 due to low detection rates in milk and dust.
PBDEs have been shown to disrupt endocrine functions in experimental animals (15, 16), but human studies are limited. The few human studies that have been conducted to date have reported associations between PBDE exposure and altered hormone levels (17, 18, 19, 20). Specifically, we recently reported that PBDE concentrations in dust from participant-collected vacuum bags were associated with altered serum hormone levels in 24 men (20). In the present study, we measured PBDE concentrations in serum and in participant vacuum bag dust from couples (male and female partners living in same household) participating in the same ongoing study. The objective was to examine the relationship between dust and serum PBDE concentrations, as well as explore whether serum PBDE concentrations were strongly correlated within-couple. Strong relationships between dust and serum PBDE concentrations may help identify dust as a primary exposure pathway and provide validation for the use of dust PBDE concentrations as estimates of exposure in epidemiological studies.
METHODS
Couples living in the same household who were seeking fertility treatment were recruited from the Massachusetts General Hospital Fertility Center as part of an ongoing study of environmental exposures and reproductive health (20). Participants included men and women from infertile couples due to a male factor, a female factor, or a combination of both male and female factors. The study protocols were approved by the committees on research ethics at all participating institutions, and all participants signed an informed consent.
House dust PBDEs
Participants donated the current-use vacuum bag from their home between 2002 and 2008. Participants wrapped the vacuum bag in aluminum foil and then sealed it in a labeled plastic bag. In the one case where a bagless vacuum was used, the participant emptied vacuum dust directly into the plastic bag. Dust samples were stored at −20C until analysis. Dust was sieved using a 150 micron mesh sieve to obtain the fine fraction and analyzed for PBDEs using the method by Stapleton et al. (21, 22). Samples were spiked with internal and recovery standards, and four laboratory blanks were also spiked and analyzed alongside samples. PBDEs were quantified using an Agilent 6890 gas chromatograph coupled to an Agilent 5975 mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) operated in negative chemical ionization mode (GC/ECNI-MS). Laboratory blanks were low enough (<1%) that blank correction was not needed. The recoveries of two surrogate standards, which were spiked into each dust sample prior to extraction, were 92 and 102%.
Serum PBDEs
Serum samples (5 mL) were collected from 12 male/female partners recruited into the ongoing study in 2007–2008. The collection time of these serum samples coincided with the collection of dust samples from these homes. The serum samples were sent on dry ice to the CDC Combustion Products and Persistent Pollutants Biomonitoring Laboratory in Atlanta, GA. The methodology for the analysis of PBDEs in serum has been published by Sjodin et al. (23). Briefly, samples were denatured with formic acid, diluted with water and fortified with internal standards prior to solid phase extraction (SPE) using a Rapid Trace modular SPE system (Caliper Life Sciences; Hopkinton, MA, USA). Determination of the target analytes was performed by gas chromatography isotope dilution high resolution mass spectrometry (GC-IDHRMS) employing a MAT95XP instrument (ThermoFinnigan MAT; Bremen, Germany). The serum lipid concentrations were determined using test kits from Roche Diagnostics (Indianapolis, IN) for total triglycerides and total cholesterol. Final determinations were made on a Hitachi 912 Chemistry Analyzer (Hitachi; Tokyo, Japan). All concentrations of PBDEs are reported as background subtracted.
Data analysis
Descriptive statistics were calculated for all PBDE congeners with at least 50% detection rates to examine the distributions by congener in house dust and serum. One half the detection limit was assigned to non-detect levels for the calculation of geometric means. Spearman's correlation coefficients were calculated to assess bivariate relationships between PBDE concentrations in house dust and serum, and between different PBDE congeners within the same matrix. Spearman's correlation coefficients were also calculated to assess within-couple (female-male) correlations of serum PBDE concentrations.
RESULTS
Table 1 presents the distribution and detection rates of PBDE congeners measured in house dust. Results are presented for 50 dust samples, although only the 12 most recently collected dust samples were from couples who also provided serum samples for PBDE analysis. The distribution of PBDE concentrations of the 12 matched dust samples is similar to the distribution of the entire 50 samples analyzed. Concentrations of PBDEs (sum of all congeners) ranged from 980 to 44,546 ng/g dust. The geometric mean of summed PBDEs was 4,742 ng/g. All of the PBDE congeners detected were log-normally distributed. BDE 209, the major congener in DecaBDE commercial mixtures, was the dominant PBDE congener, accounting for 43% on average of total PBDEs by weight in the dust samples. BDE 47 and 99, the two major constituents of the PentaBDE commercial mixture, made up 16% and 22% respectively, of total PBDEs on average. There were strong correlations (Spearman r ≥ 0.80, p < 0.05) among dust concentrations of PBDE congener groups with the same or close degree of bromination. A complete table of correlation coefficients for all detectable congeners in dust can be found in the Supporting Information (Table S1).
Table 1.
Distribution of PBDE Congeners in House Dust, ng/g (N=50)
| Selected Percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Congener | Geometric Mean |
Minimum | 10th | 25th | 50th | 75th | 90th | Maximum | DL | Detection rate (%) |
| 30 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 5.20 | 0.06 | 4 |
| 17 | 2.62 | <DL | 0.84 | 1.57 | 2.67 | 5.49 | 8.86 | 486 | 0.10 | 94 |
| 25 | NC | <DL | <DL | <DL | <DL | <DL | 2.19 | 3.95 | 0.20 | 24 |
| 28,33a | 13.0 | 3.14 | 5.36 | 6.83 | 11.7 | 21.1 | 35.2 | 84.0 | 0.10 | 100 |
| 75 | 7.54 | <DL | <DL | 5.90 | 14.6 | 24.6 | 51.3 | 100 | 0.70 | 76 |
| 49 | 21.4 | <DL | 7.51 | 12.8 | 19.6 | 41.5 | 74.7 | 195 | 0.80 | 98 |
| 71 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.30 | 0 |
| 47 | 543 | 100 | 157 | 288 | 390 | 1122 | 2299 | 8627 | 4.17 | 100 |
| 66 | 9.76 | <DL | 3.87 | 5.79 | 7.94 | 20.6 | 35.4 | 134 | 0.05 | 98 |
| 100 | 135 | 19.0 | 44.2 | 63.3 | 99.9 | 228 | 608 | 2164 | 0.19 | 100 |
| 119 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 77.3 | 0.05 | 2 |
| 99 | 643 | 79.3 | 204 | 260 | 427 | 1140 | 3241 | 12967 | 0.47 | 100 |
| 116 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.10 | 0 |
| 85155a | 33.6 | 4.61 | 10.8 | 14.4 | 24.6 | 54.2 | 169 | 544 | 0.10 | 100 |
| 154 | 63.2 | 6.69 | 18.3 | 25.4 | 51.3 | 131 | 359 | 1093 | 0.10 | 100 |
| 153 | 78.6 | 13.2 | 23.4 | 31.2 | 55.9 | 188 | 380 | 1352 | 0.10 | 100 |
| 138 | 4.28 | <DL | <DL | 3.10 | 5.45 | 13.0 | 32.7 | 81.5 | 0.10 | 86 |
| 156 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.10 | 0 |
| 183 | 20.0 | 3.76 | 5.87 | 11.3 | 17.4 | 33.9 | 61.8 | 688 | 0.10 | 100 |
| 191 | 16.9 | 0.05 | 3.66 | 9.66 | 20.7 | 41.9 | 122 | 1038 | 0.10 | 94 |
| 190 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.10 | 0 |
| 202 | 0.86 | <DL | <DL | <DL | 3.40 | 6.29 | 10.3 | 33.6 | 0.10 | 58 |
| 201 | 12.6 | 0.05 | 4.34 | 7.37 | 12.6 | 22.8 | 39.6 | 494 | 0.10 | 98 |
| 197 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.20 | 0 |
| 203200a | 12.1 | 1.25 | 3.27 | 5.90 | 11.2 | 27.5 | 36.4 | 243 | 0.25 | 100 |
| 196 | NC | <DL | <DL | <DL | <DL | <DL | <DL | <DL | 0.20 | 0 |
| 205 | NC | <DL | <DL | <DL | <DL | <DL | 0.54 | 64.1 | 0.20 | 10 |
| 208 | 35.3 | 8.48 | 15.3 | 18.5 | 30.1 | 49.9 | 93.0 | 853 | 0.89 | 100 |
| 207 | 63.1 | 12.4 | 26.6 | 31.9 | 54.9 | 97.3 | 242 | 1492 | 1.06 | 100 |
| 206 | 163 | 33.9 | 73.3 | 85.5 | 156 | 250 | 505 | 3772 | 0.50 | 100 |
| 209 | 1906 | 425 | 803 | 1146 | 1482 | 2840 | 5816 | 32366 | 14.1 | 100 |
| Total PBDEs | 4742 | 980 | 1852 | 2651 | 4458 | 7879 | 15360 | 44546 | ||
Congeners are listed together due to coelution in GC/MS analysis.
DL = Detection limit. One-half the DL was used for measurements below the DL.
NC = Not calculated. Geometric means and percentiles were not calculated for congeners with detection rates below 50%.
Table 2 presents the distribution and detection rates of PBDEs measured in serum, shown as both total serum and lipid-adjusted values. BDE 47 was found at the highest median concentration in serum, followed by BDE 153 at the next to highest median concentration. Several congeners, including BDE 209, had low detection rates (less than 30%). BDE 209 had the highest detection limit and was only detected in 2 of 24 serum samples. The congeners with high detection rates in serum (BDE 28, 47, 99, 100 and 153) are components of the PentaBDE commercial mixture. These congeners were correlated with one another (r = 0.70–0.96, p < 0.05), with the exception of BDE 153, which is also a component of the OctaBDE mixture. A complete table of correlation coefficients for all detectable congeners in serum can be found in the Supporting Information (Table S2).
Table 2.
Distribution of PBDE Congeners in Serum (N=24)
| Selected Percentiles | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Congener | Geometric Mean |
Minimum | 10th | 25th | 50th | 75th | 90th | Maximum | LOD | Detection rate (%) |
|
| Serum (pg/g) |
17 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 4.1 | 2.5 | 8 |
| 28 | 6.7 | 1.3 | <DL | 4.1 | 7.6 | 11 | 17 | 39 | 2.5 | 88 | |
| 47 | 95 | 17 | 34 | 50 | 96 | 187 | 245 | 511 | 10.9 | 100 | |
| 66 | NC | <DL | <DL | <DL | <DL | <DL | 2.7 | 5.8 | 2.5 | 17 | |
| 85 | NC | <DL | <DL | <DL | <DL | <DL | 4.0 | 6.1 | 2.5 | 25 | |
| 99 | 16 | 4.8 | 4.8 | 8.9 | 15 | 34 | 41 | 72 | 9.8 | 75 | |
| 100 | 18 | 3.1 | 7.0 | 11 | 19 | 22 | 49 | 176 | 2.5 | 100 | |
| 153 | 43 | 6.8 | 12.8 | 24 | 36 | 74 | 137 | 1151 | 2.5 | 100 | |
| 154 | NC | <DL | <DL | <DL | <DL | 2.6 | 4.1 | 8.6 | 2.5 | 29 | |
| 183 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 4.8 | 2.5 | 13 | |
| 209 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 25.1 | 25.0 | 8 | |
| Total PBDEs | 255 | 94 | 116 | 157 | 219 | 341 | 523 | 1691 | |||
| Serum, Lipid- adjusted (ng/g) |
17 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 0.7 | 0.3–0.6 | |
| 28 | 1.1 | <DL | <DL | 0.7 | 1.3 | 1.8 | 2.8 | 6.4 | 0.3–0.6 | ||
| 47 | 16 | <DL | 5.2 | 8.7 | 17 | 34 | 41 | 83 | 1.4–2.5 | ||
| 66 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 0.9 | 0.3–0.6 | ||
| 85 | NC | <DL | <DL | <DL | <DL | <DL | 0.7 | 0.9 | 0.3–0.6 | ||
| 99 | 2.6 | <DL | <DL | 1.4 | 2.4 | 5.0 | 6.9 | 12 | 1.3–2.3 | ||
| 100 | 3.0 | <DL | 1.0 | 2.1 | 3.0 | 4.3 | 8.1 | 24 | 0.3–0.6 | ||
| 153 | 7.1 | 1.3 | 1.8 | 4.3 | 7.0 | 11 | 20 | 154 | 0.3–0.6 | ||
| 154 | NC | <DL | <DL | <DL | <DL | <DL | 0.8 | 1.2 | 0.3–0.6 | ||
| 183 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 0.6 | 0.3–0.6 | ||
| 209 | NC | <DL | <DL | <DL | <DL | <DL | <DL | 6 | 3.3–5.8 | ||
| Total PBDEs | 40 | 13 | 14 | 28 | 39 | 61 | 76 | 225 | |||
DL = Detection limit. One-half the DL was used for measurements below the DL.
NC = Not calculated. Geometric means and percentiles were not calculated for congeners with detection rates below 50%.
Lipid-adjusted serum detection limits are ranges due to varying sample volumes.
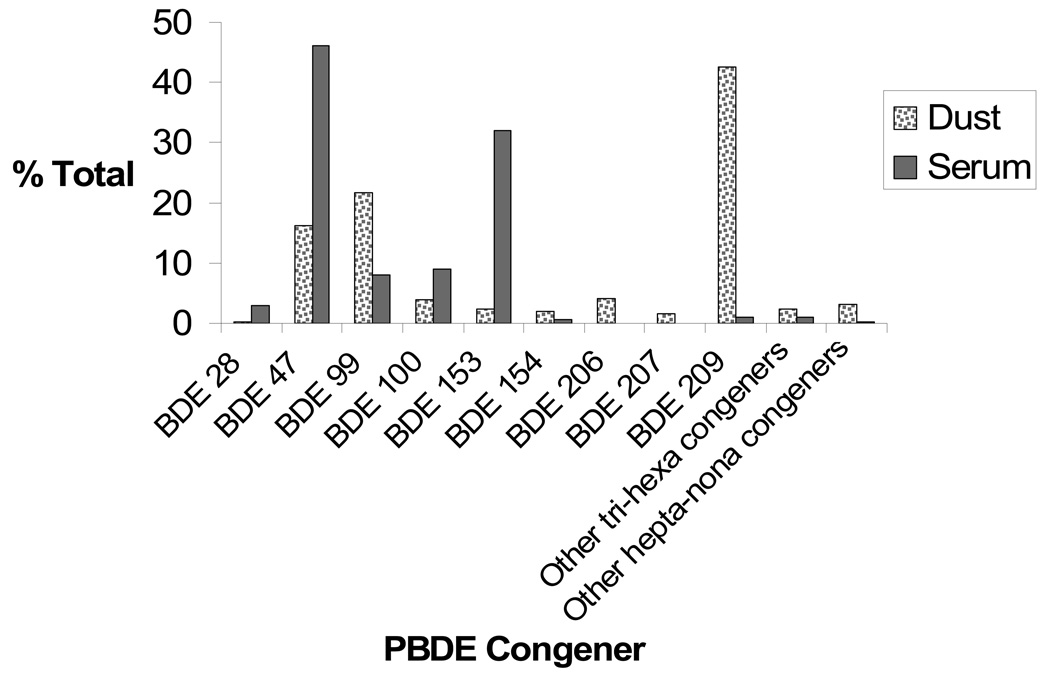
Figure 1 compares the overall PBDE congener composition profiles of dust and serum samples. The compositions are represented as percent, by mass, of each total of samples (dust or serum). The dust and serum samples followed the same pattern with some exceptions. BDE 209 was the dominant congener in the majority of dust samples, although 11 of 50 samples had levels of BDE 99 higher than the other congeners, and 7 of these samples also had levels of other congeners higher than BDE 209. In serum, BDE 47 was found at the highest concentration in 17 out of 24 samples, while in 4 females and 3 males, BDE-153 was found at a higher concentration than other congeners.
Figure 1. PBDE Congener by proportion in dust and serum.
Dust: n = 50, except those congeners with <100% detection (some of "other tri-nona congeners"). See Table 1 for detection rates.
Serum: n = 24, except those congeners with <100% detection (BDE 28, 99, 154, 209 and "other tri-nona congeners"). See Table 2 for detection rates.
Other tri-hexa and hepta-nona congeners are those listed in Tables 1 and 2.
BDE 206 and 207 were not measured in serum.
Table 3 shows Spearman's correlations between dust and serum concentrations of PBDEs. Several congeners (BDE 17, 66, 85, 154, 183 and 209) had low detection rates in serum (see Table 2) and were therefore not included in correlation matrices with dust concentrations. Of the two individuals with serum containing detectable levels of BDE 209, one of the homes had a concentration of BDE 209 in dust above the median, and the other home had a concentration below the median. There was a strong correlation (r = 0.65–0.89, p < 0.05) between dust and serum concentrations for most BDE congeners with high detection rates (BDE 47, 99 and 100). However, dust and serum levels of BDE 153 were not correlated (r < 0.01). There were strong correlations between several BDE congeners measured in dust and lower-brominated congeners measured in serum. F or example, BDE 100 in dust was correlated with BDE 28 in serum (r = 0.79, p = 0.002).
Table 3.
Spearman correlation coefficients for selected PBDE concentrations in dust and serum
| Dust PBDE Congener | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE 17 | BDE 28 | BDE 75 | BDE 49 | BDE 47 | BDE 66 | BDE 99 | BDE 100 | BDE 154 | BDE 153 | BDE 138 | BDE 183 | BDE 209 | |||
| Male Serum (lipid- adjusted) PBDE Congener (n=12) |
BDE 28 | r p-value |
0.81 0.001 |
0.49 0.10 |
0.83 0.0008 |
0.77 0.004 |
0.82 0.001 |
0.75 0.005 |
0.89 0.0001 |
0.79 0.002 |
0.87 0.0002 |
0.87 0.0002 |
0.79 0.002 |
0.55 0.06 |
0.04 0.91 |
| BDE 47 | r p-value |
0.84 0.0006 |
0.53 0.08 |
0.79 0.002 |
0.71 0.009 |
0.81 0.002 |
0.70 0.01 |
0.85 0.0004 |
0.69 0.01 |
0.81 0.001 |
0.82 0.001 |
0.69 0.01 |
0.54 0.07 |
−0.004 0.99 |
|
| BDE 99 | r p-value |
0.87 0.0003 |
0.60 0.04 |
0.76 0.004 |
0.75 0.005 |
0.84 0.0007 |
0.73 0.007 |
0.89 0.0001 |
0.72 0.008 |
0.85 0.0004 |
0.83 0.0008 |
0.73 0.008 |
0.36 0.25 |
0.07 0.84 |
|
| BDE 100 | r p-value |
0.78 0.003 |
0.64 0.02 |
0.88 0.0002 |
0.82 0.001 |
0.72 0.008 |
0.70 0.01 |
0.81 0.001 |
0.65 0.02 |
0.71 0.01 |
0.72 0.008 |
0.58 0.05 |
0.55 0.07 |
0.16 0.61 |
|
| BDE 153 | r p-value |
0.02 0.96 |
0.05 0.87 |
0.25 0.44 |
−0.01 0.97 |
0.02 0.97 |
−0.14 0.66 |
−0.04 0.91 |
−0.24 0.46 |
−0.05 0.87 |
0.00 1.00 |
−0.15 0.65 |
0.01 0.97 |
0.33 0.29 |
|
| BDE 17 | BDE 28 | BDE 75 | BDE 49 | BDE 47 | BDE 66 | BDE 99 | BDE 100 | BDE 154 | BDE 153 | BDE 138 | BDE 183 | BDE 209 | |||
| Female Serum (lipid- adjusted) PBDE Congener (n=12) |
BDE 28 | r p-value |
0.82 0.001 |
0.51 0.09 |
0.70 0.01 |
0.73 0.007 |
0.85 0.0004 |
0.82 0.001 |
0.90 0.0001 |
0.84 0.0007 |
0.94 0.0001 |
0.84 0.0007 |
0.85 0.0005 |
0.40 0.20 |
0.01 0.97 |
| BDE 47 | r p-value |
0.78 0.003 |
0.45 0.14 |
0.62 0.03 |
0.64 0.03 |
0.80 0.002 |
0.76 0.005 |
0.87 0.0002 |
0.80 0.002 |
0.90 0.0001 |
0.80 0.002 |
0.85 0.0004 |
0.34 0.30 |
0.24 0.46 |
|
| BDE 99 | r p-value |
0.56 0.06 |
0.32 0.32 |
0.34 0.28 |
0.31 0.33 |
0.64 0.03 |
0.50 0.10 |
0.69 0.01 |
0.55 0.06 |
0.67 0.02 |
0.62 0.03 |
0.71 0.01 |
0.13 0.69 |
0.45 0.14 |
|
| BDE 100 | r p-value |
0.57 0.05 |
0.31 0.33 |
0.51 0.09 |
0.47 0.12 |
0.70 0.01 |
0.66 0.02 |
0.78 0.003 |
0.71 0.01 |
0.80 0.002 |
0.71 0.01 |
0.78 0.003 |
0.29 0.35 |
0.16 0.62 |
|
| BDE 153 | r p-value |
0.05 0.88 |
0.22 0.48 |
0.10 0.76 |
0.07 0.83 |
0.13 0.68 |
0.03 0.91 |
0.08 0.80 |
0.03 0.91 |
−0.11 0.73 |
0.02 0.95 |
0.06 0.86 |
0.03 0.91 |
0.16 0.62 |
|
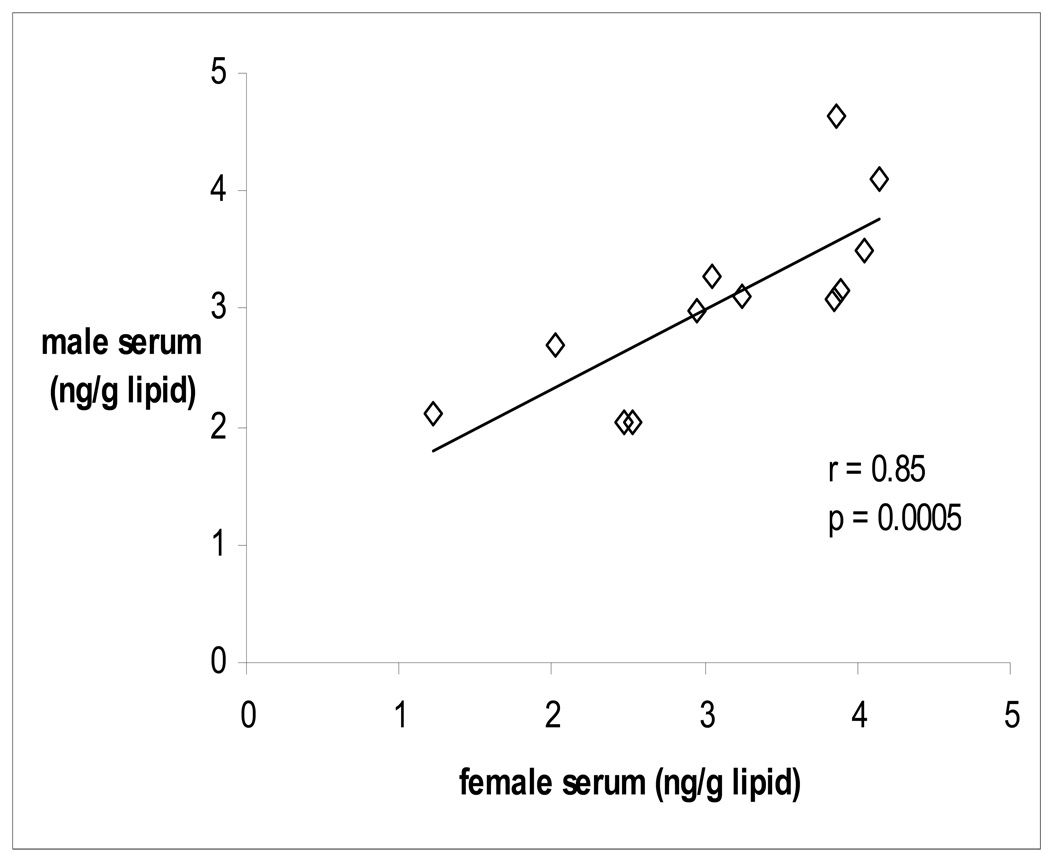
There was a strong correlation (r = 0.85, p = 0.0005) for serum concentrations of the major Penta formulation BDEs (sum of BDE 47, 99 and 100) between males and females of couples residing in the same household (Figure 2). However, serum levels of BDE 153 were not significantly correlated within couples (r = 0.40, p = 0.19). A complete table of correlations between PBDE concentrations in male and female serum which includes all detectable congeners (BDE 28, 47, 99, 100, and 153) can be found in the Supporting Information (Figure S3).
Figure 2. Within-couple serum PBDE correlation (sum of BDE 47, 99, 100).
Values in graph are transformed to the natural log. Spearman correlation coefficient, n = 12 couples.
DISCUSSION
PBDE concentration ranges in dust and serum in the present study were similar to concentrations found in other studies conducted among North Americans (21, 24, 25, 26), and therefore the PBDE exposures among individuals in this study are likely representative of exposures of the general US population. There were strong correlations between dust concentrations of PBDE congener groups with the same or close degree of bromination. These relationships resemble the congener mixtures (PentaBDE, OctaBDE, or DecaBDE) in commercial products and suggest that the congeners originated from the same sources within the home. Because BDE 202 is not present at detectable levels in commercial formulations, its detection in dust samples may be indicative of environmental debromination of BDE 209 (27). BDE 209 was measured at the highest concentrations in dust, followed by BDE 99 and BDE 47. In contrast, BDE 47 and BDE 153 were measured at the highest concentrations in serum. BDE 47 may dominate serum samples due to dietary intake, as it was often found as the dominant congener in food items analyzed as part of a market basket survey (28). The predominance of BDE 47 in serum samples may also be due in part to gaseous inhalation, as BDE 47 has been found to be a dominant congener in indoor air (13, 29). However, it is not possible to evaluate contributions to exposure from diet or inhalation of volatile gases in the present study. Our observation of a strong correlation between dust and serum BDE 47 concentrations supports the argument that dust is a major exposure pathway for BDE 47.
Several congeners, including BDE 209, had low detection rates in serum and therefore conclusions regarding dust-serum or within-couple relationships for these congeners were not possible. Several congeners (BDE 28, 47, 99, 100, 153) had high detection rates, and therefore associations with PBDE concentrations in dust could be evaluated. There were strong correlations between dust and serum concentrations of the major Penta formulation BDE congeners 47, 99 and 100, which suggests that dust is a good measure of exposure to these congeners. Serum concentrations of these congeners were also strongly correlated between males and females of couples (Figure 2), which supports the use of a serum or dust measurement from one member of a couple living together to represent their partner's exposure to these congeners. This estimate of exposure may not apply to children living in the same household, as a child's exposure to dust is expected to be greater (30). Dust and serum levels of BDE 153 were not correlated, which may be due to differences in exposure sources (e.g. diet or exposures outside the home), transformation, distribution or metabolism of this congener. BDE 153 has a long half-life as compared to the other congeners (31, 32). Serum concentrations of BDE 153 were also not correlated between males and females within couples. Conversely, BDE 153 in dust was correlated to lower brominated congeners in serum, which may support the argument that transformation is occurring. As observed by Qiu, et al. (33), different PBDE congeners may have different rates of hydroxylation, and this may be an explanation why human serum may exhibit different congener profiles than dust or commercial product mixtures. Huwe et al. (34) demonstrated that PBDE congeners have different degrees of bioconcentration in rats, possibly due to metabolism differences between congeners. The lack of BDE 209 measured in serum may be due to higher detection limits and/or to its short biological half-life. Stapleton et al. (6) demonstrated that when carp were fed BDE 209, only lower-brominated congeners, and not BDE 209, bioaccumulated in the fish. Further research into the transformation processes of individual PBDE congeners is needed to understand patterns in the biomarker profiles of these compounds.
A recent study utilizing the National Health and Nutrition Examination Survey (NHANES) dietary questionnaire responses and serum PBDE data concluded that intake of poultry and red meat is a source of PBDE exposure in the US population (35). In particular, BDE 153 was the only congener associated with total fat intake, and although vegetarians had lower total PBDE serum levels, they did not have significantly reduced levels of BDE 153. Higher levels of dietary exposure to BDE 153 may explain higher BDE 153 levels in serum in the present study population. However, US market basket surveys (28, 36) do not indicate that certain foods have higher concentrations of BDE 153. Fraser et al. (35) also found similar results to the present study in terms of serum congener correlations, with the Penta formulation congeners (BDE 28, 47, 99, 100 and 153) being strongly correlated with one another. However, the authors reported that BDE 153 had a weaker association than the other congeners. In the present study, the Penta formulation congeners were also strongly correlated, but BDE 153 was not associated with the other congeners in serum.
Limited studies have assessed relationships between dus t and serum concentrations of PBDEs. A study of only five Swedish homes reported a correlation between researcher-collected house dust and plasma levels of PBDEs, although the association was dependent on one of the five households (37). A recent study of 19 Belgian students found no correlation between PBDE concentrations in the students’ serum and dust concentrations in their dormitories (38). Another study conducted on 34 German homes found no significant correlation between dust and serum concentrations of PBDEs, and the authors concluded that diet is the main exposure pathway (39). However, although European food samples were reported to have the same level of PBDE contamination as US samples, (10, 28), dust levels in European countries are orders of magnitude lower than US levels (24), and therefore these data may not be comparable to the data in the present study. Based on pharmacokinetic modeling, Lorber (10) concluded that dietary and inhalation exposures could not explain US body burdens of PBDEs, and that exposure to indoor dust is the primary pathway.
Various studies on indoor environmental contaminants employ different methods in the collection of house dust as a measure of exposure. Specifically, researcher-collected dust has been compared to vacuum bag dust (26, 40, 41). The studies by Colt et al concluded that there was a high level of agreement between researcher-collected dust (high-volume surface sampler, HVS3) and vacuum bag dust for pesticides and other organic contaminants, including polychlorinated biphenyls (PCBs). The study by Allen et al (26) found that researcher-collected dust had varying degrees of correlation with vacuum bag dust concentrations of PBDEs (r = 0.39–0.77), depending on the room in the home and the sampling round. Furthermore, PBDE concentrations in researcher-collected dust were significantly different between rooms of the same home. Future studies on dust collection methods should focus on the validation of these methods and include biomarkers as evidence of biological relevance. It is possible that the use of vacuum bag dust in exposure assessment may be a superior method of dust collection, provided that the dust collected is a measure of longer term integrative exposure representative of the total home environment, and thus total exposure, and not limited to a specific area or time. The use of vacuum bags may also provide a much more time- and cost-efficient method for measuring dust contamination in large-scale epidemiological studies. On the other hand, researcher-collected dust samples would likely have more utility in studies aiming to define specific PBDE exposure sources in the home or other microenvironments.
This study is the first to provide empirical evidence of the association between house dust and serum concentrations of PBDEs in the US. For PBDE congeners that do not show strong correlation between dust and serum, such as BDE 153, dust may not be a good indicator of body burden. However, for other PBDE congeners such as the major Penta formulation BDEs, which were strongly correlated between dust and serum concentrations, house dust may be a good measure of exposure. This observation serves to further validate our recent finding of significant relationships between dust concentrations of PentaBDEs and circulating hormone levels in men (20). Furthermore, house dust may provide a satisfactory estimate of human exposure to BDE 209 due to its high concentrations in dust and current limitations of measuring BDE 209 in serum. The relatively short biological half-life of BDE 209 may prevent reliable measurement in serum, but because BDE 209 concentrations in dust are high, people are likely continuously exposed. Thus, dust concentrations may currently be the best marker of exposure to BDE 209 in the absence of other biomarkers.
Supplementary Material
Acknowledgements
Work supported by R01 ES009718 and R01 ES016099 from the National Institute of Environmental Health Sciences (NIEHS). The authors are very grateful to Russ Hauser (Harvard School of Public Health and Harvard Medical School), who is principal investigator of the ongoing reproductive health study, and Drs. Thomas Webster and Michael McClean (Boston University) for their guidance on study design.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supporting Information
The Supporting Information to this manuscript contains tables with correlation coefficients for relationships between all detectable congeners in dust, all detectable congeners in serum, and between PBDE concentrations in male and female serum.
REFERENCES
- 1.ATSDR. Toxicological profile for polybrominated biphenyls and polybrominated diphenyl ethers (PBBs and PBDEs) Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2004. [PubMed] [Google Scholar]
- 2.WHO. Persistent Organic Pollutants (POPs) in Human Milk; Fact Sheet No. 4.3, Code: RPG4_Food_Ex2. World Health Organization, European Environment and Health Information System; 2007
- 3.EPA. www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 4.BSEF. www.bsef.com/regulation/north-america/deca-bde-3/
- 5.Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K. Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers. Environ. Health Perspect. 2006;114:176–181. doi: 10.1289/ehp.8350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stapleton HM, Alaee M, Letcher RJ, Baker JE. Debromination of the flame retardant decabromodiphenyl ether by juvenile carp (Cyprinus carpio) following dietary exposure. Environ. Sci. Technol. 2004;38:112–119. doi: 10.1021/es034746j. [DOI] [PubMed] [Google Scholar]
- 7.Soderstrom G, Sellstrom U, de Wit CA, Tysklind M. Photolytic debromination of decabromodiphenyl ether (BDE 209) Environ. Sci. Technol. 2004;38:127–132. doi: 10.1021/es034682c. [DOI] [PubMed] [Google Scholar]
- 8.Betts KS. Unwelcome guest: PBDEs in indoor dust. Environ. Health Perspect. 2008;116:A202–A208. doi: 10.1289/ehp.116-a202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster TF, Harrad S, Millette JR, Holbrook RD, Davis JM, Stapleton HM, Allen JG, McClean MD, Ibarra C, Abdallah MA, Covaci A. Identifying transfer mechanisms and sources of decabromodiphenyl ether (BDE 209) in indoor environments using environmental forensic microscopy. Environ. Sci. Technol. 2009;43:3067–3072. doi: 10.1021/es803139w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorber M. Exposure of Americans to polybrominated diphenyl ethers. Journal of Expo. Sci. Environ. Epidemiol. 2008;18:2. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- 11.Jones-Otazo HA, Clarke JP, Miriam LD, Archbold JA, Ferguson G, Harner T, Richardson GM, Ryan JJ, Wilford B. Is house dust the missing exposure pathway for PBDEs? An analysis of the urban fate and human exposure to PBDEs. Environ. Sci. Technol. 2005;39:5121–5130. doi: 10.1021/es048267b. [DOI] [PubMed] [Google Scholar]
- 12.Webster TF, Vieira V, Schecter A. Estimating exposure to PBDE-47 via air, food and dust using Monte Carlo methods. Organohalogen Compd. 2005;67:505–508. [Google Scholar]
- 13.Johnson-Restrepo B, Kannan K. An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States. Chemosphere. 2009;76:542–548. doi: 10.1016/j.chemosphere.2009.02.068. [DOI] [PubMed] [Google Scholar]
- 14.Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey LE, La Guardia M, McClean MD, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ. Sci. Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- 15.Darnerud PO. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008;31:152. doi: 10.1111/j.1365-2605.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 16.Legler J. New insights into the endocrine disrupting effects of brominated flame retardants. Chemosphere. 2008;73:216. doi: 10.1016/j.chemosphere.2008.04.081. [DOI] [PubMed] [Google Scholar]
- 17.Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Jr, Anderson HA. Hormone disruption in adult male sport fish consumers. Environ. Health Perspect. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bloom M, Spliethoff H, Vena J, Shaver S, Addink R, Eadon G. Environmental exposure to PBDEs and thyroid function among New York anglers. Environ. Toxicol. Pharm. 2008;25:386–392. doi: 10.1016/j.etap.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Yuan J, Chen L, Chen D, Guo H, Bi X, Ju Y, Jiang P, Shi J, Yu Z, Yang J, Li L, Jiang Q, Sheng G, Fu J, Wu T, Chen X. Elevated serum polybrominated diphenyl ethers and thyroid-stimulating hormone associated with lymphocytic micronuclei in Chinese workers from an E-waste dismantling site. Environ. Sci. Technol. 2008;42:2195–2200. doi: 10.1021/es702295f. [DOI] [PubMed] [Google Scholar]
- 20.Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009;407:3425–3429. doi: 10.1016/j.scitotenv.2009.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Polybrominated dihenyl ethers in house dust and clothes dryer lint. Environ. Sci. Technol. 2005;39:925–931. doi: 10.1021/es0486824. [DOI] [PubMed] [Google Scholar]
- 22.Stapleton HM, Harner T, Shoeib M, Keller JM, Schantz MM, Leigh SD, Wise SA. Determination of polybrominated diphenyl ethers in indoor dust standard reference materials. Anal. Bioanal. Chem. 2006;384:791–800. doi: 10.1007/s00216-005-0227-y. [DOI] [PubMed] [Google Scholar]
- 23.Sjodin A, Jones RS, Lapeza CR, Focant J-F, McGahee EE, III, Patterson DG., Jr Semi-automated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls and polychlorinated biphenyls in human serum. Anal Chem. 2004;76:1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- 24.Sjödin A, Päpke O, McGahee E, Focant J, Jones RS, Pless-Mulloli T, Toms LL, Herrmann T, Müller J, Needham LL, Patterson DG., Jr Concentration of polybrominated diphenyl ethers (PBDEs) in household dust from various countries. Chemosphere. 2008;73:S131–S136. doi: 10.1016/j.chemosphere.2007.08.075. [DOI] [PubMed] [Google Scholar]
- 25.Sjodin A, Wong L-Y, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated biphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States Population: 2003–2004. Environ. Sci. Technol. 2008;42:1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- 26.Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ. Int. 2008;34:1085–1091. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Stapleton HM, Dodder NG. Photodegradation of decabromodiphenyl ether in house dust by natural sunlight. Environ. Toxicol. Chem. 2008;27:306–312. doi: 10.1897/07-301R.1. [DOI] [PubMed] [Google Scholar]
- 28.Schecter A, Papke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006;114:1515–1520. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toms LL, Hearn L, Kennedy K, Harden F, Bartkow M, Temme C, Mueller JF. Concentrations of polybrominated diphenyl ether (PBDEs) in matched samples of human milk, dust and indoor air. Environ. Int. 2009;35:864–869. doi: 10.1016/j.envint.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 30.U.S. EPA. Exposure Factors Handbook. E PA/600/P-95/002Fa,b,c. Washington, DC: Office of Research and Development; 1997. [Google Scholar]
- 31.Geyer HJ, Schramm K-W, Darnerud PO, Aune M, Feicht A, Fried KW, Henkelmann B, Lenoir D, Schmid P, McDonald T. Terminal elimination half-lives of the brominated flame retardants TBBPA, HBCD, and lower brominated PBDEs in humans. Organohalogen Compounds. 2004;66:3867–3872. [Google Scholar]
- 32.Van den Steen E, Covaci A, Jaspers VL, Dauwe T, Voorspoels S, Eens M, Pinxten R. Accumulation, tissue-specific distribution and debromination of decabromodiphenyl ether (BDE 209) in European starlings (Sturnus vulgaris) Environ. Pollut. 2007;148:648–653. doi: 10.1016/j.envpol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Qui X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers (PBDEs) in human blood samples from the United States. Environ. Health Perspect. 2006;117:93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huwe JK, Hakk H, Smith DJ, Diliberto JJ, Richardson V, Stapleton HM, Birnbaum LS. Comparative absorption and bioaccumulation of polubrominated diphenyl ethers following ingestion via dust and oil in male rats. Environ. Sci. Technol. 2008;42:2694–2700. doi: 10.1021/es702644k. [DOI] [PubMed] [Google Scholar]
- 35.Fraser AJ, Webster T, McClean MD. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ. Health Perspect. 2009;117:1520–1525. doi: 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schecter A, Haffner D, Colacino J, Patel K, Papke O, Opel M, Birnbaum L. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD) in composite U.S. food samples. Environ. Health Perspect. doi: 10.1289/ehp.0901345. In press, doi: 10.1289/ehp.0901345 (available at http://dx.doi.org/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson M, Julander A, van Bavel B, Hardell L. Levels of brominated flame retardants in blood in relation to levels in household air and dust. Environ. Int. 2007;33:62–69. doi: 10.1016/j.envint.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Roosens L, Abdallah MA, Harrad S, Neels H, Covaci A. Factors influencing concentrations of polybrominated diphenyl ethers (PBDEs) in students from Antwerp, Belgium. Environ. Sci. Technol. 2009;43:3535–3541. doi: 10.1021/es900571h. [DOI] [PubMed] [Google Scholar]
- 39.Fromme H, Korner W, Shahin N, Wanner A, Albrecht M, Boehmer S, Parlar H, Mayer R, Liebl B, Bolte G. Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ. Int. 2009;35:1125–1135. doi: 10.1016/j.envint.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Colt JS, Zahm SH, Camann DE, Hartge P. Comparison of pesticides and other compounds in carpet dust samples collected from used vacuum cleaner bags and from a high-volume surface sampler. Environ. Health Perspect. 1998;106:721–724. doi: 10.1289/ehp.98106721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colt JS, Gunier RB, Metayer C, Nishioka MG, Bell EM, Reynolds P, Buffler PA, Ward MH. Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environ. Health. 2008 doi: 10.1186/1476-069X-7-6. doi:10.1186/1476-069X-7-6, available online from: http://www.ehjournal.net/content/7/1/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.