Abstract
The bone and bone marrow are among the most frequent sites of cancer metastasis. It is estimated that 350,000 patients die with bone metastases annually in the US. The ability of tumor cells to colonize the bone marrow and invade the bone is the result of close interactions between tumor cells and the bone marrow microenvironment. In this article, we review the contribution of interleukin-6 (IL-6) produced in the bone marrow microenvironment to bone metastasis. This cytokine has a strong pro-tumorigenic activity due to its multiple effects on bone metabolism, tumor cell proliferation and survival, angiogenesis, and inflammation. These effects are mediated by several signaling pathways, in particular the Janus kinase/signal transducer and transcription activator (JAK/STAT-3), Ras/mitogen activated protein kinase (MAPK), and phosphoinositol-3 kinase (PI3K)–protein kinase B/Akt (PkB/Akt), which are activated by IL-6 and amplified in the presence of soluble IL-6 receptor (sIL-6R). Supporting the role of IL-6 in human cancer is the observation of elevated serum levels of IL-6 and sIL-6R in patients with bone metastasis and their association with a poor clinical outcome. Over the last decade several large (monoclonal antibodies) and small (inhibitors of IL-6 mediated signaling) molecules that inhibit IL-6 activity in preclinical models have been developed. Several of these inhibitors are now undergoing phase I and II clinical trials, which will determine their inclusion in the list of effective targeted agents in the fight against cancer.
Keywords: Interleukin-6, tumor microenvironment, bone metastasis
1. Bone marrow microenvironment and bone metastasis
It is estimated that 350,000 patients die with bone metastases annually in the US.1 Considering its stiff structure and composition, it is surprising that the bone is among the most common sites for the establishment of cancer metastasis.2,3 However, it is in part explained by the unique microenvironment provided by the bone marrow. The bone marrow is the site of niches where hematopoietic stem cells (HSCs) reside. These niches consist of osteoblasts that line the endosteal surface of the bone and are in close interaction with HSCs, with whom they maintain contact via cell–cell adhesion molecules like osteopontin and integrins, and which they attract via soluble factors like stromal-derived factor (SDF)-1, the ligand for the chemokine receptor CXCR4 present at the surface of HSCs.4–6 Like HSCs, many circulating tumor cells express CXCR4 and home in on the SDF-1 rich environment of the bone marrow and the osteoblastic niche.7–9 However, the homing of tumor cells into the bone marrow does not necessarily indicate that these cells will be able to proliferate and form bone metastases. The formation of bone metastases requires a significant alteration of the bone metabolism that affects the balance between bone formation and bone degradation in favor of one (osteoblastic metastasis) or the other (osteolytic metastasis). In many cancers, like breast and prostate cancers, this process is the result of the direct production by tumor cells of hormones and growth factors like parathyroid hormone-related peptides (PTHrP), receptor activator of nuclear factor kappa B (NFκB) ligand (RANKL), granulocyte macrophage colony stimulatory factor (GMCSF), IL-1, IL-6, and macrophage inflammatory protein (MIP)-1α, which activate osteoblasts and osteoclasts and disrupt the homeostatic balance that controls bone formation and degradation. However, in other cancers like multiple myeloma and neuroblastoma, it is the interaction between tumor cells and bone marrow mesenchymal cells (BMMCs) that plays a critical role.10,11 These cells are a source of growth factors, chemokines, and cytokines, which affect tumor cells and whose production is controlled by tumor cells via adhesion-dependent and -independent mechanisms. The interaction and cross talk between tumor cells and BMMCs play a critical role in tumor cell proliferation and survival, and in the progression toward bone metastasis.12,13 In this review article, we will focus on IL-6, one of the soluble factors that is expressed by BMMCs in the presence of tumor cells. This cytokine plays multiple roles in cancer progression and metastasis. We will primarily focus here on its contributory role in the establishment of bone metastasis.
2. IL-6 and its signaling mechanism
2.1. IL-6 signaling and transsignaling
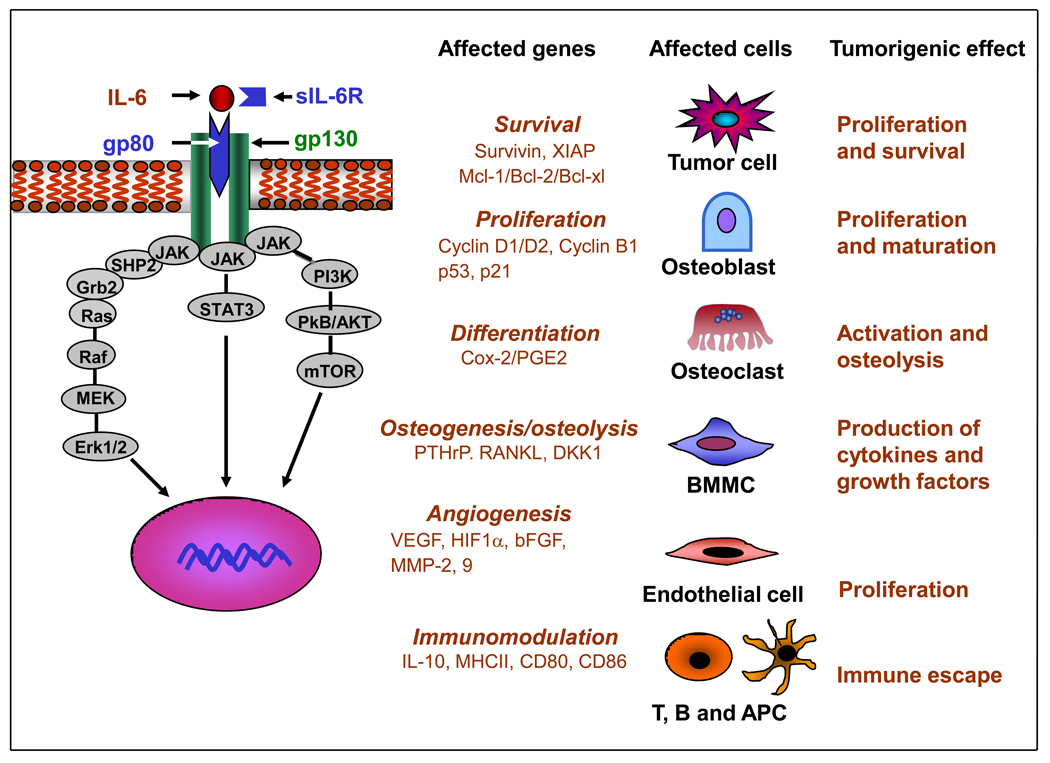
Interleukin-6 is a pleiotropic cytokine overexpressed in response to injury, inflammation, and infection.14 It was originally cloned as a B cell stimulatory factor and designated Interferon β2. It was later found to stimulate cytotoxic T cells and to induce the differentiation of osteoclast precursor cells into mature and active osteoclasts.15,16 IL-6 is produced by many cells including osteoblasts, monocytes and macrophages, and BMMCs. Serum levels of IL-6 are low or undetectable under normal physiological conditions. However, the production of IL-6 is regulated by several physiological factors like diet, exercise, and stress. IL-6 production by skeletal muscle increases 100-fold during physical activity 17 and adipose tissues are another main source of IL-6. In muscles IL-6 sensitizes myotubes to insulin and enhances glycogen synthesis and glucose uptake, whereas in adipose tissues it reduces insulin-dependent hepatic glycogen synthesis, decreases glucose uptake, increases triglyceride release, and down-regulates lipoprotein lipase, thus promoting obesity and insulin-resistant type 2 diabetes.18 Elevated levels of serum IL-6 concomitantly with elevated levels of acute phase C reactive protein, are reported to be associated with depression, chronic inflammation, and cardiac diseases.19 IL-6 interacts with a heterotrimeric membrane-associated receptor, member of the class I cytokine receptor family (Figure 1). This receptor is composed of an α subunit (IL-6Rα/gp80), which binds the soluble ligand IL-6 and β2 subunits (gp130), which, through their cytoplasmic domain, function as the signal-transducing component of the complex.20 Whereas gp130 is ubiquitously expressed by cells, IL-6Rα/gp80 is expressed in selected cells like B cells, macrophages, and osteoclasts that respond to IL-6.21 IL-6Rα/gp80 also exists in a soluble form designated sIL-6R, produced either by alternate splicing or by shedding via proteolytic cleavage mediated by metalloproteinases such as a disintegrin and metalloproteinase (ADAM) 10 and 17 (TACE).22,23 In contrast to most soluble receptors that trap the ligand and act as antagonists, sIL-6R stabilizes IL-6, promotes the formation of a functional multimolecular complex with gp130, and enhances signaling.24 This mechanism known as transsignaling allows cells that do not express the specific IL-6R/gp80 receptor protein to respond to IL-6.25 The source of sIL-6R in cancer patients is not entirely known, but it is shed by inflammatory cells like neutrophils, monocytes/macrophages, and T cells.26,27 Gp130 can also be solubilized, but in contrast to sIL-6R, soluble gp130 prevents the binding of IL-6 to the receptor and has an antagonistic activity on IL-6 signaling.28,29 IL-6 activates several intracellular signaling pathways. Binding of IL-6 to its receptor activates the Janus family of kinases (JAK1, JAK2, and TYK2) bound to the cytoplasmic domain of gp130.30 These kinases phosphorylate signal transduction and activator of transcription (STAT)-3 at Tyr705, promoting its nuclear transfer and transcriptional function.31 IL-6 also activates Ras and promotes its translocation to the plasma membrane where it activates Raf, mitogen-activated protein kinase kinase (MEK), and MAP (Erk1/2).32 A third pathway activated by IL-6 is the phosphoinositol 3 kinase (PI3K)–protein kinase B (PkB/Akt) pathway as JAK can phosphorylate PI3K.33,34 Binding of STAT-3 to a specific DNA domain promotes the expression of a large variety of genes (Figure 1). Among those are survival proteins like survivin, X-linked inhibitor of apoptosis (XIAP), Bcl-2, Bcl-XL, and Mcl-1; proteins involved in cell proliferation like cyclins and MYC and proangiogenic factors like hypoxia-inducible factor (HIF)-1α, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and matrix metalloproteinase (MMP)-2 and -9.
Figure 1. IL-6-mediated signaling, gene expression, and its cellular effects.
IL-6 activates three pathways, STAT-3, Erk1/2, and PkB/Akt. This results in the upregulation of a number of genes that affect survival, proliferation, differentiation, osteogenesis/osteolysis, angiogenesis, and immune modulation, in a variety of target cells. The expression of these genes has several pro-tumorigenic effects.
2.2. Interaction between IL-6 and other regulatory pathways
Interleukin-6 interacts with several pathways that also contribute to its pro-tumorigenic activity, in particular cyclooxygenase (Cox)-2, Wnt, transforming growth factor-β (TGF-β), and NFκB. IL-6 stimulates the expression of Cox-2 in osteoblasts, osteoclasts, and tumor cells, and the production of prostaglandin E2 (PGE2). PGE2 acts as a mediator of osteoclast activation by increasing the expression of RANKL in osteoblasts and the expression of RANK in osteoclasts. In addition, IL-6 induces the expression of PGE2 receptors, EP2, and EP4 in osteoblasts, triggering a positive feedback loop where more IL-6 results in production of PGE2 via Cox-2 and at the same time enhances PGE2 response by increasing the number of PGE2 receptors at the cell surface. PGE2 then stimulates the expression of IL-6, creating a cascade of signals that increases osteolysis.35,36 IL-6 also interacts with the Wnt signaling pathway. Wnt plays a regulatory role in osteogenesis by promoting the differentiation of BMMCs into osteoblasts and the synthesis of collagen by these cells. Wnt signaling itself is controlled by several inhibitors, in particular Dickkopf-1 (DKK-1), a soluble protein that, when bound to Wnt, prevents its interaction with Frizzled and the lipoprotein co-receptor protein LRP5/6, and thus inhibits Wnt signaling.37 DKK-1 is expressed by many metastatic cancer cells like breast cancer cells and myeloma, and is responsible for the inhibition of osteogenesis associated with osteolytic bone metastasis. By stimulating the production of DKK-1 in myeloma cells, IL-6 prevents the differentiation of osteoblast progenitor cells into mature osteoblasts and prevents bone-promoting osteolysis.38 The bone matrix is also a reservoir of growth factors and in particular transforming growth factor-β (TGF-β), with which IL-6 interacts. TGF-β is released in a soluble and active form upon proteolytic degradation of the bone by osteoclasts.39,40 TGF-β upregulates IL-6 expression in several cell types like fibroblasts and osteoblasts, and prostate cancer cells, and stimulates the production of PTHrP in tumor cells. TGF-β̣ and IL-6 thus act synergistically to potentiate bone degradation.41 IL-6/STAT-3 interacts with NFκB as STAT-3 prolongs NFκB retention through acetyltransferase p300-mediated p65 NFκB acetylation, which inhibits the nuclear export of NFκB. STAT-3 is therefore necessary to maintain NFκB activity not only in cancer cells, but also in tumor-associated hematopoietic cells.42 STAT-3 also binds to p53 and represses its function as a regulator of apoptosis.43
3. IL-6 and cancer metastasis
3.1. Autocrine and paracrine mechanisms of IL-6 activity
The role of IL-6 in cancer is complex and includes autocrine and paracrine mechanisms. Many tumor cells from prostate, breast, and colon cancer produce large amounts of IL-6 and express the IL-6R/gp80 and gp130 receptor subunits, which allows them to respond to IL-6 stimulation in an autocrine manner. STAT-3 is also persistently activated in tumor cells.44,45 However, in other cancers, in particular myeloma and neuroblastoma, most tumor cells do not produce IL-6, but express a functional IL-6 receptor complex and thus respond to IL-6 produced in the tumor microenvironment in a paracrine manner (Figure 2). In myeloma, adhesion contact between myeloma cells and stromal cells induces the expression of IL-6 by stromal cells.46 We have shown that in neuroblastoma, the production of IL-6 is induced in BMMCs in the presence of neuroblastoma cells, but in contrast to myeloma this induction does not require cell–cell contact and is mediated by soluble factors including galectin-3 binding protein (Gal-3BP)/Mac2.11,47 IL-6 has multiple effects on tumor progression. Some are the result of its direct action on tumor cells; others are the result of its activity on normal cells in the tumor microenvironment, in particular osteoblasts, osteoclasts, endothelial cells, and immune cells.
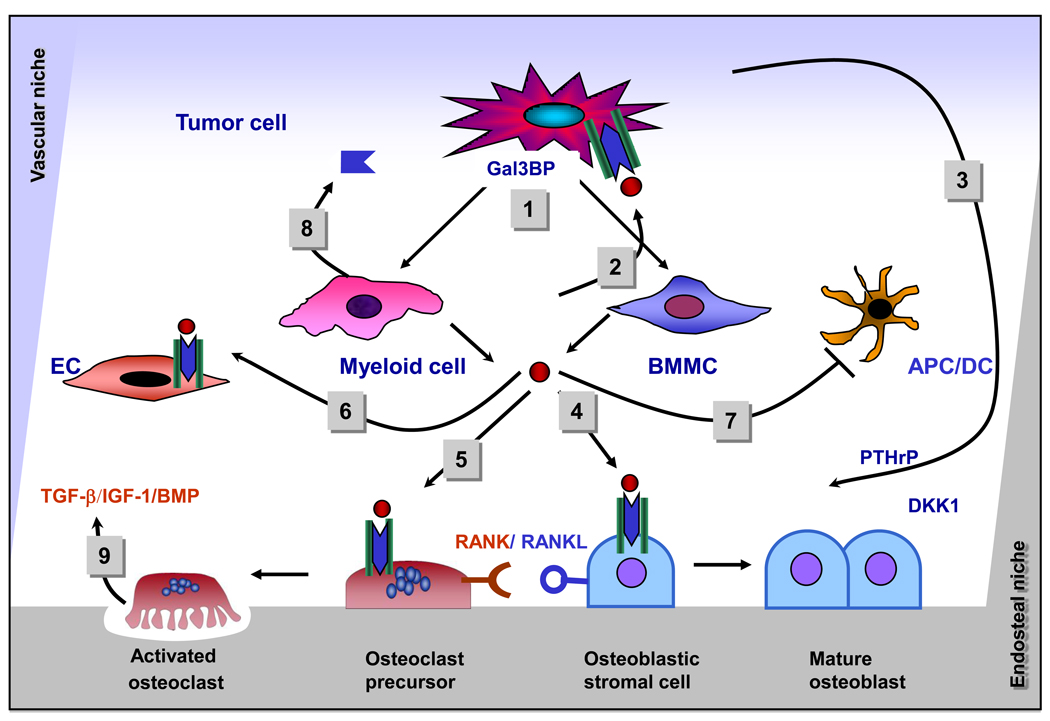
Figure 2. Paracrine effects of IL-6 in the bone marrow microenvironment.
(1) Tumor cells induce the production of IL-6 in BMMCs and myeloid cells in the bone marrow microenvironment, (2) IL-6 stimulates the proliferation and enhances the survival of tumor cells, (3) IL-6 increases the production of PTHrP and DKK-1 by tumor cells, (4) IL-6 promotes the degradation of the bone matrix by inducing the expression of RANKL in osteoblasts, (5) IL-6 activates osteoclasts, (6) IL-6 stimulates endothelial cells and endothelial progenitor cells, promoting angiogenesis and vasculogenesis, (7) IL-6 inhibits the maturation of APC and dendritic cells, (8) myeloid cells produce sIL-6R, which potentiates the effect of IL-6, (9) growth factors like TGF-β, IGF-1, and BMP are released from degraded bone, and enhance IL-6 production, contributing to the vicious circle of bone metastasis.
3.2. Effect of IL-6 on tumor cell proliferation and survival
Interleukin-6 has a direct growth stimulatory effect on many tumor cells through the activation of several signaling pathways. By activating Ras/Raf/MEK/Erk1/2, IL-6 stimulates tumor cell proliferation.36,48,49 Activation of STAT-3 by IL-6 upregulates the expression of cyclins D1, D2, and B1, and MYC, and downregulates the expression of cdk inhibitor p21Cip1, thus promoting entry into the cell cycle.50–52 IL-6 is also an important regulator of cell survival, providing tumor cells with a mechanism to escape cell death induced by stress and cytotoxic drugs. IL-6 increases the expression of several survival proteins, including Bcl-2, Bcl-XL, Mcl-1, survivin, and XIAP.53 Overexpression of these proteins is commonly associated with increased chemoresistance and constitutive activation of STAT-3 are resistant to chemotherapeutic agents.54 IL-6 thus contributes to a sanctuary effect in the bone marrow, where tumor cells acquire resistance to cytotoxic chemotherapy.55
3.3. IL-6 promotes osteolysis
Interleukin-6 enhances bone degradation in many ways. First, it induces the production of RANKL by BMMCs and osteoblasts. Under physiological conditions, these cells express low levels of IL-6R, but in the presence of sIL-6R, STAT-3 is activated and the expression of RANKL is induced.56–59 The binding of RANKL to its receptor RANK activates NFκB, Erk1/2, and p38/MAP kinase signaling, and induces osteoclast maturation and the expression of osteoclast-associated receptors (OSCAR), an Ig-like surface receptor that acts as a co-stimulatory receptor for osteoclast differentiation.60,61 Second, IL-6 induces in tumor cells the expression of several proteins involved in bone resorption such as PTHrP, IL-8, IL-11, RANKL, and Cox-2. IL-6 and sIL-6R stimulate PTHrP production by osteoblastic stromal cells via MEK/Erk1/2 pathways. PTHrP increases the expression of RANKL and downregulates the expression of osteoprotegerin (OPG), the decoy receptor for RANKL by osteoblasts tipping the bone metabolism toward osteolysis.59,62 Third, by stimulating the expression of DKK-1 in tumor cells, IL-6 inhibits Wnt-mediated osteogenesis and further imbalances the bone homeostasis toward excessive degradation.38 IL-6 also increases the activity of estradiol 17 β-hydroxysteroid dehydrogenase, thereby inhibiting the anti-osteoclast activity of estrogens, promoting osteolysis and hypercalcemia in breast cancer patients. Furthermore, IL-6 downregulates the synthesis of genes like type II collagen and aggrecan, contributing to a decrease in new bone formation.56,63
3.4. Role of IL-6 in metastasis to other organs
Interleukin-6 also plays an important role in promoting metastasis to organs other than the bone. Overexpression of IL-6 in specific organs like the lungs, brain or liver will attract circulating tumor cells to these organs and promote their establishment into metastatic tumors. For example, IL-6-dependent STAT-3 activation in human melanoma promotes the growth of metastatic tumors in the brain by inducing the overexpression of bFGF, MMP-2, and VEGF, which contribute to invasion and angiogenesis.64 NFκB activation in Kupffer cells stimulates their production of IL-6, which promotes the growth of metastatic Lewis lung carcinoma cells in the liver. Interestingly, it has also been recently demonstrated that the production of IL-6 (and IL-8) in a primary tumor promotes the recruitment of circulating tumor cells back into their primary tumor, creating a process called “tumor self-seeding” that accelerates tumor growth, angiogenesis, and stromal cell recruitment.65
3.5. IL-6 stimulates angiogenesis and vasculogenesis
Interleukin-6 plays multiple functions in angiogenesis and vascular remodeling. IL-6 enhances in vitro the proliferation, migration and matrigel tube formation of endothelial progenitor cells isolated from adult human circulating blood in a dose-dependent manner, suggesting a role in vasculogenesis.66 IL-6 increases angiogenesis by transcriptional upregulation of VEGF in a JAK/STAT-3- and HIF-1α-dependent manner in tumor cells and the expression of bFGF and MMP-9 in tumor-associated myeloid cells and endothelial cells that contribute to tumor angiogenesis.67–70
3.6. Immunomodulatory role of IL-6
Interleukin-6 belongs to the group of inflammatory cytokines and chemokines associated with a Th2 and M2 response of the immune system and to an inflammatory reaction that is pro-tumorigenic. 71 IL-6-mediated activation of STAT-3 in regulatory T cells is responsible for the production of several pro-inflammatory cytokines like IL-10 that help tumor cells escaping immune surveillance. IL-6-induced STAT-3 activation inhibits the expression of MHC class II, CD80, CD86, and IL-12 expression in dendritic cells, preventing their maturation and compromising their ability to trigger cytotoxic CD8+T cells and natural killer (NK) cells. IL-6 downregulates the activity of NK cells and their anti-tumor function.72 By promoting inflammation and immune escape, IL-6 thus contributes to an immune microenvironment that is favorable to tumor progression.
4. Prognostic significance of IL-6 and IL-6R levels in peripheral blood of cancer patients
Considering the pro-tumorigenic roles of IL-6, it is therefore not surprising that elevated serum levels of IL-6 and sIL-6R have been associated with poor clinical outcome in many human cancers, including in breast and prostate cancer, multiple myeloma, hepatocellular carcinoma, lymphoma, and pediatric solid tumors.73–76 The levels of IL-6 typically found in the serum of cancer patients is within the picogram range (100–500 pg/ml), at which there is very little evidence in vitro that IL-6 activates STAT-3. In contrast, the concentration of sIL-6R found in the serum of patients with cancer is within the ng/ml range. These observations suggest that in the absence of sIL-6R most tumor cells could remain insensitive to IL-6 in vivo because of its low concentration and instability. By stabilizing IL-6 and enhancing IL-6-mediated signaling, sIL-6R could be a critical regulator of IL-6 activity in the tumor microenvironment. The source of sIL-6R in cancer is presently unclear. Whereas several tumor cells can shed IL-6R or produce it as a result of alternate splicing,28 inflammation is likely to play a key role, as monocytes, and in particular neutrophils, can produce sIL-6R.26,77,78
5. Targeting IL-6
The abundance of evidence supporting a pro-tumorigenic effect of IL-6 in tumor progression and bone metastasis has prompted the initiation of clinical trials testing the safety and therapeutic efficacy of inhibitors of IL-6 and IL-6 signaling in cancer treatment. Currently, the strategies focus on large proteins like humanized monoclonal antibodies (mAb) and small molecules that inhibit IL-6-mediated signaling or the production of IL-6 (Table 1).
Table 1.
Inhibitors of IL-6 and IL-6-mediated signaling: preclinical and clinical trials
| Agent | Description | Target | Characteristics | Clinical | Clinical efficacy |
|---|---|---|---|---|---|
| CNTO 328 | Humanized anti- IL-6 mAb |
IL-6 | 1/2 life of twoweeks |
Phase I – III in MM, hematological malignancies and Castleman’s disease |
65% five-year survival in MM |
| Tocilizumab/Actemra | Humanized recombinant anti- IL-6R antibody |
IL-6R/gp- 80 |
Inhibits IL-6R and sIL-6R |
Phases I & II in advanced cancers, Castleman’s disease and phase III in rheumatoid arthritis |
Well tolerated |
| INCB20 | JAK inhibitor | JAK1/2 | Inhibits JAK dependent STAT3 and Akt activation |
INCB 018424 tested in Phase I & II in RA |
Well tolerated |
| S31–201 (NSC 74859) |
STAT-3 inhibitor | STAT-3 | Inhibits STAT-3 dimerization (SH2) |
Not tested | NA |
| Sorafenib | Multikinase inhibitor |
Multiple | Inhibits Raf/MEK/ERK and STAT-3 |
Phases I and II in solid tumors (Renal Cell Ca, Lung Ca, Hepato Ca). |
Well tolerated |
| Lenalidomide | Immunomodulator | Multiple | Inhibits IL-6 production in monocytes |
Myeloma and MDS |
Well tolerated |
5.1. Large molecules: mAb
Several humanized mAb against IL-6 and IL-6R have been developed. A humanized mAb against IL-6 developed by Centocor (CNTO 328) has shown good activity in preclinical models of myeloma when used alone or in combination with bortezomib or dexamethasone.79–81 It has been FDA-approved for a phase II multicenter trial in multiple myeloma. A similar anti-IL-6 antibody (B-E8) has been developed by Diaclone (Besancon, France) and has been tested in a non-randomized trial in combination with dexamethasone and melphalan in 24 patients with multiple myeloma. It has improved overall survival (68.2% at five years).82 A humanized anti IL-6Rα/gp80 mAb (Tocilizumab/Actemra®) developed by the Japanese company Chugai and licensed to Genentech and its parent company Roche, has been used in Japan since 2005 to treat patients with Castelman’s disease and arthritis. It has recently been tested in three concomitant but independent European and international studies in non-cancer patients (≥18 years of age) with rheumatoid arthritis. All three studies have been recently reported.83–85 These studies show that this mAb is well tolerated and effective. It is being currently tested in children (aged 2 to 19) with juvenile rheumatoid arthritis and seems to be similarly well tolerated and effective.86 The reported adverse effects seem relatively mild and reversible, and primarily associated with the anticipated immunosuppressive effect of blocking IL-6. The most frequent side effects reported were respiratory and skin infections, gastrointestinal disorders, psychiatric disorders, and hypertension associated with elevated cholesterol levels. Serious side effects leading to discontinuation of the therapy, like gastrointestinal hemorrhage and perforation, were reported in less than 1.8% of treated patients.87 Actemra has very recently (January 2010) been approved by the Food and Drug Administration to treat rheumatoid arthritis. Although not yet approved for cancer, the abundant data supporting the pro-tumorigenic role of IL-6 in cancer make the testing of Actemra in cancer patients very attractive.
5.2. Small molecules
Several small molecule inhibitors of IL-6/IL-6R-mediated signaling have been developed. Some have only been tested in preclinical models of cancer, but others are currently being tested in patients.88 Among these is INCB20 (Incyte Corp., Wilmington, DE, USA), a synthetic compound that inhibits JAK family members.89 It inhibits IL-6-dependent proliferation of INA-6 myeloma cells in vitro at concentrations between 0.1 and 1 µM and when administered in myeloma-bearing mice, it inhibits tumor growth and survival. This inhibitor also inhibits the Ras/Raf/MEK/Erk1/2 and the PI3K–PkB/Akt pathways, all downstream of JAK1/2 activation. 531–201 (NSC 74859) is an inhibitor that was identified by the structure-based high throughput virtual screen of the National Cancer Institute chemical library (and was named 531–201 when resynthesized as a pure compound). It selectively inhibits the DNA binding activity of STAT-3 in vitro with an IC50 value of 86 µM. It induces apoptosis in tumor cells that constitutively express active STAT-3.90 When administered to MDA-MB-231-bearing mice, it significantly inhibits tumor growth and STAT-3 phosphorylation in tumor tissues. It is also active in hepatocellular cancer in mice91, but has not been tested yet in patients. Sorafenib (Nexavar, Bay 43-9006) is a multikinase inhibitor that was originally developed by Bayer on the basis of its inhibitory effect on Ras-signaling. It has been recently shown to inhibit STAT-3 phosphorylation in primary medulloblastoma cells in vitro and the growth of human medulloblastoma tumors in nude mice.92 It has been tested in several phase I and II clinical trials in patients with solid tumors and shown to be safe.93,94 Sunitinib (Su11248) is a similar multikinase inhibitor available orally that targets several receptor tyrosine kinases like platelet-derived growth factor receptor (PDGFR), kit, FLT3 or VEGFR, and has been shown to inhibit STAT-3 phosphorylation in renal cell carcinoma cells.95 It has been tested in phase I clinical trials in patients with metastatic renal cell carcinoma in combination with bevacizumab with an objective response rate of 52%.96 Lenalidomide (Revlimid, Celgene, NJ, USA) is an immunomodulatory drug derivative of thalidomide that has been approved by the Food and Drug Administration (FDA), and is currently undergoing clinical trials in multiple myeloma, myelodysplastic syndromes, and melanoma.97 Its mechanism of action is not entirely known, but it has anti-angiogenic, immunosuppressive, and anti-metastatic activities, in part via inhibition of HIF-1α.98 It is a potent inhibitor of IL-6 expression in tumor cells and myeloid cells.
5.3 Combining anti-IL-6 with other strategies to combat bone metastasis
There have been two other main strategies developed over the last decade to combat bone metastasis, inhibition of osteoclast activity and inhibition of RANKL. Nitrogen-containing bisphosphonates are pyrophosphoric acid-based compounds that have a high affinity for the bone, are potent inducers of apoptosis in osteoclasts,99,100 and have been used in clinical practice for patients with osteoporosis and in cancer patients with bone metastasis.101,102 Denosumab (AMG162), a humanized monoclonal antibody against RANKL, has been used in phase I and II clinical trials in patients with metastatic prostate and breast cancer and multiple myeloma.103,104 It is safe and effective in reducing bone metastasis. Whether denosumab and zoledronic acid should be used in combination with Actemra or other anti-IL-6-based therapies is an interesting question considering that they have different targets that are part of the same pathway responsible for the formation of osteolytic metastases.
Conclusion
Over the last decade, IL-6 has emerged as an important contributor to the tumor microenvironment and inflammation contributing to pro-tumorigenic activity. IL-6 function involves multiple cell–cell interactions and signaling pathways that together promote osteolytic bone metastasis, tumor cell proliferation and survival, angiogenesis and vasculogenesis, and immune escape. As a result, inhibition of IL-6 and IL-6R-mediated signaling has been the subject of intense investigation. We are at a time when several clinical trials testing the safety and clinical efficacy of inhibitors of IL-6 are ongoing, and will soon determine the validity of targeting IL-6 in cancer treatment.
Acknowledgments
The authors thank Mrs. J. Rosenberg for typing and editing the manuscript. This work was supported by grant CA 084103 from the National Institutes of Health (YDC), and the Children’s Cancer Research Fund and Children’s Neuroblastoma Cancer Foundation (TA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Reddi AH, Roodman D, Freeman C, Mohla S. Mechanisms of tumor metastasis to the bone: challenges and opportunities. J Bone Miner Res. 2003;18:190–194. doi: 10.1359/jbmr.2003.18.2.190. [DOI] [PubMed] [Google Scholar]
- 4.Hattori K, Heissig B, Rafii S. The regulation of hematopoietic stem cell and progenitor mobilization by chemokine SDF-1. Leuk Lymphoma. 2003;44:575–582. doi: 10.1080/1042819021000037985. [DOI] [PubMed] [Google Scholar]
- 5.Juarez J, Bendall L, Bradstock K. Chemokines and their receptors as therapeutic targets: the role of the SDF-1/CXCR4 axis. Curr Pharm Des. 2004;10:1245–1259. doi: 10.2174/1381612043452640. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 7.Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747–4757. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 8.Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- 9.Strahm B, Durbin AD, Sexsmith E, Malkin D. The CXCR4-SDF1alpha axis is a critical mediator of rhabdomyosarcoma metastatic signaling induced by bone marrow stroma. Clin Exp Metastasis. 2007:1–10. doi: 10.1007/s10585-007-9094-6. [DOI] [PubMed] [Google Scholar]
- 10.Teoh G, Anderson KC. Interaction of tumor and host cells with adhesion and extracellular matrix molecules in the development of multiple myeloma. Hematol Oncol Clin North Am. 1997;11:27–42. doi: 10.1016/s0889-8588(05)70413-5. [DOI] [PubMed] [Google Scholar]
- 11.Sohara Y, Shimada H, Minkin C, et al. Bone marrow mesenchymal stem cells provide an alternate pathway of osteoclast activation and bone destruction by cancer cells. Cancer Res. 2005;65:1129–1135. doi: 10.1158/0008-5472.CAN-04-2853. [DOI] [PubMed] [Google Scholar]
- 12.Blouin S, Basle MF, Chappard D. Interactions between microenvironment and cancer cells in two animal models of bone metastasis. Br J Cancer. 2008;98:809–815. doi: 10.1038/sj.bjc.6604238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Scheller J, Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195:173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 15.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for Translational Therapeutics. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 16.Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4 Suppl 3:S233–S242. doi: 10.1186/ar565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 18.Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- 19.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 20.Yamasaki K, Taga T, Hirata Y, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 21.Hilbert DM, Kopf M, Mock BA, Kohler G, Rudikoff S. Interleukin 6 is essential for in vivo development of B lineage neoplasms. J Exp Med. 1995;182:243–248. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knupfer H, Preiss R. sIL-6R: more than an agonist? Immunol Cell Biol. 2008;86:87–91. doi: 10.1038/sj.icb.7100113. [DOI] [PubMed] [Google Scholar]
- 23.Holub MC, Szalai C, Polgar A, Toth S, Falus A. Generation of 'truncated' interleukin-6 receptor (IL-6R) mRNA by alternative splicing; a possible source of soluble IL-6R. Immunol Lett. 1999;68:121–124. doi: 10.1016/s0165-2478(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 24.Peters M, Meyer zum Buschenfelde KH, Rose-John S. The function of the soluble IL-6 receptor in vivo. Immunol Lett. 1996;54:177–184. doi: 10.1016/s0165-2478(96)02669-7. [DOI] [PubMed] [Google Scholar]
- 25.Rose-John S, Scheller J, Elson G, Jones SA. Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol. 2006;80:227–236. doi: 10.1189/jlb.1105674. [DOI] [PubMed] [Google Scholar]
- 26.Chalaris A, Rabe B, Paliga K, et al. Apoptosis is a natural stimulus of IL6R shedding and contributes to the proinflammatory trans-signaling function of neutrophils. Blood. 2007;110:1748–1755. doi: 10.1182/blood-2007-01-067918. [DOI] [PubMed] [Google Scholar]
- 27.Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008;180:7102–7106. doi: 10.4049/jimmunol.180.11.7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol. 2006;63:321–329. doi: 10.1111/j.1365-3083.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 29.Richards PJ, Nowell MA, Horiuchi S, et al. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis Rheum. 2006;54:1662–1672. doi: 10.1002/art.21818. [DOI] [PubMed] [Google Scholar]
- 30.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 32.Hu L, Shi Y, Hsu JH, et al. Downstream effectors of oncogenic ras in multiple myeloma cells. Blood. 2003;101:3126–3135. doi: 10.1182/blood-2002-08-2640. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Li Y, Shen B. PI3-K/Akt pathway contributes to IL-6-dependent growth of 7TD1 cells. Cancer Cell Int. 2003;3:1. doi: 10.1186/1475-2867-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jee SH, Chu CY, Chiu HC, et al. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004;123:1169–1175. doi: 10.1111/j.0022-202X.2004.23497.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoprotegerin/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 36.Ara T, Song L, Shimada H, et al. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall CL, Keller ET. The role of Wnts in bone metastases. Cancer Metastasis Rev. 2006;25:551–558. doi: 10.1007/s10555-006-9022-2. [DOI] [PubMed] [Google Scholar]
- 38.Gunn WG, Conley A, Deininger L, et al. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 39.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 40.Guise TA, Chirgwin JM. Transforming growth factor-beta in osteolytic breast cancer bone metastases. Clin Orthop Relat Res. 2003:S32–S38. doi: 10.1097/01.blo.0000093055.96273.69. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T, Matsuda T, Muraguchi A, Miyazono K, Kawabata M. Cross-talk between IL-6 and TGF-beta signaling in hepatoma cells. FEBS Lett. 2001;492:247–253. doi: 10.1016/s0014-5793(01)02258-x. [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niu G, Wright KL, Ma Y, et al. Role of Stat3 in regulating p53 expression and function. Mol Cell Biol. 2005;25:7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mora LB, Buettner R, Seigne J, et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Res. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 45.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 46.Hideshima T, Chauhan D, Richardson P, Anderson KC. Identification and validation of novel therapeutic targets for multiple myeloma. J Clin Oncol. 2005;23:6345–6350. doi: 10.1200/JCO.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 47.Fukaya Y, Shimada H, Wang LC, Zandi E, DeClerck YA. Identification of Gal-3 binding protein as a factor secreted by tumor cells that stimulates interleukin-6 expression in the bone marrow stroma. J Biol Chem. 2008 doi: 10.1074/jbc.M803115200. [DOI] [PubMed] [Google Scholar]
- 48.Ogata A, Chauhan D, Teoh G, et al. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J Immunol. 1997;159:2212–2221. [PubMed] [Google Scholar]
- 49.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 50.Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001–7110. doi: 10.1038/sj.onc.1205859. [DOI] [PubMed] [Google Scholar]
- 52.Quintanilla-Martinez L, Kremer M, Specht K, et al. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol. 2003;162:1449–1461. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gritsko T, Williams A, Turkson J, et al. Persistent activation of stat3 signaling induces survivin gene expression and confers resistance to apoptosis in human breast cancer cells. Clin Cancer Res. 2006;12:11–19. doi: 10.1158/1078-0432.CCR-04-1752. [DOI] [PubMed] [Google Scholar]
- 54.Barre B, Vigneron A, Perkins N, et al. The STAT3 oncogene as a predictive marker of drug resistance. Trends Mol Med. 2007;13:4–11. doi: 10.1016/j.molmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 55.Shain KH, Yarde DN, Meads MB, et al. Beta1 integrin adhesion enhances IL-6-mediated STAT3 signaling in myeloma cells: implications for microenvironment influence on tumor survival and proliferation. Cancer Res. 2009;69:1009–1015. doi: 10.1158/0008-5472.CAN-08-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanchard F, Duplomb L, Baud'huin M, Brounais B. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009;20:19–28. doi: 10.1016/j.cytogfr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 57.O'Brien CA, Gubrij I, Lin SC, Saylors RL, Manolagas SC. STAT3 activation in stromal/osteoblastic cells is required for induction of the receptor activator of NF-kappaB ligand and stimulation of osteoclastogenesis by gp130-utilizing cytokines or interleukin-1 but not 1,25-dihydroxyvitamin D3 or parathyroid hormone. J Biol Chem. 1999;274:19301–19308. doi: 10.1074/jbc.274.27.19301. [DOI] [PubMed] [Google Scholar]
- 58.Guillen C, de Gortazar AR, Esbrit P. The interleukin-6/soluble interleukin-6 receptor system induces parathyroid hormone-related protein in human osteoblastic cells. Calcif Tissue Int. 2004;75:153–159. doi: 10.1007/s00223-004-0113-1. [DOI] [PubMed] [Google Scholar]
- 59.Erices A, Conget P, Rojas C, Minguell JJ. Gp130 activation by soluble interleukin-6 receptor/interleukin-6 enhances osteoblastic differentiation of human bone marrow-derived mesenchymal stem cells. Exp Cell Res. 2002;280:24–32. doi: 10.1006/excr.2002.5627. [DOI] [PubMed] [Google Scholar]
- 60.Kim K, Lee J, Kim JH, et al. Protein inhibitor of activated STAT 3 modulates osteoclastogenesis by down-regulation of NFATc1 and osteoclast-associated receptor. J Immunol. 2007;178:5588–5594. doi: 10.4049/jimmunol.178.9.5588. [DOI] [PubMed] [Google Scholar]
- 61.Bommert K, Bargou RC, Stuhmer T. Signalling and survival pathways in multiple myeloma. Eur J Cancer. 2006;42:1574–1580. doi: 10.1016/j.ejca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 62.Itoh S, Udagawa N, Takahashi N, et al. A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone. 2006;39:505–512. doi: 10.1016/j.bone.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 63.Legendre F, Dudhia J, Pujol JP, Bogdanowicz P. JAK/STAT but not ERK1/ERK2 pathway mediates interleukin (IL)-6/soluble IL-6R down-regulation of Type II collagen, aggrecan core, and link protein transcription in articular chondrocytes. Association with a down-regulation of SOX9 expression. J Biol Chem. 2003;278:2903–2912. doi: 10.1074/jbc.M110773200. [DOI] [PubMed] [Google Scholar]
- 64.Xie TX, Huang FJ, Aldape KD, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 65.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei LH, Kuo ML, Chen CA, et al. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 69.Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- 70.Huang SP, Wu MS, Shun CT, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 71.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 72.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 73.Egler RA, Burlingame SM, Nuchtern JG, Russell HV. Interleukin-6 and soluble interleukin-6 receptor levels as markers of disease extent and prognosis in neuroblastoma. Clin Cancer Res. 2008;14:7028–7034. doi: 10.1158/1078-0432.CCR-07-5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kovacs E. Investigation of interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R) and soluble gp130 (sgp130) in sera of cancer patients. Biomed Pharmacother. 2001;55:391–396. doi: 10.1016/s0753-3322(01)00079-8. [DOI] [PubMed] [Google Scholar]
- 75.Robak T, Wierzbowska A, Blasinska-Morawiec M, Korycka A, Blonski JZ. Serum levels of IL-6 type cytokines and soluble IL-6 receptors in active B-cell chronic lymphocytic leukemia and in cladribine induced remission. Mediators Inflamm. 1999;8:277–286. doi: 10.1080/09629359990289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stasi R, Brunetti M, Parma A, et al. The prognostic value of soluble interleukin-6 receptor in patients with multiple myeloma. Cancer. 1998;82:1860–1866. [PubMed] [Google Scholar]
- 77.Dowdall JF, Winter DC, Andrews E, et al. Soluble interleukin 6 receptor (sIL-6R) mediates colonic tumor cell adherence to the vascular endothelium: a mechanism for metastatic initiation? J Surg Res. 2002;107:1–6. doi: 10.1006/jsre.2001.6222. [DOI] [PubMed] [Google Scholar]
- 78.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 79.Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111:592–595. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- 80.Voorhees PM, Chen Q, Kuhn DJ, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res. 2007;13:6469–6478. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- 81.Voorhees PM, Chen Q, Small GW, et al. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. Br J Haematol. 2009 doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moreau P, Harousseau JL, Wijdenes J, et al. A combination of anti-interleukin 6 murine monoclonal antibody with dexamethasone and high-dose melphalan induces high complete response rates in advanced multiple myeloma. Br J Haematol. 2000;109:661–664. doi: 10.1046/j.1365-2141.2000.02093.x. [DOI] [PubMed] [Google Scholar]
- 83.Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371:987–997. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 85.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008;58:2968–2980. doi: 10.1002/art.23940. [DOI] [PubMed] [Google Scholar]
- 86.Yokota S, Imagawa T, Mori M, et al. Efficacy and safety of tocilizumab in patients with systemic-onset juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled, withdrawal phase III trial. Lancet. 2008;371:998–1006. doi: 10.1016/S0140-6736(08)60454-7. [DOI] [PubMed] [Google Scholar]
- 87.Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aggarwal BB, Sethi G, Ahn KS, et al. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 89.Burger R, Le Gouill S, Tai YT, et al. Janus kinase inhibitor INCB20 has antiproliferative and apoptotic effects on human myeloma cells in vitro and in vivo. Mol Cancer Ther. 2009;8:26–35. doi: 10.1158/1535-7163.MCT-08-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Siddiquee K, Zhang S, Guida WC, et al. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–7396. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin L, Amin R, Gallicano GI, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang F, Van Meter TE, Buettner R, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther. 2008;7:3519–3526. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–437. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 94.Gridelli C, Maione P, Del Gaizo F, et al. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist. 2007;12:191–200. doi: 10.1634/theoncologist.12-2-191. [DOI] [PubMed] [Google Scholar]
- 95.Xin H, Zhang C, Herrmann A, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–2513. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Lenalidomide in multiple myeloma. Expert Rev Anticancer Ther. 2006;6:1165–1173. doi: 10.1586/14737140.6.8.1165. [DOI] [PubMed] [Google Scholar]
- 98.Lu L, Payvandi F, Wu L, et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc Res. 2009;77:78–86. doi: 10.1016/j.mvr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 99.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 100.Oades GM, Senaratne SG, Clarke IA, Kirby RS, Colston KW. Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol. 2003;170:246–252. doi: 10.1097/01.ju.0000070685.34760.5f. [DOI] [PubMed] [Google Scholar]
- 101.Perry CM, Figgitt DP. Zoledronic acid: a review of its use in patients with advanced cancer. Drugs. 2004;64:1197–1211. doi: 10.2165/00003495-200464110-00004. [DOI] [PubMed] [Google Scholar]
- 102.Coleman RE. Bisphosphonates: clinical experience. Oncologist. 2004;9 Suppl 4:14–27. doi: 10.1634/theoncologist.9-90004-14. [DOI] [PubMed] [Google Scholar]
- 103.Body JJ, Facon T, Coleman RE, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 104.Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]