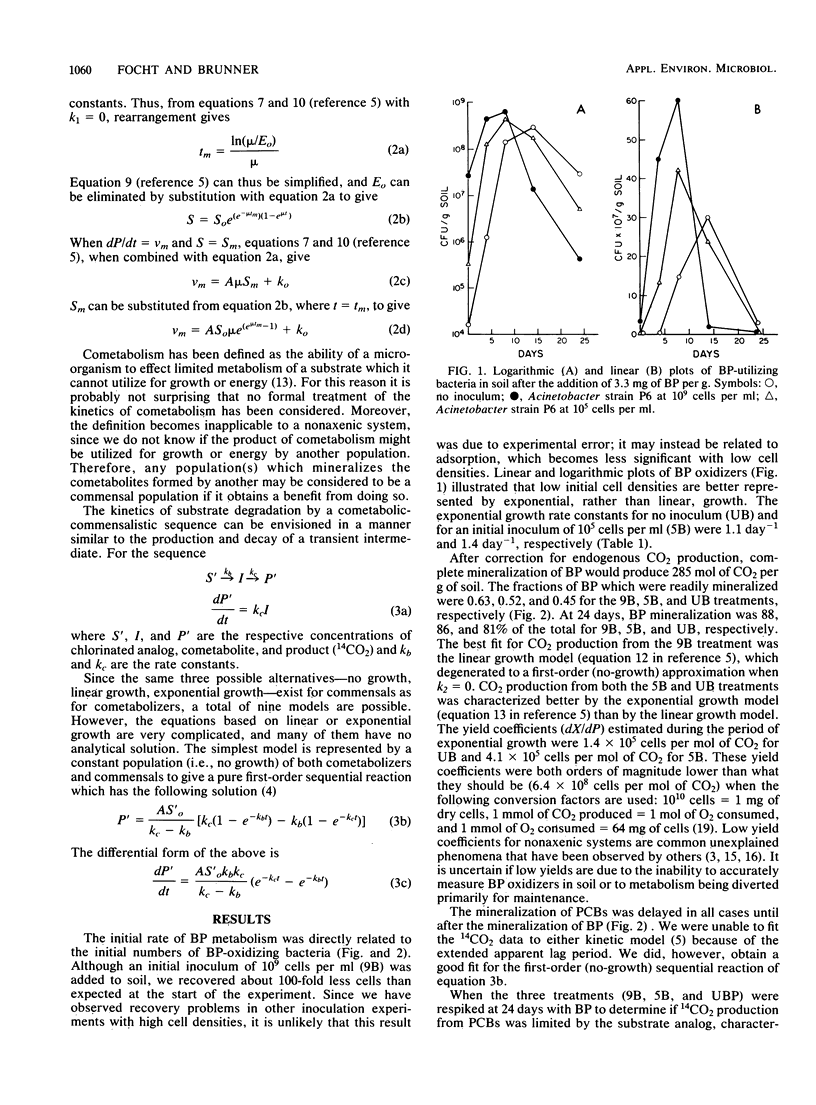
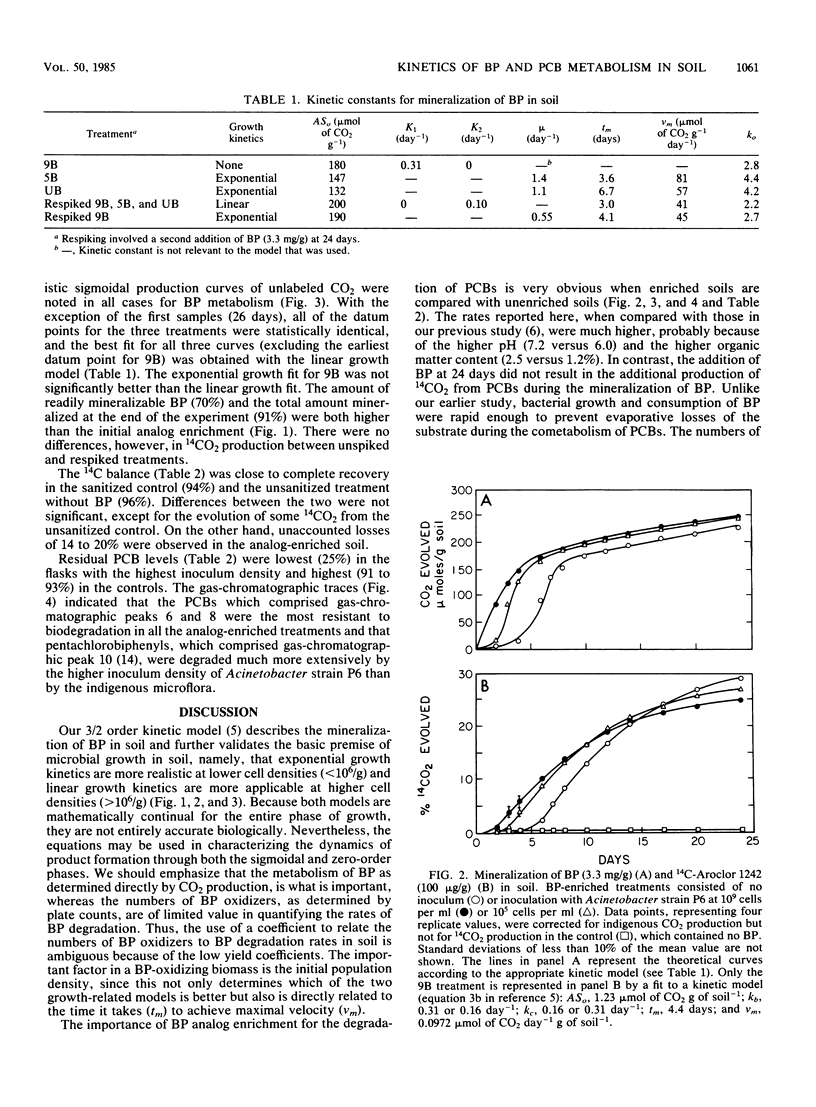
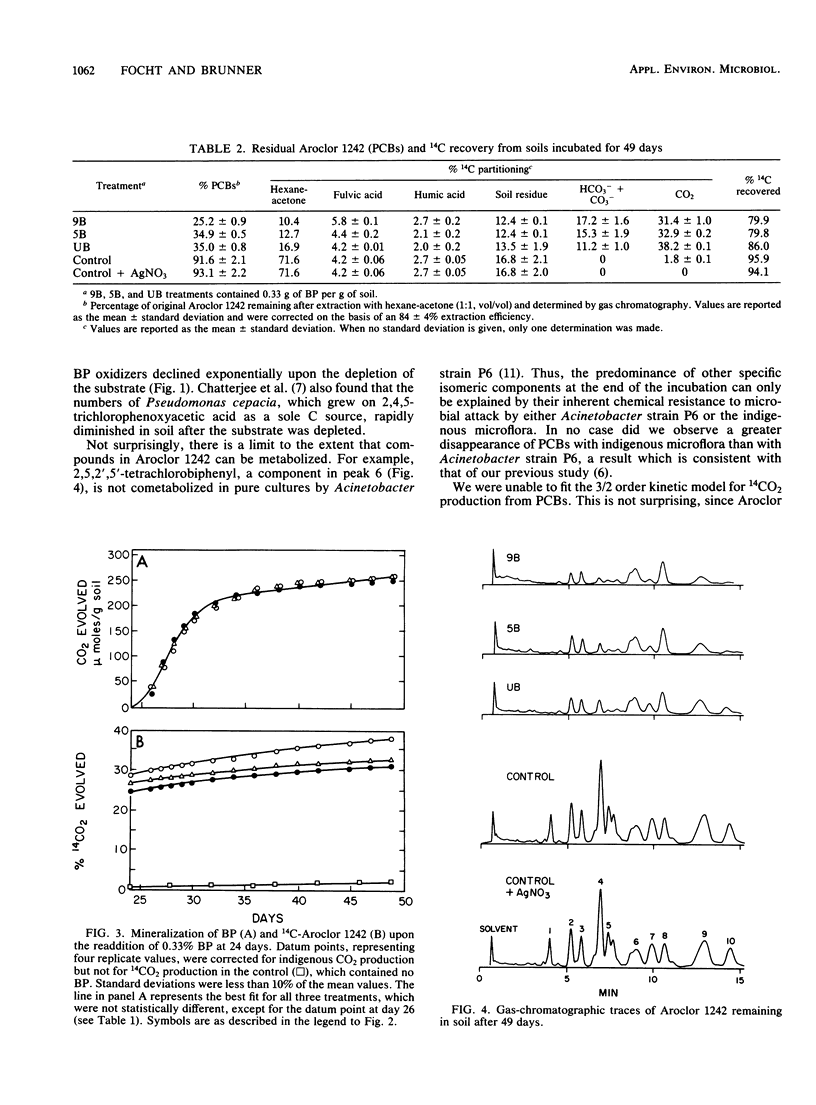
Abstract
The metabolism of 14C-labeled PCBs (polychlorinated biphenyls), which comprised the Aroclor 1242 mixture, was greatly enhanced by the addition of biphenyl (BP) to soil. After 49 days, only 25 to 35% of the original PCBs remained in the soil, and 48 to 49% was converted to 14CO2 (including soil carbonates) in treatments enriched with BP; by contrast, 92% of the PCBs remained and less than 2% was converted to 14CO2 in the unenriched control. Although the mineralization of PCBs in soils inoculated with Acinetobacter strain P6 was not greater than that in uninoculated BP-enriched soils, the initial and maximum mineralization rates and the disappearance of more highly chlorinated PCBs were greater with Acinetobacter strain P6. The mineralization of BP was consistent with kinetic models based upon linear-no growth and exponential growth; lower cell densities (<106/g) of BP-oxidizing bacteria gave a better fit for exponential growth, whereas the highest cell density (109/g) gave a better fit for linear-no growth. The numbers of BP-oxidizing bacteria declined exponentially upon depletion of the substrate. Since the mineralization of the chlorinated cometabolites was brought about by microorganisms (commensals) other than BP oxidizers, 14CO2 production could not be fit to either of the two growth models. However, 14CO2 production from the highest-density inoculum could be fit to a first-order (no-growth) sequential-reaction series. Although the population dynamics of the commensals could not be determined, the rate-limiting step in the cometabolic-commensal metabolism of PCBs to CO2 had to be the initial oxidation, since the rate of 14CO2 production was directly related to the population density of BP oxidizers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Ahmed M., Focht D. D. Oxidation of polychlorinated biphenyls by achromobacter pCB. Bull Environ Contam Toxicol. 1973 Aug;10(2):70–72. doi: 10.1007/BF01685874. [DOI] [PubMed] [Google Scholar]
- Belser L. W., Mays E. L. Use of nitrifier activity measurements to estimate the efficiency of viable nitrifier counts in soils and sediments. Appl Environ Microbiol. 1982 Apr;43(4):945–948. doi: 10.1128/aem.43.4.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner W., Focht D. D. Deterministic three-half-order kinetic model for microbial degradation of added carbon substrates in soil. Appl Environ Microbiol. 1984 Jan;47(1):167–172. doi: 10.1128/aem.47.1.167-172.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D. K., Kilbane J. J., Chakrabarty A. M. Biodegradation of 2,4,5-trichlorophenoxyacetic acid in soil by a pure culture of Pseudomonas cepacia. Appl Environ Microbiol. 1982 Aug;44(2):514–516. doi: 10.1128/aem.44.2.514-516.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- Focht D. D., Alexander M. Aerobic cometabolism of DDT analogues by Hydrogenomonas sp. J Agric Food Chem. 1971 Jan-Feb;19(1):20–22. doi: 10.1021/jf60173a042. [DOI] [PubMed] [Google Scholar]
- Francis A. J., Spanggord R. J., Ouchi G. I., Bramhall R., Bohonos N. Metabolism of DDT analogues by a Pseudomonas sp. Appl Environ Microbiol. 1976 Aug;32(2):213–216. doi: 10.1128/aem.32.2.213-216.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Metabolic breakdown of Kaneclors (polychlorobiphenyls) and their products by Acinetobacter sp. Appl Environ Microbiol. 1983 Jul;46(1):140–145. doi: 10.1128/aem.46.1.140-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Maier W. J. Kinetics of microbial growth on pentachlorophenol. Appl Environ Microbiol. 1985 Jan;49(1):46–53. doi: 10.1128/aem.49.1.46-53.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Favre J. S., Focht D. D. Conservation in soil of h(2) liberated from n(2) fixation by hup nodules. Appl Environ Microbiol. 1983 Aug;46(2):304–311. doi: 10.1128/aem.46.2.304-311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massé R., Messier F., Péloquin L., Ayotte C., Sylvestre M. Microbial biodegradation of 4-chlorobiphenyl, a model compound of chlorinated biphenyls. Appl Environ Microbiol. 1984 May;47(5):947–951. doi: 10.1128/aem.47.5.947-951.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J. Energy yields and growth of heterotrophs. Annu Rev Microbiol. 1970;24:17–52. doi: 10.1146/annurev.mi.24.100170.000313. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Appl Environ Microbiol. 1984 Feb;47(2):395–402. doi: 10.1128/aem.47.2.395-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. A., Tiedje J. M. Nonlinear estimation of Monod growth kinetic parameters from a single substrate depletion curve. Appl Environ Microbiol. 1983 May;45(5):1453–1458. doi: 10.1128/aem.45.5.1453-1458.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


