Abstract
Objective
To compare the recovery of mobility and self-care functions among veteran amputees according to the timing and type of rehabilitation services received.
Design
Observational study of inpatient rehabilitation care patterns of 2 types (specialized and consultative) with 2 timings (early and late).
Setting
Data from inpatient specialized rehabilitation units (SRUs) and consultative services within 95 Veterans Affairs Medical Centers across the United States during fiscal years 2003-2004.
Patients
Medical records of 1,502 patients who received early or late consultative or specialized rehabilitation.
Assessment of risk factors
Hypotheses were established and general categories of negative and positive risk factors specified a priori from available clinical characteristics. Linear mixed effects models were used to model motor Functional Independence Measure (FIM™) gain scores on patient-level variables accounting for the correlation within the same facility.
Main outcome measures
Recovery of activities of daily living (ADLs) and mobility (physical functioning) expressed as the magnitudes of gains in motor FIM™ scores achieved by rehabilitation discharge.
Results
After adjustment, amputees who received specialized rehabilitation had motor FIM™ gains that were on average 8.0 points higher than those amputees who received consultative rehabilitation. Although patients whose rehabilitation was delayed until after discharge from the index surgical stay tended to be more clinically complex, they had comparable gains to patients who received early rehabilitation. Advanced age, trans-femoral amputation, paralysis, serious nutritional compromise, and psychosis were associated with lower motor FIM™ gains. The variance for the random effect for facility was statistically significant, suggesting extraneous variation within facility that was not explainable by observed patient-level variables.
Conclusion
Based on this analysis, those patients who receive specialized rehabilitation can be expected to make comparatively higher gains than patients who receive consultative services, regardless of timing and clinical complexity. Findings highlight the need for clinicians to adjust prognostic expectations to both clinical severity and the type of rehabilitation patients receive.
Introduction
Approximately 1.6 million, or one in every 190 Americans, was estimated to have limb loss in 2005.1 The aging of the population and the high prevalence of diabetes mellitus could more than double the number to 3.6 million by the year 2050. Amputation is physiologically and functionally devastating with extraordinary economic costs and reductions in quality of life. It is generally believed that rehabilitation, by enhancing functional recovery, can improve quality of life and reduce the economic burden associated with major limb loss. Yet, there is ongoing concern that health care professionals have little evidence of the value of many treatment alternatives, including rehabilitation. Consequently, the current Federal Administration strongly endorses comparative effectiveness research as a way to address how patients with a particular medical condition react to alternative “real world” approaches to care.2 The comparison of outcome expectations associated with differences in the timing and type (intensities) of rehabilitation received by patients with lower extremity amputation is essential for the upcoming healthcare reform debate.2
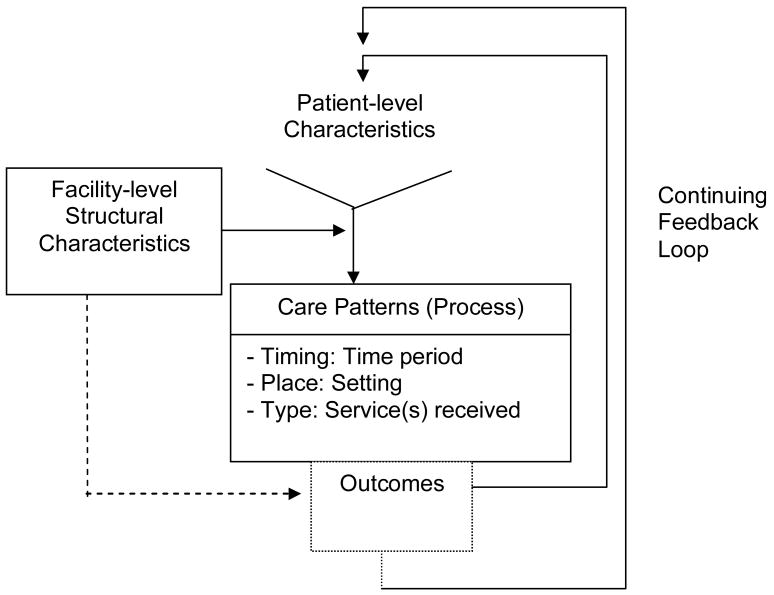
Variation in care has been documented in many medical fields, particularly where knowledge about best practices is most uncertain.3 Clearly, knowledge about best practices in rehabilitation is limited. Rehabilitation involves a long process with phases that are not well defined, sharply delineated, or standardized and the level of services provided varies.4 Moreover, high intensity care, as shown in other fields, is not necessarily associated with higher quality care or improved outcomes.5 We previously developed the Type, Place, Timing (TPT) framework6 to help delineate rehabilitation patterns and phases of care, and to study the degree to which rehabilitation service variability is associated with outcome differences among clinically similar groups of patients (Figure 1). Outcomes, using this framework, are expected to vary according to (1) the Type of rehabilitation received, (2) the Place where rehabilitation occurs, and (3) the Timing of rehabilitation. The TPT framework expands on the Donabedian domains of structure, process, and outcomes by visualizing linkages between rehabilitation and other types of health care services and by focusing on the environment where care occurs. This framework encourages a paradigm shift away from visualizing single episodes towards visualizing the continuum of services and further focusing on how care setting environments might influence patients' outcomes.7
Figure 1. The Timing, Place, and Type (TPT) Framework.
The outcomes of each service form the patient-level characteristics for the next service.
The TPT framework is supported by previous findings that patient complexity, as well as structural elements, influence both the types of rehabilitation received by patients and their outcomes in stroke and amputation.8-10 The decision as to whether patients receive specialized or consultative rehabilitation relates to clinical traits. For example, among veteran amputees, those with either the most profound or mild physical disabilities are more likely to receive consultative, while those with intermediately severe disabilities are more likely to receive specialized rehabilitation.11
These patterns would seem logical. Ambulation after limb loss is demanding. It might be impractical for those with profound disabilities. Conversely, those with mild disabilities are more likely capable of moving directly to outpatient services. There is also evidence that the type of rehabilitation received depends on service availability. While 26% of veterans whose surgical amputations occurred at Veterans Affairs Medical Centers (VAMCs) with specialized rehabilitation unit (SRU) beds received specialized services, only 11% of those whose amputation occurred in a VAMC without those beds received specialized care.8 Patient complexity across multiple domains (demand)12 and facility-level structural characteristics (supply)13 within the TPT framework are seen as combining with the medical-surgical care received to influence rehabilitation professionals' decisions about when, where, and what services to provide (Figure 1). The results of such decisions could influence the level of functional outcomes achieved.
The TPT framework classifies rehabilitation processes by type, place, and timing. The “Type” (T) of rehabilitation approximates what is done for the patient, i.e., the “Black Box of services.”14, 15 In this study, type contrasts specialized and consultative rehabilitation services. In specialized rehabilitation, patients are discharged from medical or surgical services and transferred to specific SRUs set aside for comprehensive inpatient rehabilitation. Primary responsibility for care shifts to Physical Medicine and Rehabilitation (PM&R) professionals with functional recovery becoming the main focus of the hospital stay. SRUs must meet explicitly defined standards, as outlined in the “CARF (Commission on Accreditation of Rehabilitation Facilities) Medical Rehabilitation Standards Manual.”16, 17 These continuously updated field-driven standards are aimed at constantly improving the value and responsiveness of the programs delivered to the people served.
SRU care in the Veterans Health Administration (VHA) tends to be highly coordinated and highly intensive with PM&R professionals seeing patients daily at set intervals. It is comparable to treatment in an inpatient rehabilitation facility (IRF) in the private sector. Consultation rehabilitation, by contrast, occurs when PM&R professionals see patients while they remain on other acute hospital services. Medical and surgical care remain the primary focus of the hospital stay. The patients' overall care remains the primary responsibility of those services. Consultation rehabilitation services are not required to follow CARF technical standards. During consultation rehabilitation, PM&R professions typically do not see patients daily but rather according to assessed need and staff availability.
The “Place” (P) reflects the setting where rehabilitation services are rendered, including inpatient, outpatient, long-term care facility, or home.15 Place is particularly relevant to the rehabilitation process and the capacity of that setting to produce a quality outcome. Even more important than to other healthcare fields, the environmental contexts,18 i.e., where rehabilitation occurs, will determine how well the functional outcomes achieved generalize to the individual's eventual real world living circumstances. Place is primarily the inpatient setting in this study.
The “Timing” (T) of rehabilitation relates to the onset of the disability and/or to the receipt of previous fundamental non-rehabilitative health care services, such as surgery in the case of amputation. In this study, the surgical amputation date and the hospital discharge date of the index surgical stay (the hospital stay in which amputation occurs) define onset and timing. Immediate postoperative inpatient rehabilitation occurs directly after the surgical amputation while patients are still hospitalized. With this early pattern, because they receive rehabilitation directly after surgery, patients can presumably avoid the development of maladaptive compensatory gait or transfer patterns which could theoretically complicate later prosthetic rehabilitation. Late rehabilitation begins during a separate hospitalization after discharge from the index surgical stay. In the late pattern, patients experience life in a non-hospital circumstance (either long-term care facility or home) before beginning rehabilitation, thereby gaining more of a real-life context to the process. Timing is introduced as essential to understanding how rehabilitation fits within the continuum of care along with medical, surgical, and other types of healthcare episodes.
In this study, we explored differences in expectation for motor Functional Independence Measure (FIM™) gain for patients receiving single episodes of inpatient specialized or consultative rehabilitation occurring either in the immediate postoperative period (early) or after acute hospital discharge (late). Motor FIM™ gain was selected as the outcome of interest because the recovery and preservation of physical functioning is considered the primary objective of rehabilitation treatment.19 We hypothesize that because of its greater coordination and intensity, patients who receive specialized rehabilitation can be expected to realize higher gains in physical functioning by discharge than patients who receive consultative rehabilitation, controlling for timing and clinical characteristics. We also anticipate that patients who receive early compared to late rehabilitation will achieve slightly higher motor FIM™ gains.
Methods
This observational study was approved by the Institutional Review Boards at the University of Pennsylvania in Philadelphia, Pennsylvania and the Samuel S. Stratton Veterans Affairs Medical Center (VAMC) in Albany, New York. This study was a quantitative epidemiological analysis in which data were collected through observations.20 It is considered prognostic because patients' initial motor FIM™ scores and all other clinical characteristics were measured prior to rehabilitation treatment and the final motor FIM™ score was measured at the conclusion of rehabilitation treatment at discharge.
Database Description
Data were obtained from 7 VHA administrative databases used to track veterans' health status and health care utilization. The databases included 4 inpatient datasets referred to as the Patient Treatment Files (PTF) (main, procedure, bed section, and surgery),21 2 outpatient datasets (visit and event), and the Functional Status and Outcomes Database (FSOD).22 Our methods of data extraction have been previously described.12, 23, 24 The FSOD, in particular, was most essential to this study since it tracks the timing and type of rehabilitation services received along a continuum and functional status regardless of the type(s) of rehabilitation received.
Definition of the Care Patterns Studied
This analysis focused on outcome expectations for persons who received inpatient rehabilitation care patterns of 2 types (specialized and consultative) with 2 timings (early and late). It is limited to those with a single episode of rehabilitation, either consultation only or specialized during the early or late time period.
Type = Specialized pattern
These patients were seen in consultation first, and then referred to the higher level specialized services. Individuals are discharged from specialized rehabilitation services when the team believed they achieved their goals or could not achieve them.
Type = Consultative pattern
Patients in the consultative groups completed their rehabilitation services on the consultative service and had no evidence of admission onto a SRU. Individuals are discharged from consultative rehabilitation services when the team believed they achieved their goals or could not achieve them.
Place
The patterns we studied were constrained to one place; that is, the inpatient VAMC setting, with continuation in the outpatient setting in some cases.
Timing = Early pattern
This is the acute or immediate postoperative rehabilitation pattern. Patients who had early timing met the following criteria: 1) had FSOD evidence of treatment on a SRU or consultation service with admission dates that fell after the surgical amputation date but on or before the index surgical discharge date; and 2) had no evidence of a SRU or consultation admission date after the index surgical discharge date.
Timing = Late pattern
This is a post-acute rehabilitation pattern. Patients who had late timing met the following criteria: 1) had FSOD rehabilitation admission and discharge dates that fell at least 1 day after discharge from the index surgical stay; and 2) had no evidence of consultative or SRU rehabilitation during the index surgical stay.
Subjects
There were a total of 4,727 veterans with a new lower extremity amputation identified from two waves of data spanning from October 1, 2002 to September 30, 2004 (fiscal years [FY] 2003 and 2004). A new major amputation was defined as the individual having no evidence of an amputation within the year preceding the index surgical date with amputation above the toes being considered a major amputation.25
The process to reach our final sample is illustrated in Figure 2. There were 370 individuals who had some evidence of rehabilitation, but had to be excluded because they did not have a rehabilitation discharge date or had incomplete rehabilitation treatment information (V57 codes indicating some form of inpatient rehabilitation but no formal FSOD record), leaving 4,357 who could be assigned to our study groups. The full population was first described according to rehabilitation timing using the rehabilitation admission and discharge dates along with the surgical date. Timing of inpatient rehabilitation was classified broadly as preoperative (with or without rehabilitation at other periods), early (after surgery, but beginning services before discharge from the index surgical stay), late (after surgery and after discharge from the index surgical stay), or both early and late.
Figure 2. Inpatient Rehabilitation Care Patterns Classified According to the TPT Framework (Initial n=4,357).
Early = rehabilitation occurring after surgery but on or before discharge from the index surgical stay; Late = rehabilitation occurring after readmission following discharge from the index surgical stay; Early + late = rehabilitation occurring both early and late; Pre-op = patients seen prior to surgery; No evidence = indicates no record of any form of inpatient rehabilitation. SRU = Specialized rehabilitation unit, Consultative = Consultative rehabilitation. Bolded patterns indicate those compared in this study. Of the 96 missing data (= 80 + 16), 58 were missing because of death. Type is specified only for the early and late timing patterns studied.
Since the primary intention of this study was to compare outcomes of patients who received inpatient rehabilitation based on two types (specialized or consultative) with two timings (early or late), those patients who did not receive rehabilitation according to one of these patterns were not included in the study. Specifically, we excluded patients with timing being preoperative for both specialized and consultative. There were 1,598 of the 4,357 veterans (36.68%) identified who received one of the patterns selected to be studied here. To generate the final analytic data set, 96 individuals with missing discharge FIM™ scores or living location prior to the surgical hospitalization had to be removed. There were 1,502 veterans (i.e., 94.0% of the 1,598 patients) with complete data available for analysis.
Explanatory Variables
Facility-level structural characteristics
The variable identifying the 95 VAMCs where surgery occurred was the single facility-level structural variable included as a random effect.
Patient level-characteristics
Patient-level characteristics were organized into domains of related information through the multidimensional diagnostic-specific Post Amputation Quality-of-Life (PAQ) framework12 intended to characterize patients' relative need for rehabilitation. The dimensions include sociodemographics, illness burden, and functional status.
Sociodemographic variables include age, gender, marital status (married versus not married), and living location prior to the surgical hospitalization (extended care versus home or hospital).
Illness burden encompasses contributing amputation etiologies, comorbidities, amputation level, and medical acuity. Diagnoses (both amputation etiologies and comorbidities) were identified using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes from the outpatient care files beginning three months prior to the hospitalization and from the main and bed section files up to the surgical date. Ten of the original twelve etiologies were included in our analyses.23 Congenital deformity and lower-limb cancer etiologies had insufficient prevalence for inclusion.
For the comorbidities, we used the 2003 version of the Elixhauser comorbidity measure.26, 27 There were no cases with the ICD-9-CM code for the comorbid condition of obesity. Thus, obesity was not included in the analyses.
Amputation level distinguished between type and number: unilateral trans-tibial (reference category), unilateral trans-femoral, bilateral trans-tibial, and bilateral trans-femoral. Patients with a trans-tibial and trans-femoral amputation were combined and classified as bilateral trans-femoral amputees because of low prevalence.
Medical acuity was intended to adjust for the extent of active illness during the index surgical stay. The acuity classification system combined procedural codes combining diagnostic workups and treatments of certain organ systems, such as cardiopulmonary, expected to affect an individual's tolerance of therapy or capacity to recover function.12
Initial functional status was determined at the time of rehabilitation admission as specified by the 18 item 7 performance level FIM™.28 The FIM™ is the standard measure of functional status applied in assessing VHA and private sector rehabilitation patients consisting of motor and cognitive domains. The 13 motor FIM™ summed score (range 13-91) expresses patients' physical abilities to manage activities of daily living (ADLs) and to move around in their environments. The 5 cognitive FIM™ summed score (range 5-35) expresses patients' abilities to communicate and perform basic cognitive functions. The FIM™ sub-scales are reliable and internally consistent.29, 30 Low scores in either the motor or cognitive FIM™ signify greater disabilities (profoundly severe), and higher scores indicate patients have milder disabilities.
Outcomes
The dependent variable was magnitude of gain in the 13 functions expressed as the motor FIM™ score change. The magnitude of gain was calculated by subtracting the patient's motor FIM™ score at admission from his or her motor FIM™ score at discharge. A larger positive number is associated with greater gains in physical functioning.
Analysis
To understand how the characteristics of patients differed across 2 types and 2 timings of rehabilitation, a table was developed to show patient-level characteristics (frequencies and/or means) by type and then by timing. P-values obtained from Chi-square tests or t-tests were shown in the table.
Next, we compared differences in initial and discharge motor FIM™ score, and the outcome of interest, i.e., motor FIM™ gain score, by types and timings of rehabilitation received. P-values were obtained from t-tests.
We then fit linear mixed effects models. In the models, the motor FIM™ gain score was the outcome. Prior to model building, the histogram of motor FIM™ gain score was shown to be skewed to the left. Transformations provided little correction for this skewness. However, once covariates were entered into the model, the histogram of the residuals was close to a normal distribution. Therefore, we fit the model to the original motor FIM™ gain score without transforming it.
The two main predictors were the type (specialized versus consultative) and the timing (early versus late) of rehabilitation which entered the models as two dummy variables. Our hypothesis related primarily to the influence of the timing and type of rehabilitation services on the outcome. Consequently, the main effects model was the focus of analysis. The statistical interaction between type and timing was tested secondarily to show if the influence of the type of rehabilitation was similar at both time periods.
The other predictors that we considered were summarized earlier in this paper. Specifically, because we assumed that there could be a non-linear association between the outcome and patient's initial motor FIM™ score, we tested the significance of the quadratic term. We also included a dummy variable to indicate year (1 or 2) to adjust for change in the patterns of service utilization.
The 95 VAMCs in which surgery occurred were entered as a random effect to adjust for any potential correlations among the outcomes of patients receiving care at the same site.
To build the final model, we entered all predictors in a linear mixed effects model first. Then backward model selection was conducted manually. Specifically, variables with p≥0.05 were removed one at a time, starting with the variable with the highest p-value. The significance of the remaining variables was checked, recognizing that previously insignificant variables may become significant as other variables were removed. The manual selection stopped when all variables in the final model were significant (defined as p<0.05).
To illustrate the degree to which it is important to consider the rehabilitation care pattern when estimating prognosis, we used the reduced model to estimate average motor FIM™ gains for 3 simulated case types, assuming each received consultative versus specialized rehabilitation services. PROC MIXED in SAS 9.1 was used for all adjusted analyses.
Results
Of the 1,502 cases in this analysis, the vast majority (79.1%) received consultation services, while 314 (20.9%) were admitted onto a SRU for specialized rehabilitation. Also, most patients received the early pattern of care (89.1%). For those in the late pattern, the average time between discharge from the index surgical stay and admission to late rehabilitation was 73.5 days (inter-quartile range [IQR]: 13.0-118.0 days). Of the 164 persons who received late rehabilitation, 21 patients (12.8%) had either a re-amputation (n=19) or surgical revision (n=2) prior to their late admission date.
The characteristics of patients who received specialized versus consultation rehabilitation and early versus late rehabilitation are shown in Tables 1 and 2. Patients who received specialized compared to those who received consultation services were younger (p<0.001), and more likely living at home before hospitalization (p=0.04). They were also more likely to have unilateral trans-tibial amputations (p<0.0001), and to have device infections and/or diabetes mellitus as contributing causes (p<0.05). With regard to comorbidities, lower proportions of persons who had specialized rehabilitation had iron deficiency anemia, other neurological conditions, renal failure, tumor without metastasis, ongoing cardiac pathology, or serious nutritional compromise (all p-values<0.05). They were more likely to have substance abuse or mental health issues (p<0.01). In contrast, persons who received specialized rehabilitation also had strikingly higher average motor and cognitive FIM™ scores at admission than those patients who received consultative rehabilitation (p<0.0001). There was also a difference in rehabilitation length of stay (LOS) between the two types of rehabilitation determined as the time between rehabilitation admission and discharge dates. Those patients who had specialized received services, on average, for 16.8 days compared to 14.3 days for those patients who received consultative rehabilitation services (p=0.02). [Note: This information is not in Table 1 and we did not include this in our models either as we designed our models to be prognostic.]
Table 1. Patient-level Characteristics by Type and Timing of Rehabilitation Services.
| Variable | Specialized | Consultative | p-value | Early | Late | p-value |
|---|---|---|---|---|---|---|
| Sample Size (N) Demographics | 314 | 1,188 | 1,338 | 164 | ||
| Age, years average ± SD | 64.8 ± 10.4 | 67.6 ± 11.4 | <0.0001 | 67.0 ± 11.3 | 66.8 ± 12.4 | 0.78 |
| Gender, n (%) | ||||||
| Male | 314 (100.0) | 1,173 (98.7) | 0.04 | 1,326 (99.1) | 161 (98.2) | 0.26 |
| Female | 0 (0.0) | 15 (1.3) | 12 (0.9) | 3 (1.8) | ||
| Marital status, n (%) | ||||||
| Married | 138 (44.0) | 565 (47.6) | 0.25 | 619 (46.3) | 84 (51.2) | 0.30 |
| Not married | 176 (56.1) | 623 (52.4) | 719 (53.8) | 80 (48.8) | ||
| Living location before the surgical hospitalization (categorical variable), n (%) | ||||||
| Extended care | 13 (4.1) | 100 (8.4) | 0.02 | 77 (5.8) | 36 (22.0) | <0.0001 |
| Home | 295 (94.0) | 1,051 (88.5) | 1,226 (91.6) | 120 (73.2) | ||
| Hospital | 6 (1.9) | 37 (3.1) | 35 (2.6) | 8 (4.9) | ||
| Amputation level (categorical variable), n (%) | ||||||
| Unilateral trans-tibial | 189 (60.2) | 509 (42.9) | <0.0001 | 672 (50.2) | 74 (45.1) | 0.23 |
| Unilateral trans-femoral | 111 (35.4) | 509 (42.9) | 550 (41.1) | 70 (42.7) | ||
| Bilateral trans-tibial | 7 (2.2) | 34 (2.9) | 37 (2.8) | 4 (2.4) | ||
| Bilateral trans-femoral | 7 (2.2) | 88 (7.4) | 79 (5.9) | 16 (9.8) | ||
| Contributing etiologies (individual variables), n (%) | ||||||
| Chronic osteomyelitis | 85 (7.2) | 15 (4.8) | 0.13 | 92 (6.9) | 8 (4.9) | 0.33 |
| Device Infection | 45 (14.3) | 121 (10.2) | 0.04 | 146 (10.9) | 20 (12.2) | 0.62 |
| Diabetes mellitus type I | 54 (17.2) | 180 (15.2) | 0.37 | 210 (15.7) | 24 (14.6) | 0.72 |
| Diabetes mellitus type II | 234 (74.5) | 770 (64.8) | <0.01 | 892 (66.7) | 112 (68.3) | 0.68 |
| Local significant infection | 244 (77.7) | 913 (76.9) | 0.75 | 1,026 (76.7) | 131 (79.9) | 0.36 |
| Peripheral vascular disease | 1,045 (88.0) | 270 (86.0) | 0.35 | 1,168 (87.3) | 147 (89.6) | 0.39 |
| Previous amputation complication | 32 (10.2) | 109 (9.2) | 0.58 | 125 (9.3) | 16 (9.8) | 0.86 |
| Skin breakdown | 201 (64.0) | 773 (65.1) | 0.73 | 861 (64.4) | 113 (68.9) | 0.25 |
| Systemic sepsis | 21 (6.7) | 132 (11.1) | 0.02 | 134 (10.0) | 19 (11.6) | 0.53 |
| Trauma | 50 (15.9) | 156 (13.1) | 0.20 | 182 (13.6) | 24 (14.6) | 0.72 |
| Comorbidities (individual variables), n (%) | ||||||
| Alcohol abuse | 22 (7.0) | 61 (5.1) | 0.20 | 75 (5.6) | 8 (4.9) | 0.70 |
| Arrhythmias | 49 (15.6) | 211 (17.8) | 0.37 | 219 (16.4) | 41 (25.0) | <0.01 |
| Chronic pulmonary disease | 51 (16.2) | 2256 (21.6) | 0.04 | 275 (20.6) | 32 (19.5) | 0.76 |
| Congestive heart failure | 60 (19.1) | 282 (23.7) | 0.08 | 294 (22.0) | 48 (29.3) | 0.04 |
| Deficiency anemias | 55 (17.5) | 273 (23.0) | 0.04 | 292 (21.8) | 36 (22.0) | 0.97 |
| Depression | 32 (10.2) | 105 (8.8) | 0.46 | 125 (9.3) | 12 (7.3) | 0.40 |
| Fluid and electrolyte disorders | 65 (20.7) | 247 (20.8) | 0.97 | 271 (20.3) | 41 (25.0) | 0.16 |
| Hypothyroidism | 11 (3.5) | 45 (3.8) | 0.81 | 47 (3.5) | 9 (5.5) | 0.21 |
| Hypertension | 206 (65.6) | 769 (64.7) | 0.77 | 868 (64.9) | 107 (65.2) | 0.93 |
| Hypertension with complication | 4 (1.3) | 4 (0.3) | 0.04 | 6 (0.5) | 2 (1.2) | 0.20 |
| Paralysis | 6 (1.9) | 61 (5.1) | 0.01 | 62 (4.6) | 5 (3.1) | 0.35 |
| Psychoses | 14 (4.5) | 85 (7.2) | 0.09 | 92 (6.9) | 7 (4.3) | 0.20 |
| Renal failure | 42 (13.4) | 216 (18.2) | 0.04 | 218 (16.3) | 40 (24.4) | <0.01 |
| Solid tumor without metastasis | 18 (5.7) | 118 (9.9) | 0.02 | 126 (9.4) | 10 (6.1) | 0.16 |
| Valvular disease | 8 (2.6) | 60 (5.1) | 0.06 | 55 (4.1) | 13 (7.9) | 0.03 |
| Weight loss | 19 (6.1) | 67 (5.6) | 0.78 | 71 (5.3) | 15 (9.2) | 0.04 |
| Procedures (individual variables), n (%) | ||||||
| Active pulmonary pathology | 1 (0.3) | 16 (1.4) | 0.13 | 15 (1.1) | 2 (1.2) | 0.91 |
| Acute central nervous system | 30 (9.6) | 100 (8.4) | 0.52 | 117 (8.7) | 13 (7.9) | 0.73 |
| Ongoing active cardiac pathology | 47 (15.0) | 128 (10.8) | 0.04 | 159 (11.9) | 16 (9.8) | 0.42 |
| Ongoing wound problems | 18 (5.7) | 76 (6.4) | 0.67 | 89 (6.7) | 5 (3.1) | 0.07 |
| Serious nutritional compromise | 4 (1.3) | 61 (5.1) | <0.01 | 55 (4.1) | 10 (6.1) | 0.24 |
| Severe renal disease | 15 (4.8) | 106 (8.9) | 0.02 | 107 (8.0) | 14 (8.5) | 0.81 |
| Substance abuse or mental health | 10 (3.2) | 12 (1.0) | <0.01 | 19 (1.4) | 3 (1.8) | 0.68 |
| Cognitive and communication status at rehabilitation admission | ||||||
| Cognitive FIM™ average ± SD | 28.9 ± 6.1 | 25.7 ± 10.4 | <0.0001 | 26.3 ± 9.8 | 26.9 ± 9.8 | 0.45 |
Percentages shown are of those with each characteristic that were included in each care pattern.
SD = standard deviation
Age, local significant infection, arrhythmias, fluid and electrolyte disorders, hypertension, hypothyroidism, psychoses, and ongoing active cardiac pathology were not significantly different across the care patterns. They were significant in the adjusted model predicting motor FIM™ gain and are included in this table for reference. The following comorbidities were not included because their prevalence was 5% or less in all cells and they showed no statistically significant associations: AIDS, chronic blood loss anemia, coagulopathy, drug abuse, hypothyroidism, liver disease, lymphoma, metastatic cancer, other neurological disorders, and rheumatoid arthritis.
Table 2. Unadjusted Cognitive and Motor FIM™ Scores by Type and Timing of Rehabilitation Services.
| By type | By timing | |||||
|---|---|---|---|---|---|---|
| Variable | Specialized N = 314 |
Consultative N = 1,188 |
p-value | Early N = 1,338 |
Late N = 164 |
p-value |
| Initial motor FIM™ | ||||||
| Mean (SD) | 49.5 (14.9) | 37.1 (20.2) | <0.0001 | 38.9 (19.3) | 45.8 (23.2) | <0.01 |
| Median (IQR) | 51 (41-60) | 34 (18-53) | 39 (21-54) | 49.5 (22.5-65.5) | ||
| Initial cognitive FIM™ | ||||||
| Mean (SD) | 28.9 (6.1) | 25.7 (10.4) | <0.0001 | 26.3 (9.8) | 26.9 (9.8) | 0.45 |
| Median (IQR) | 30 (26-34) | 30 (18-35) | 30 (20-35) | 31.5 (22-35) | ||
| Discharge motor FIM™ | ||||||
| Mean (SD) | 67.9 (15.4) | 46.6 (25.0) | <0.0001 | 50.6 (24.6) | 54.9 (27.2) | 0.03 |
| Median (IQR) | 73 (62-78) | 46.5 (21-71) | 55 (26-73) | 66 (24.5-79.5) | ||
| Change in motor FIM™ | ||||||
| Mean (SD) | 18.4 (10.2) | 9.5 (14.7) | <0.0001 | 11.6 (14.1) | 9.1 (16.2) | 0.05 |
| Median (IQR) | 18 (12-25) | 3 (0-17) | 8 (0-20) | 7 (0-17) | ||
SD=standard deviation; IQR = Inter-quartile range (25th and 75th percentile)
Compared to patients who received early rehabilitation, those patients who received late rehabilitation were more likely residing in an extended care facility at the time of admission for their surgical amputation (p<0.0001). They were more likely to have cardiac arrhythmias, valvular disease, congestive heart failure, weight loss, or renal failure noted at the time of surgery (all p-values<0.05). Patients who received rehabilitation late, on average, were less physically disabled at the time of rehabilitation than those who received rehabilitation early, and had significantly less change in motor FIM™ by rehabilitation discharge (p<0.05).
Table 3 shows estimates from the final model after backward selection and testing the significance of the quadratic terms of the initial motor and cognitive FIM™ scores and the interaction between type and timing. The timing variable was not selected (p-value=0.51 in the full model with all predictors) and the type by timing interaction was not significant either (p=0.99 in the full model). The quadratic term of initial motor FIM™ score was significant but not the quadratic term for the initial cognitive FIM™ score. Thus, the final model has type and the quadratic term of initial motor FIM™ score. The timing variable, the quadratic term of initial cognitive FIM™ score, and the interaction between type and timing were not included in the final model.
Table 3. Final Model for Predicting Motor FIM™ Gains on the Basis of Patient-level Factors Known at Admission to Rehabilitation.
| Predictors | Estimated parameter | Standard error | P-value |
|---|---|---|---|
| Intercept | 19.9 | 3.1 | <0.001 |
| Rehabilitation service (ref: consultative) | |||
| Specialized | 8.0 | 0.97 | <0.0001 |
| Demographics | |||
| Age | -0.2 | 0.03 | <0.0001 |
| Amputation level (ref: unilateral trans-tibial) | |||
| Unilateral trans-femoral | -1.7 | 0.7 | 0.05 |
| Bilateral trans-tibial | 0.08 | 2.0 | |
| Bilateral trans-femoral | -2.7 | 1.4 | |
| Contributing etiologies | |||
| Local significant infection | -2.4 | 0.8 | <0.01 |
| Comorbidities | |||
| Arrhythmias | -2.5 | 0.9 | <0.01 |
| Fluid and electrolyte disorders | -1.7 | 0.8 | 0.04 |
| Hypertension | 1.7 | 0.7 | 0.01 |
| Hypothyroidism | -3.6 | 1.7 | 0.04 |
| Paralysis | -4.3 | 1.6 | 0.01 |
| Psychoses | -3.6 | 1.3 | 0.01 |
| Procedures | |||
| Ongoing active cardiac pathology | -2.3 | 1.0 | 0.03 |
| Serious nutritional compromise | -3.7 | 1.7 | 0.03 |
| Functional status | |||
| Initial motor FIM™ score | 0.5 | 0.1 | <0.0001 |
| Initial motor FIM™ score squared | -0.008 | 0.001 | <0.0001 |
| Initial cognitive FIM™ score | 0.2 | 0.05 | <0.0001 |
After adjusting for clinical differences and the facilities where patients received surgical amputation, the type of rehabilitation received was a strong predictor of motor FIM™ gains (p<0.0001). Patients receiving specialized rehabilitation compared to consultative rehabilitation would be expected to have on average 8.0 points higher motor FIM™ gain scores. The significance of the quadratic term of initial motor FIM™ score shows that the influence of the severity of initial physical disability was complex. Those patients whose initial physical disabilities were profoundly severe (lower initial motor FIM™ scores) or mild (higher initial motor FIM™ scores) made lower gains in physical functioning compared to those patients in the middle band (with intermediate severity of initial physical disability).
After adjusting for other clinical covariates and the facilities where patients had their surgical amputations, persons who were elderly or who had cognitive disabilities made lower gains in physical function. Veterans with either unilateral or bilateral trans-femoral amputations made lower gains (an average 1.7 and 2.7 motor FIM™ points lower, respectively) than veterans with a unilateral trans-tibial amputation. In contrast, those patients with bilateral trans-tibial amputations were able to make comparable gains to those patients with unilateral trans-tibial amputations. Veterans who had serious nutritional compromise with a feeding gastrostomy, enterostomy, or parenteral nutrition during the index surgical stay recovered less physical function (by an average of 3.7 motor FIM™ points), as did those patient with paralysis (by an average of 4.3 motor FIM™ points) or a history of psychosis (by an average of 3.6 motor FIM™ points). Finally, the variance of the random effect of facility was statistically significant (p=0.04), suggesting that there was extraneous variations within facility that was not explainable by observed patient-level variables.
Applying the model parameters from Table 3, we illustrate how expectations for motor FIM™ gain differ for 3 case types depending on whether they receive specialized or consultative care.
Case type1 profile includes patients who are 80 years old, with bilateral trans-femoral amputation, local significant infection, paralysis, an initial motor FIM™ = 35, initial cognitive FIM™ = 30, and serious nutritional compromise. Applying these parameters to forecast for this subgroup, an average gain of 9.0 motor FIM™ points for rehabilitation on a SRU would be expected compared to only a gain of 1.0 motor FIM™ point if consultation is provided in the same facility.
Case type 2 profile is for patients who are 55 years old, with unilateral trans-tibial amputation, initial motor FIM™ = 80, and initial cognitive FIM™ = 35. An average gain of 10.2 motor FIM™ points is projected for specialized compared to 2.2 motor FIM™ points for consultative rehabilitation within the same facility.
Case type 3 profile is identical to the case type 2 profile except for the much lower initial motor FIM™ = 70. The forecasted average motor FIM™ gains is 17.5 motor FIM™ points assuming specialized and 9.5 motor FIM™ points assuming consultative rehabilitation within the same facility.
Discussion
The Effects of Service Type on Gains in Physical Function
Results confirmed our primary hypothesis. After controlling for clinical differences, and strongly dependent on initial severity of disabilities, amputees who receive a single episode of specialized rehabilitation services can be expected to achieve gains in physical function that are on average 8.0 motor FIM™ points higher than those patients who receive consultative rehabilitation. Amputees see residual difficulty in physical functioning as the strongest negative influence of limb loss on quality of life.31 Also, a one point increase in the FIM™ is estimated to be associated with an average decrease in the required care from a second person for 2.2-5.0 minutes per day.28, 32-35 Thus, an 8.0 FIM™ point improvement is clinically meaningful. The expected higher gains associated with specialized rehabilitation is consistent with previous findings36 and highlights the need for clinicians to consider the types of rehabilitation planned before attempting to prognosticate functional recovery.
The Effects of Other Types of Clinical Characteristics on Gains
Findings also identified essential clinical information that can be applied to project the likely magnitude of functional gains. Veterans' initial severity of physical disability after amputation represents the single most important prognostic factor. Results highlight a middle band effect. Patients with either severe or mild initial physical disabilities can be expected to have less recovery of physical functioning than those with intermediate levels of physical disability. Logically at the extremes of severity, limitations are so profound that it becomes difficult for individuals to mount sufficient efforts to make large gains. Moreover, with mild disability, it is impossible to measure recovery beyond the measurement ceiling of the motor FIM™. The facilitating effects of high cognitive function on the recovery of physical abilities empirically support the common clinical assumptions that patients need memory, recall, and problem solving skills to benefit from therapy.
Many of the patient-related factors shown by association to independently retard gains are known to forecast a variety of adverse outcomes. Advanced age, the presence of many illnesses, and above the knee amputation have previously been noted to be associated with reduced functional recovery after amputation.10, 37-39 In addition to harboring reduced gains as shown in our study, malnourishment is associated with higher rates of postoperative infection after revascularization procedures.40 Cardiac dysfunction, shown to both delay rehabilitation and reduce gains in our study, is the most common attributed cause of death after surgical amputation.41 A history of psychiatric disturbance appearing as a negative prognostic factor for gains in physical function has also been linked to reduced capacity and recurrent symptoms following revascularization procedures of the legs.42 Finally, paralysis, high amputation level, cognitive deficits, and nutritional compromise, all of which were demonstrated to be negative prognostic factors for gain in this study, also reduce the likelihood of patients being prescribed a prosthetic limb.12 Furthermore, there are likely additional non-measured variables, such as motivation, that are contributing to prognosis. Our finding that amputees in whom hypertension is coded make higher gains is less clinically intuitive and harder to explain. It could be that the medical management of and therapies provided to those with hypertension are more carefully monitored. Alternatively, it may be that among most medically complex amputees with hypertension that the coding of more serious conditions would take precedence. This later interpretation is consistent with previous studies on mortality. Patients with a variety of conditions including amputation who have hypertension documented in administrative records are repeatedly shown to have significantly lower rates of mortality. 23, 43,44
The Effects of Rehabilitation Timing on Gains
Although patients who had early rehabilitation made higher motor FIM™ gains than those individuals who had later rehabilitation, the apparent benefit of early rehabilitation on functional recovery disappeared after adjusting for illness burden and initial severity of disability. With regard to the published literature, most studies on the effects of early rehabilitation focus on traumatic and non-traumatic brain injury. In basic science models, early rehabilitation has been shown to reduce neural degeneration and be associated with better recovery from traumatic brain injury.45 Earlier rehabilitation is consistently associated with higher functional outcomes in retrospective studies of stroke rehabilitation.46-48 But evidence supporting its benefit on improved functioning and mortality is equivocal. Based on systematic review, which identified only a single trial, fewer patients died and/or were disabled after earlier post stroke mobilization but the effect was not statistically significant.49 Early rehabilitation does appear associated with reduced costs with post stroke and lumbar spine surgery.50, 51 We could not find any studies that addressed the influence of early rehabilitation on post amputation outcomes.
Our findings suggest that any apparent benefit of early over late rehabilitation among amputees relates more to differences in severity of patients at presentation than to the effects of rehabilitation timing. Those patients whose rehabilitation was delayed until the late period were more likely to be living in extended care facilities prior to hospitalization and have greater illness burden (particularly heart and renal disease) peri-operatively, but tended to have less severe physical disabilities at rehabilitation admission. Moreover, a number of veterans who presented to the late pattern had re-amputations or revisions suggesting ongoing ischemia and healing problems.
The decision for early versus late rehabilitation appears to be appropriately driven by patient circumstances and clinical differences. Timing should be driven more by the amputees' clinical readiness for these services and ongoing needs than by expectations for higher outcome. Yet, a large proportion of elderly persons die within a year of their amputations.23 Consequently, early rehabilitation should be provided when feasible to help maximize function quickly. Moreover, it is important to recognize that although the effects of early rehabilitation on motor FIM™ gains was at best modest in this study, it is possible that early rehabilitation may prove beneficial in other care patterns or for other types of outcomes.
Clinical and Policy Applicability and Impact
The TPT framework offers a means to compare outcome expectations associated with alternative patterns of rehabilitation care resulting naturally from differences in provider practice style or geographic variability. As health care systems evolve, the TPT framework can help PM&R professionals, researchers, and policy analysts conceptualize how rehabilitation services fit within the larger continuum of services and contribute to outcomes along with other types of services. Verville and Thomas recently suggested that the Academy of PM&R serves a leadership role in quality of care research addressing function.52 Our findings confirm the central importance of initial function to the forecasting of functional gains but also highlight the need to simultaneously include information from many clinical domains when estimating prognosis. Simulations through the statistical model developed here can be applied to compare expectations for motor FIM™ gain for groups of individuals according to clinical severity at admission to rehabilitation.
Moreover, explicit expectations for motor FIM™ gain according to patients' clinical presentations and the types of rehabilitation services to be provided can empower patients, PM&R practitioners, and policy makers. Such knowledge can empirically guide rehabilitation treatment and evidence-based practices. PM&R professionals might apply outcome predictions in quality monitoring or to help make rational decisions about the value of alternative services relative to the outcomes achieved. Similarly, policy makers might apply actual versus predicted gains when monitoring the influence of policy changes on costs and population outcomes. Reductions in IRF care in response to ongoing changes in Medicare policy are already occurring in the private sector. Functional gains have decreased, rates of long-term care discharge have increased, and 180 day post-rehabilitation mortality has increased among people receiving rehabilitation for a variety of conditions.53-55
The case type examples presented in results show how statistical models like the one presented might be applied to quantify prognosis applying 3 distinct symptom constellations. Predicted gains show that expectations for motor FIM™ gain differ strikingly and in ways that are clinically plausible according to patient complexity and the types of rehabilitation services provided. The expectations for recovery of physical functioning were lower for all 3 case types assuming the receipt of consultation rather than specialized services. With regard to clinical complexity, the first case type illustrates how severe illness, severe disability, and older age in combination harbor a relatively poor prognosis for recovery of physical functioning. Case type 2 shows how amputees with mild physical disabilities can be expected to make relatively fewer gains in physical function because of the measurement ceiling of the motor FIM™ instrument. Case type 3, identical to case type 2 except for a much lower initial motor FIM™, demonstrates the degree to which amputees with severe physical disabilities at rehabilitation admission but no other negative prognostic factors have the potential to make relatively high gains.
Strengths, Limitations, and Future Research
The strength of this study rests in its large sample, the multi-centered nature of data, richness of available clinical information, the capacity to define distinct types of rehabilitation services, and the feasibility of addressing linkages between rehabilitation and acute medical-surgical management. This study is also limited in several ways. Analyses were based on FY 2003 and FY 2004 patient outcomes. Service use patterns and outcomes may differ today and findings might not generalize to women and non-veterans. Concepts about the clinical determinants of prognosis for motor FIM™ gain according to different levels of rehabilitation can inform VHA and private sector clinicians. It is essential to recognize that the statistical models should not be expected to forecast outcomes for amputees in the private sector without recalibration. Also, only a small number of people received the late specialized pattern. Finally, without randomization or reduction of selection bias, it is impossible to claim that the incremental improvements in functional gain can be attributed to specialized rehabilitation services. This study was directed to the analysis of outcome prognosis and is not intended to assume causality. There is; however, growing evidence that more intensive versus less intensive inpatient rehabilitation services for amputees are associated with better outcomes across multiple dimensions.6, 12, 56, 57
Further research is needed to address the prognostic implications of additional rehabilitation care patterns. Only a small proportion of patients after amputation in the private sector or VHA ever receive high intensity rehabilitation on a SRU or IRF.36, 58 Also, we only studied care patterns from two types and two timings. The patterns selected for study were those limited to single episodes of inpatient rehabilitation. Just over one third of the amputee population treated within the VHA received one of these patterns. Significant proportions of patients have either no evidence of or multiple episodes of inpatient rehabilitation (29.0% and 27.9%, respectively). Future work will need to focus on the determinants of multiple episodes and expectations for motor FIM™ gain. It will further be essential to drill down to the smaller elements that define the processes of rehabilitation.59, 60
Conclusion
The Federal Coordinating Council for Comparative Effectiveness Research specifically identified people with disabilities as a priority population.61 Rehabilitation was cited as essential to helping people with disabilities live in their communities with added years of high quality of life. Inpatient rehabilitation is particularly important to these objectives because services are explicitly targeted to enhancing function so that people who are at risk for long-term care placement can transition from the hospital back to the community.
Our findings, combined with results from others, show that facility-level structural characteristics,8 patient-level characteristics,10, 37-39 and the rehabilitation care pattern received determine the services received and the magnitude of functional gains achieved. Evidence that this complex combination of determinants appears to drive resource use and outcomes supports the TPT framework (Figure 1). We evaluated expectations for motor FIM™ gain according to inpatient care patterns differing by service timing and type, all beginning in the same place (i.e., an inpatient hospital). In contrast, Dillingham et al. recently compared expectations across care patterns among amputees receiving rehabilitation in 3 different places.56 Their findings of outcome differences according to place combined with ours according to type emphasize the importance of estimating prognoses according to the pattern of care received. We hope that the TPT framework will facilitate the design of comparative effectiveness studies in PM&R, ultimately guiding delivery of the right type of rehabilitation services, to the right patient, at the right time, in the most effective setting. We further hope the framework will encourage a vision of rehabilitation as occurring within the larger care continuum contributing to patient outcomes along with medical and surgical services.
Acknowledgments
We would like to thank Ms. Janice A. Duglas who provided support in the preparation of the manuscript, and Mr. Clifford Marshall who developed and guided the group in applications of the FSOD data.
The research for this article was supported by the National Institutes of Health (R01-HD042588). It was also supported by resources and the use of facilities at the Samuel S. Stratton Department of Veterans Affairs Medical Center in Albany, NY. No medical devices are described in the study. Material from this study was not presented at an AAPM&R Annual Assembly.
Footnotes
There are no personal conflicts of interest of any of the authors and no authors reported disclosures. The opinions and conclusions of the authors are not necessarily those of the sponsoring agencies.
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422–9. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Agency for Healthcare Research and Quality. American Recovery and Reinvestment Act: Comparative Effectiveness Funding 2009. 2009 May 1; Available from: URL: http://www.ahrq.gov/fund/cefarra.htm.
- 3.Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46(1):69–79. doi: 10.1353/pbm.2003.0004. [DOI] [PubMed] [Google Scholar]
- 4.Eldar R. Quality of Care in Rehabilitation Medicine. Int J Qual Care. 1999;11(1):73–9. doi: 10.1093/intqhc/11.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Wennberg J, Gittelsohn Small area variations in health care delivery. Science. 1973;182(117):1102–8. doi: 10.1126/science.182.4117.1102. [DOI] [PubMed] [Google Scholar]
- 6.Stineman M, Kwong P, Kurichi J, Prvu-Bettger, Vogel W, Maislin G, et al. The effectiveness of inpatient rehabilitation in the acute postoperative phase of care after trans-tibial or trans-femoral amputation: Study of an integrated health care delivery system. Arch Phys Med Rehabil. 2008;89:1863–72. doi: 10.1016/j.apmr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stineman MG. A model of health environmental integration. Top Stroke Rehabilitation. 2001;8(2):34–45. doi: 10.1310/0L5G-NQHY-GH4K-HV58. [DOI] [PubMed] [Google Scholar]
- 8.Bates B, Kurichi J, Marshall C, Reker D, Maislin G, Stineman M. Does the presence of a specialized rehabilitation unit in a Veterans Affairs facility impact referral for rehabilitative care after a lower-extremity amputation? Arch Phys Med Rehabil. 2007;88(10):1249–55. doi: 10.1016/j.apmr.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoenig H, Sloane R, Horner RD, Zolkewitz M, Reker D. Differences in rehabilitation services and outcomes among stroke patients cared for in veterans hospitals. Health Serv Res. 2001;35(6):1293–318. [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg. 2005;42(2):227–35. doi: 10.1016/j.jvs.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Bates BE, Kwong PL, Kurichi JE, Bidelspach DE, Reker DM, Maislin G, et al. Factors influencing decisions to admit patients to veterans affairs specialized rehabilitation units after lower-extremity amputation. Arch Phys Med Rehabil. 2009;90(12):2012–8. doi: 10.1016/j.apmr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurichi JE, Kwong PL, Reker DM, Bates BE, Marshall CR, Stineman MG. Clinical factors associated with prescription of a prosthetic limb in elderly veterans. J Am Geriatr Soc. 2007;55(6):900–6. doi: 10.1111/j.1532-5415.2007.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donabedian A. Evaluating the quality of medical care. Milbank Memorial Fund Quarterly. 1966;44(3):166–206. [PubMed] [Google Scholar]
- 14.DeJong G, Horn SD, Conroy B, Nichols D, Healton EB. Opening the black box of post-stroke rehabilitation: stroke rehabilitation patients, processes, and outcomes. Arch Phys Med Rehabil. 2005;86(12 Suppl 2):S1–S7. doi: 10.1016/j.apmr.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Shojania KG, Showstack J, Wachter RM. Assessing hospital quality: a review for clinicians. Eff Clin Pract. 2001;4(2):82–90. [PubMed] [Google Scholar]
- 16.CARF International. The 2000 Medical Rehabilitation Standards Manual. Tucson, AZ: CARF; 2000. Commission on Accreditation of Rehabilitation Facilities. [Google Scholar]
- 17.CARF International. The 2009 Medical Rehabilitation Standards Manual. Tucson, AZ: CARF; 2009. Commission on Accreditation of Rehabilitation Facilities. [Google Scholar]
- 18.World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 19.Weinstein S. PM&R Represents a New Frontier for the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):1. doi: 10.1016/j.pmrj.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Black N. Why we need observational studies to evaluate the effectiveness of health care. Bmj. 1996;312(7040):1215–8. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hines Edward J., Jr, editor. VIReC Research User Guide. FY2000 VHA Medical SAS Inpatient Datasets. VA Hospital, Hines, IL: Veterans Affairs Information Resource Center; 2003. [Google Scholar]
- 22.VHA Office of Information. VHA Corporate Databases Monograph. 2006 [cited 2007 April 18]. Available from: URL: http://www.virec.research.va.gov/References/links/VHACorporateDatabaseMonograph2006Final.pdf.
- 23.Bates B, Stineman MG, Reker D, Kurichi JE, Kwong PL. Risk Factors Associated with Mortality in a Veteran Population Following Trans-tibial or Trans-femoral Amputation. J Rehabil Res Dev. 2006;43(7):917–28. doi: 10.1682/jrrd.2006.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurichi JE, Stineman MG, Kwong PL, Bates BE, Reker DM. Assessing and Using Comorbidity Measures in Elderly Veterans with Lower Extremity Amputations. Gerontology. 2007;53(5):255–9. doi: 10.1159/000101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng CL, Helmer D, Rajan M, Tiwari A, Miller D, Crystal S, et al. Evaluation of regional variation in total, major, and minor amputation rates in a national health-care system. Int J Qual Health Care. 2007;19(6):368–76. doi: 10.1093/intqhc/mzm044. [DOI] [PubMed] [Google Scholar]
- 26.Agency for Healthcare Research and Quality. Comorbidity Software 3.1. 2005 [cited 2006 December 19]. Available from: URL: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp#variables.
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Granger CV, Hamilton BB, Keith RA, Zielezny M, Sherwin FS. Advances in Functional Assessment for Medical Rehabilitation. Top Geriatr Rehabil. 1986;1:59–74. [Google Scholar]
- 29.Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7-level functional independence measure (FIM)[see comment] Scand J Rehabil Med. 1994;26(3):115–9. [PubMed] [Google Scholar]
- 30.Stineman MG, Shea JA, Jette A, Tassoni CJ, Ottenbacher KJ, Fiedler R, et al. The functional independence measure: Tests of scaling assumptions, structure, and reliability across 20 diverse impairment categories. Arch Phys Med Rehabil. 1996;77(11):1101–8. doi: 10.1016/s0003-9993(96)90130-6. [DOI] [PubMed] [Google Scholar]
- 31.Zidarov D, Swaine B, Gauthier-Gagnon C. Quality of life of persons with lower-limb amputation during rehabilitation and at 3-month follow-up. Arch Phys Med Rehabil. 2009;90(4):634–45. doi: 10.1016/j.apmr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Granger CV, Cotter AC, Hamilton BB, Fiedler RC. Functional assessment scales: a study of persons after stroke. Arch Phys Med Rehabil. 1993;74(2):133–8. [PubMed] [Google Scholar]
- 33.Granger CV, Cotter AC, Hamilton BB, Fiedler RC, Hens MM. Functional assessment scales: a study of persons with multiple sclerosis. Arch Phys Med Rehabil. 1990;71(11):870–5. [PubMed] [Google Scholar]
- 34.Granger CV, Divan N, Fiedler RC. Functional assessment scales. A study of persons after traumatic brain injury. Am J Phys Med Rehabil. 1995;74(2):107–13. [PubMed] [Google Scholar]
- 35.Hamilton BB, Deutsch A, Russell C, Fiedler RC, Granger CV. Relation of disability costs to function: spinal cord injury. Arch Phys Med Rehabil. 1999;80(4):385–91. doi: 10.1016/s0003-9993(99)90274-5. [DOI] [PubMed] [Google Scholar]
- 36.Kurichi JE, Small DS, Bates BE, Prvu-Bettger JA, Kwong PL, Vogel WB, et al. Possible incremental benefits of specialized rehabilitation bed unit among veterans following lower extremity amputations. Med Care. 2009;47(4):457–65. doi: 10.1097/MLR.0b013e31818b08c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacKenzie EJ, Bosse MJ, Castillo RC, Smith DG, Webb LX, Kellam JF, et al. Functional outcomes following trauma-related lower-extremity amputation. J Bone Joint Surg Am. 2004;86-A(8):1636–45. doi: 10.2106/00004623-200408000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Nehler MR, Coll JR, Hiatt WR, Regensteiner JG, Schnickel GT, Klenke WA, et al. Functional outcome in a contemporary series of major lower extremity amputations. Journal of Vascular Surgery. 2003;38(1):7–14. doi: 10.1016/s0741-5214(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 39.Weiss GN, Gorton TA, Read RC, Neal LA. Outcomes of lower extremity amputations. J Am Geriatr Soc. 1990;38(8):877–83. doi: 10.1111/j.1532-5415.1990.tb05703.x. [DOI] [PubMed] [Google Scholar]
- 40.Westvik TS, Krause LK, Pradhan S, Westvik HH, Maloney SP, Rutland R, et al. Malnutrition after vascular surgery: are patients with chronic renal failure at increased risk? Am J Surg. 2006;192(5):e22–7. doi: 10.1016/j.amjsurg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Cruz CP, Eidt JF, Capps C, Kirtley L, Moursi MM. Major lower extremity amputations at a Veterans Affairs hospital. Am J Surg. 2003;186(5):449–54. doi: 10.1016/j.amjsurg.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Cherr GS, Wang J, Zimmerman PM, Dosluoglu HH. Depression is associated with worse patency and recurrent leg symptoms after lower extremity revascularization. J Vasc Surg. 2007;45(4):744–50. doi: 10.1016/j.jvs.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 43.Iezzoni LI, Foley SM, Daley J, et al. Comorbidities, complications, and coding bias. Does the number of diagnosis codes matter in predicting in-hospital mortality? Jama. 1992;267:2197–203. doi: 10.1001/jama.267.16.2197. [DOI] [PubMed] [Google Scholar]
- 44.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data: the role of length of stay and comorbidities. Journal of the American Medical Association. 1988;260(15):2240–6. [PubMed] [Google Scholar]
- 45.Lippert-Gruner M, Maegele M, Pokorny J, Angelov DN, Svestkova O, Wittner M, et al. Early rehabilitation model shows positive effects on neural degeneration and recovery from neuromotor deficits following traumatic brain injury. Physiol Res. 2007;56(3):359–68. doi: 10.33549/physiolres.930971. [DOI] [PubMed] [Google Scholar]
- 46.Maulden SA, Gassaway J, Horn SD, Smout RJ, DeJong G. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil. 2005;86(12 Suppl 2):S34–S40. doi: 10.1016/j.apmr.2005.08.119. [DOI] [PubMed] [Google Scholar]
- 47.Paolucci S, Antonucci G, Grasso MG, Morelli D, Troisi E, Coiro P, et al. Early versus delayed inpatient stroke rehabilitation: a matched comparison conducted in Italy. Arch Phys Med Rehabil. 2000;81(6):695–700. doi: 10.1016/s0003-9993(00)90095-9. [DOI] [PubMed] [Google Scholar]
- 48.Salter K, Jutai J, Hartley M, Foley N, Bhogal S, Bayona N, et al. Impact of early vs delayed admission to rehabilitation on functional outcomes in persons with stroke. J Rehabil Med. 2006;38(2):113–7. doi: 10.1080/16501970500314350. [DOI] [PubMed] [Google Scholar]
- 49.Bernhardt J, Thuy MN, Collier JM, Legg LA. Very Early Versus Delayed Mobilization After Stroke. Stroke. 2009 doi: 10.1002/14651858.CD006187.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen PR, Andreasen J, Asmussen M, Tonnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Serv Res. 2008;8:209. doi: 10.1186/1472-6963-8-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tay-Teo K, Moodie M, Bernhardt J, Thrift AG, Collier J, Donnan G, et al. Economic evaluation alongside a phase II, multi-centre, randomised controlled trial of very early rehabilitation after stroke (AVERT) Cerebrovasc Dis. 2008;26(5):475–81. doi: 10.1159/000155984. [DOI] [PubMed] [Google Scholar]
- 52.Verville RE, Thomas PW. A New Era in Health Care: Opportunities and Challenges. AAPM&R. 2009;1(6):511–5. doi: 10.1016/j.pmrj.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Gillen R, Tennen H, McKee T. The impact of the inpatient rehabilitation facility prospective payment system on stroke program outcomes. Am J Phys Med Rehabil. 2007;86(5):356–63. doi: 10.1097/PHM.0b013e31804a7e2f. [DOI] [PubMed] [Google Scholar]
- 54.Gornick M, Hall MJ. Trends in Medicare use of post-hospital care. Health Care Financ Rev. 1988;Spec No:27–38. [PMC free article] [PubMed] [Google Scholar]
- 55.Ottenbacher KJ, Smith PM, Illig SB, Linn RT, Ostir GV, Granger CV. Trends in length of stay, living setting, functional outcome, and mortality following medical rehabilitation. Jama. 2004;292(14):1687–95. doi: 10.1001/jama.292.14.1687. [DOI] [PubMed] [Google Scholar]
- 56.Dillingham TR, Pezzin LE. Rehabilitation setting and associated mortality and medical stability among persons with amputations. Arch Phys Med Rehabil. 2008;89(6):1038–45. doi: 10.1016/j.apmr.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 57.Pezzin LE, Dillingham TR, MacKenzie EJ. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch Phys Med Rehabil. 2000;81(3):292–300. doi: 10.1016/s0003-9993(00)90074-1. [DOI] [PubMed] [Google Scholar]
- 58.Dillingham TR, Pezzin LE, MacKenzie EJ. Incidence, acute care length of stay, and discharge to rehabilitation of traumatic amputee patients: an epidemiologic study. Archives of Physical Medicine & Rehabilitation. 1998;79(3):279–87. doi: 10.1016/s0003-9993(98)90007-7. [DOI] [PubMed] [Google Scholar]
- 59.Horn SD, DeJong G, Smout RJ, Gassaway J, James R, Conroy B. Stroke rehabilitation patients, practice, and outcomes: is earlier and more aggressive therapy better? Arch Phys Med Rehabil. 2005;86(12 Suppl 2):S101–S14. doi: 10.1016/j.apmr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Zanca J. Naming and Conceptual Organization of Inpatient Rehabilitation Interventions Described in Practice-Based Evidence Data Collection Tools. Arch Phys Med Rehabil. 2009;90(10):e23–e4. [Google Scholar]
- 61.Health and Human Services. Federal Coordinating Council for Comparative Effectiveness Research Membership. 2009 http://www.hhs.gov/recovery/programs/os/cerbios.html.