Abstract
Background
Daily evaluation of multiple organ dysfunction syndrome has been performed in critically ill adults. We evaluated the clinical course of multiple organ dysfunction over time in critically ill children using the Pediatric Logistic Organ Dysfunction (PELOD) score and determined the optimal days for measuring scores.
Methods
We prospectively measured daily PELOD scores and calculated the change in scores over time for 1806 consecutive patients admitted to seven pediatric intensive care units (PICUs) between September 1998 and February 2000. To study the relationship between daily scores and mortality in the PICU, we evaluated changes in daily scores during the first four days; the mean rate of change in scores during the entire PICU stay between survivors and nonsurvivors; and Cox survival analyses using a change in PELOD score as a time-dependent covariate to determine the optimal days for measuring daily scores.
Results
The overall mortality among the 1806 patients was 6.4%. A high PELOD score (≥ 20 points) on day 1 was associated with an odds ratio (OR) for death of 40.7 (95% confidence interval [CI] 20.3–81.4); a medium score (10–19 points) on day 1 was associated with an OR for death of 4.2 (95% CI 2.0–8.7). Mortality was 50% when a high score on day 1 increased on day 2. The course of daily PELOD scores differed between survivors and nonsurvivors. A set of seven days (days 1, 2, 5, 8, 12, 16 and 18) was identified as the optimal period for measurement of daily PELOD scores.
Interpretation
PELOD scores indicating a worsening condition or no improvement over time were indicators of a poor prognosis in the PICU. A set of seven days for measurement of the PELOD score during the PICU stay provided optimal information on the progression of multiple-organ dysfunction syndrome in critically ill children.
Almost all patients in intensive care units (ICUs) have some organ dysfunction.1–4 Adult and pediatric studies have shown that mortality increases with the number of organs involved.2,4,5 Thus, multiple-organ dysfunction syndrome (dysfunction involving two or more organs) has been viewed as the inexorable pathway to death.6 Primary multiple-organ dysfunction syndrome (present at admission or occurring within the first week after admission to the ICU) accounts for 88% of children with the syndrome; secondary multiple-organ dysfunction syndrome is less common (12%) but is associated with higher morbidity and mortality.7
Organ dysfunction scores were first developed for use in critically ill adults to describe and quantify the severity of organ dysfunction, not to predict mortality. Two scores have been proposed for critically ill children: the Pediatric Logistic Organ Dysfunction (PELOD) score and the Pediatric Multiple Organ Dysfunction Score (P-MODS).8–10 These scores quantify organ dysfunction precisely and can be used as indicators of the severity of illness throughout the clinical course. They can also be used as baseline and outcome measures in clinical studies conducted in ICUs11,12 and pediatric ICUs (PICUs).13
The PELOD score calculated with data collected over the entire PICU stay has been validated (using the most abnormal value of each variable during the entire PICU stay).10 However, the PELOD score over the entire PICU stay cannot be calculated before discharge from the unit; therefore, it cannot be used to characterize and follow the severity of organ dysfunction on a daily basis. Measurements repeated daily may provide more useful information.14 The optimal period for measuring daily scores for multiple organ dysfunction in adults has been studied.15–17 Indeed, trends in the Sequential Organ Failure Assessment score over the first 48 hours in the ICU was found to be a sensitive indicator of outcome, with decreasing scores associated with a decrease in mortality from 50% to 27%.17 Similar data for critically ill children are lacking.
We conducted this study to describe the clinical course of multiple organ dysfunction over time as measured by the daily PELOD score. Because the time and effort necessary to ensure accurate daily assessments and data entry can be substantial,18 we also aimed to determine the optimal days for measuring daily scores during the PICU stay.
Methods
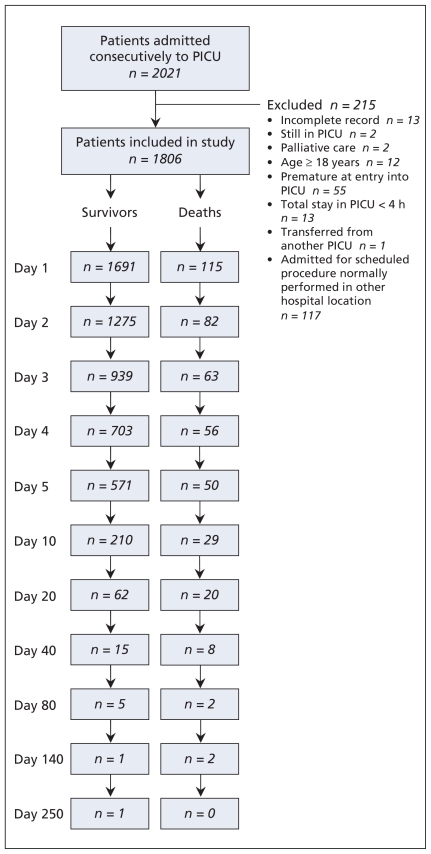
We included all 2021 consecutive patients admitted to seven multidisciplinary, tertiary care PICUs of university-affiliated hospitals (three in Canada, two in France and two in Switzerland) between September 1998 and February 2000. We excluded 215 patients for reasons described in Figure 1. The ethics committee of each participating hospital approved the study design.
Figure 1.
Selection of critically ill children for the study population. PICU = pediatric intensive care unit.
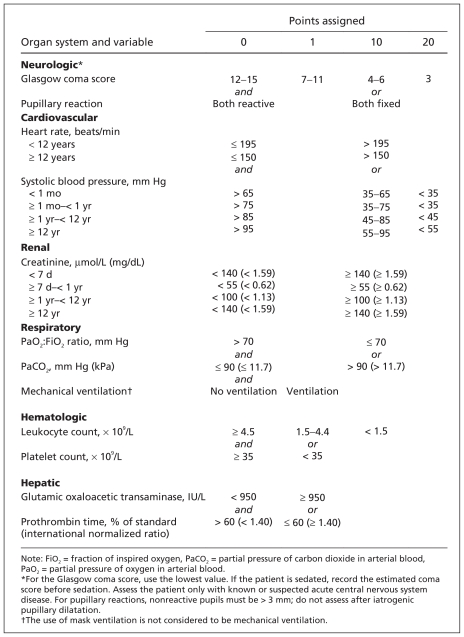
We collected data on the remaining 1806 patients’ baseline characteristics and their length of stay in the PICU. We calculated daily PELOD scores. For the PELOD score, six organ systems (neurologic, cardiovascular, renal, respiratory, hematologic and hepatic) are considered, each with up to 3 variables (total 12 variables). Each variable is assigned points (0, 1, 10 or 20) based on the level of severity (Figure 2). Levels of severity and relative weights of each organ dysfunction were determined by means of logistic regression.10 For each variable, the most abnormal value of each day was used to calculate the daily PELOD score. Variables were measured only if requested by the attending physician; if a variable was not measured, it was assumed to be identical to the previous measurement or normal.10
Figure 2.
Calculation of the daily PELOD (Pediatric Logistic Organ Dysfunction) score. Each organ dysfunction receives points for the variable associated with the highest points. For example, if the worst heart rate of the day was 200 beats/min (10 points) and the systolic blood pressure remained at 30 mm Hg (20 points), then 20 points is assigned. When a variable is measured more than once in the 24 hours, the most severe value is used in calculating the score. The maximum number of points for an organ is 20, and the maximum daily PELOD score is 71. Adapted, with permission, from Leteurtre et al9 (Copyright © 1999 SAGE Publications).
The primary outcome was the patient’s vital status at discharge from the PICU.
Statistical analysis
Results are expressed as frequencies and percentages for categorical variables and as medians and interquartile ranges (IQRs) for numeric variables. A p value of less than 0.05 was considered statistically significant.
We investigated the relationship between the PELOD score on day 1 and outcome (survival v. death) using logistic regression analysis. Thereafter, we used three analytical strategies to study the relationship between daily PELOD scores and mortality: (a) changes in daily PELOD scores during the first four days; (b) the mean rate of change in PELOD scores during the entire PICU stay between survivors and nonsurvivors; and (c) survival analyses (Cox model), with the change in daily PELOD score from day 1 as a time-dependent covariate, to determine the optimal period for measuring daily PELOD scores. In the second and third strategies, we analyzed the entire PICU stay and adjusted for centre (the 7 participating sites were considered a possible confounding variable). The statistical analyses are described in detail in Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.081715/DC1).
Results
The median age of the 1806 patients was 24 months (IQR 5–90). Characteristics of the study population are given in Table 1. The median and mean PELOD scores differed between the seven PICUs (median range 10–11, mean range 8–14; both p < 0.001).
Table 1.
Characteristics of 1806 critically ill children admitted to pediatric intensive care units
| Characteristic | No. (%) of patients*n = 1806 |
|---|---|
| Age, mo, median (IQR) | 24 (5–90) |
| Age group | |
| < 28 d (neonates) | 171 (9.5) |
| 1 mo–< 1 yr (infants) | 525 (29.1) |
| 1– < 12 yr (children) | 853 (47.2) |
| ≥ 12 yr (adolescents) | 257 (14.2) |
| Male:female ratio | 1.2 |
| Surgical patients | 882 (48.8) |
| Mechanical ventilation required | 921 (51.0) |
| PRISM score, median (IQR) | 6 (2–10) |
| Organ system of primary dysfunction on admission | |
| Cardiovascular | 485 (26.9) |
| Neurologic | 335 (18.6) |
| Respiratory | 631 (34.9) |
| Hepatic | 34 (1.9) |
| Genitourinary | 35 (1.9) |
| Gastrointestinal | 91 (5.0) |
| Endocrine | 22 (1.2) |
| Musculoskeletal | 68 (3.8) |
| Hematologic | 24 (1.3) |
| Other/undetermined | 81 (4.5) |
| Primary category of illness on admission | |
| Infection | 439 (24.3) |
| Trauma | 175 (9.7) |
| Congenital disease | 663 (36.7) |
| Chemical injury | 25 (1.4) |
| Drug | 12 (0.7) |
| Cancer | 60 (3.3) |
| Diabetes | 18 (1.0) |
| Allergic or immunologic disease | 42 (2.3) |
| Other/undetermined | 372 (20.6) |
| No. who died in PICU | 115 (6.4) |
| Length of stay in PICU, d, median (IQR) | 3 (2–6) |
Note: IQR = interquartile range, PICU = pediatric intensive care unit, PRISM = pediatric risk of mortality.
Unless stated otherwise.
Daily PELOD scores were measured on a total of 10 361 days. The median and mean values for days 1 to 7, day 14 and day 21 among survivors and nonsurvivors are given in Table 2. The maximum daily score occurred on average on day 3 (median day 2 [IQR 1–3]). The maximum daily score differed between nonsurvivors and survivors (nonsurvivors: mean 8.6, median 2 [IQR 1–7] v. survivors: mean 2.6, median 2 [IQR 1–3]; p < 0.001).
Table 2.
Daily PELOD scores among critically ill children admitted to pediatric intensive care units*
| Day | Patients; PELOD score |
|||
|---|---|---|---|---|
| Nonsurvivors |
Survivors |
|||
| Mean | Median (IQR) | Mean | Median (IQR) | |
| 1 | 22.7* | 21 (11–31) | 6.9 | 2 (0–11) |
| 2 | 20.3* | 21 (11–31) | 6.2 | 2 (0–11) |
| 3 | 16.8* | 13 (10–22) | 6.1 | 1 (0–11) |
| 4 | 15.6* | 12 (6–21) | 5.7 | 1 (0–11) |
| 5 | 14.5* | 12 (10–21) | 5.4 | 1 (0–10) |
| 6 | 14.9* | 11 (10–22) | 5.5 | 1 (0–11) |
| 7 | 13.1* | 11 (1–21) | 5.6 | 1 (0–11) |
| 14 | 11.1* | 11 (1–21) | 5.5 | 1 (0–10) |
| 21 | 11.7* | 11 (2–21) | 5.6 | 1 (0–10) |
Note: IQR = interquartile range, PELOD score = Pediatric Logistic Organ Dysfunction score.
p < 0.001, Wilcoxon two-sample test (survivors v. nonsurvivors).
Of the 499 children without any organ dysfunction on day 1, 113 (22.6%) had dysfunction of one or more organs during their PICU stay; the mortality was 4.4% among these children, as compared with 0.2% among the 386 who did not have organ dysfunction during their stay (p = 0.003). Among the 1042 patients without multiple organ dysfunction syndrome on day 1, 135 (13.0%) acquired the syndrome during their PICU stay; the mortality was 8.9% among these children, as compared with 0.6% among the 907 who did not acquire the syndrome during their stay (p < 0.001). On day 1, multiple organ dysfunction syndrome was present in 764 (42.3%) of the patients. The syndrome worsened during the PICU stay in 133 (17.4%) and remained unchanged or improved in 631 (82.6%); the mortality was 25.6% among those in whom it worsened and 10.1% among the other children; p < 0.001). New or progressive multiple organ dysfunction syndrome was reported in 899 (48.9%) of the patients.
PELOD score on day 1 and mortality
Logistic regression analysis showed that the PELOD score on day 1 was a significant prognostic factor (odds ratio [OR] per point 1.16, 95% confidence interval [CI] 1.13–1.18, after adjustment for centre). The analysis of the distribution of the day 1 scores identified three groups of scores (low [< 10 points], medium [10–19 points] and high [≥ 20 points]), with cutoff values of 10 and 20 points associated with increasing mortality (Table 3). The OR for death among children with a medium PELOD score on day 1 was 4.2 (95% CI 2.0–8.7); the OR among those with a high score on day 1 was 40.7 (95% CI 20.3–81.4). These findings show that the PELOD score on day 1 was an important predictor of the patients’ outcome.
Table 3.
Mortality associated with PELOD score on day 1 and change in score from day 1 to day 2
| Change in score from day 1 to day 2 | PELOD score on day 1 |
|||||
|---|---|---|---|---|---|---|
| Low (< 10 points) |
Medium (10–19 points) |
High (≥ 20 points) |
||||
| No. of deaths, n/N | Mortality, % (95% CI) | No. of deaths, n/N | Mortality, % (95% CI) | No. of deaths, n/N | Mortality, % (95% CI) | |
| Baseline (day 1) | 10/887 | 1.1* (0.5–2.1) | 31/660 | 4.7 (3.2–6.6) | 74/259 | 28.6 (23.2–34.5) |
| Increase | 6/167 | 3.6 (1.3–7.7) | 7/53 | 13.2 (5.5–25.3) | 19/38 | 50.0 (33.4–66.6) |
| No change | 2/336 | 0.6 (0.1–2.1) | 12/219 | 5.5 (2.7–9.4) | 11/51 | 21.6 (11.3–35.3) |
| Decrease | 1/122 | 0.8 (0.0–4.5) | 8/245 | 3.3 (1.4–6.3) | 16/126 | 12.7 (7.4–19.8) |
Note: CI = confidence interval, PELOD score = Pediatric Logistic Organ Dysfunction score.
For the 10 patients with a low PELOD score on day 1 who died, the underlying disease on admission was chronic cardiac disease (n = 7), chronic respiratory disease (n = 1), immunodeficiency (n = 1) and acute hepatic failure (n = 1).
Changes in daily scores during the first four days
Our analysis of trends in daily PELOD scores during the first four days in PICU are shown in Table 3 and in Table e1 of Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.081715/DC1). After adjustment for the day 1 score, the mortality was 50% (19/38) among children with a high score on day 1 when the score increased on day 2 (Table 3). Without adjustment for the day 1 score (owing to the great number of categories), the mortality was 50% (7/14) when there was an increase in score on day 2 and when the score increased from day 2 to day 4; the mortality was 7% (49/745) in other situations (p < 0.001) (Appendix 1, Table e1). Increasing daily PELOD scores during the first four days in the PICU indicated a poor prognosis (Appendix 1, Table e1).
Mean rate of change in PELOD score during the entire PICU stay
The changes in daily PELOD score over time in the three score groups (low, medium and high day 1 scores) differed between survivors and nonsurvivors. In all three groups, the difference in daily score between survivors and nonsurvivors increased over time (see Figure e1 in Appendix 1, available at www.cmaj.ca/cgi/content/full/cmaj.081715/DC1). In the group of patients with a low PELOD score on day 1 (< 10 points), the mean rate of change in daily score was 0 points per day among survivors and 0.3 points per day among non-survivors (p < 0.001). The corresponding mean rate of change among patients with a medium score on day 1 (10–19 points) was −0.5 points per day among survivors and 0 points per day among nonsurvivors (p < 0.001); among those with a high score on day 1 (≥ 20 points), it was −0.9 points per day among survivors and −0.5 points per day among nonsurvivors (p < 0.001). These findings show that even small increases in the PELOD score and a lack of improvement in score were worrisome signs.
Optimal days for measuring daily scores
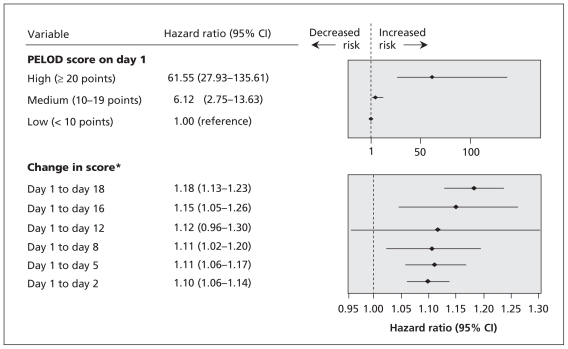
Graphic distributions of the median PELOD scores over time are shown in Figures e2 and e3 in Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.081715/DC1). A set of seven days was found to be associated with the highest hazard ratios for death: days 1, 2, 5, 8, 12, 16 and 18 (Figure 3).
Figure 3.
Serial evaluation of the change in the daily PELOD (Pediatric Logistic Organ Dysfunction) score from day 1, adjusted for baseline value (PELOD score on day 1). *Hazard ratio (HR) of death and 95% confidence interval (CI) are reported per point increase in the change in score. The cumulative HR of death was calculated as follows: (HR of PELOD score on day 1) × (HR of change in score from day 1 to specified day). For example, for a child whose score is 23 on day 1 and 13 on day 5, the change in score is −10; the HR for death would be 21.48 [61.55 × (1.11−10) = 61.55 × 0.35]. For a child whose score is 12 on day 1 and 32 on day 16, the change in score is 20; in this instance, the HR for death would be 98.37 [6.12 × (1.1520) = 6.12 × 16.08].
Interpretation
We found that the PELOD score on day 1 was a significant prognostic factor of death. In the three groups of patients stratified by low (< 10 points), medium (10–19 points) and high (≥ 20 points) PELOD score on day 1, the change in the severity of organ dysfunction over time differed between survivors and nonsurvivors: not just an increasing score, but also a score showing no improvement, was an adverse prognostic factor. Moreover, regardless of what the initial PELOD score was on day 1, an increase in the score from day 1 to day 2 and from day 2 to day 4 was associated with a mortality of 50%. Finally, we found that a set of seven days for daily measurement of the PELOD score provided optimal information on the progression of multiple organ dysfunction syndrome during the stay in the PICU.
Analyses of the trends of multiple organ dysfunction syndrome over time have been performed with several methodologic strategies and different times of data collection in adult patients.10,14–17,19,20 Many studies have shown that daily monitoring of organ dysfunction can be useful for estimating the response to therapy.21–23 We believe that the model proposed in our study — to measure the PELOD score on days 1, 2, 5, 8, 12, 16 and 18 — provides the best balance between the workload of assessing daily scores and the optimal association with prognosis throughout the PICU stay. Findings from studies involving critically ill adults support such an approach. In a study by Timsit and colleagues, the severity of organ dysfunction on any given day during the first week in ICU was an important predictor of in-hospital mortality.24 In a study by Wagner and associates, the acute physiology score on a day in the first week in ICU contributed to 54% of the prediction of in-hospital mortality, as compared with only 5% for the acute physiology score at admission.25 Moreover, in studies involving patients with long ICU stays, severity scores at admission failed to predict mortality.24,26 The late events could not be predicted with scores at admission or on the first day. This suggests that, for patients with prolonged stays in the ICU, the calculation of scores on later days may be more useful.
Rates of death have been reported to be higher among critically ill adults (> 20%)17,27 than among critically ill children (4%–5%).8,28 This may explain why a mortality of at least 50% was associated with an increase in the Sequential Organ Failure Assessment score among adult patients during the first two days in ICU17 and, in our study, with an increase in the PELOD score during the first four days in PICU (occurring in only 1.8% of our sample). Because the length of stay in a PICU is usually short (median three days in our study), we converted time into discrete day intervals to determine the ideal sequence of measurement of daily PELOD scores. Cook and associates converted time into discrete week intervals.15
Measurement of the severity of multiple organ dysfunction syndrome is needed to describe the clinical course of groups of critically ill children.13 Our study showed that the progression of the daily PELOD score provided prognostic information in addition to the highest PELOD score for the entire PICU stay. These data support the concept that the PELOD score and its progression in the PICU can be an outcome measure of interest. Measurement of organ dysfunction with scores such as the PELOD can be useful both for administrative PICU management29–31 and as a secondary outcome measure of studies conducted in the ICU,12,32 particularly the PICU,21,33 where the death rate is low. Two recent examples can be given. Among critically ill children, a restrictive transfusion strategy with a hemoglobin threshold of 7 g/dL, compared with a liberal transfusion strategy, resulted in a 96% decrease in the number of patients who had any transfusion exposure and a 44% decrease in the number of units of red blood cells transfused; these results were not associated with increased rates of new or progressive multiple organ dysfunction syndrome or increasing daily PELOD scores.21
The second example concerns the H1N1 influenza pandemic. With scarce resources, the change in PELOD score over several days may be of help to policy-makers faced the decision of triaging care between patients with severe H1N1 influenza. In such circumstances, medical policy can be informed by PELOD scores. Similar use of the Sequential Organ Failure Assessment score as an initial, 48-hour and 120-hour triage tool for critical care of adults during an influenza pandemic has been proposed.29,34
Strengths and limitations
The strengths of our study was that it was prospective and conducted in seven PICUs over three countries. Also, we included a large number of critically ill children whose combined stay in the PICUs was more than 10 000 consecutive days. We used three different statistical methods to analyze the changes in daily PELOD score, all of which gave consistent results.
Our study is not without limitations. First, we monitored only mortality in the PICU and have no data for patients discharged from the PICU. However, the number of children who die in hospital after discharge from the PICU is small (0.9% in the study by Kanter and colleagues35). Second, the number of deaths among the 338 patients who stayed in the PICU longer than seven days was low (n = 33). The validity of the daily PELOD scores after one week in the PICU (on days 8, 12, 16 and 18) should be validated in future prospective studies involving more critically ill children with long-term stays.
Conclusion
Measuring changes in organ dysfunction over time is important. First, a daily PELOD score that shows a worsening condition or no improvement over time is a strong prognostic factor for death. This information may be helpful at the bedside, particularly within the first four days in the patient’s course. Second, changes in the PELOD score describe the patterns and trajectories of multiple organ dysfunction over time. Such changes can also be used as a marker of severity of illness in clinical studies. We found that a set of seven days for the measurement of daily PELOD scores provided optimal information on the progression of multiple organ dysfunction syndrome during the stay in the PICU. This information could be used for epidemiologic and administrative purposes.
Supplementary Material
Acknowledgement
These data were presented in part at the 5th World Congress on Pediatric Critical Care, June 24–28, 2007, in Geneva, Switzerland.
Footnotes
Funding: This study was supported by the Programme Hospitalier de Recherche Clinique 1997 of the French Health Ministry. The funders had no role in the design or conduct of the study, the collection, management, analysis or interpretation of the data, or the preparation, review or approval of the manuscript. The researchers are independent from the funders.
Previously published at www.cmaj.ca
Competing interests: None declared.
Contributors: Stéphane Leteurtre, Francis Leclerc and Bruno Grandbastien contributed to the study concept and design. Stéphane Leteurtre, François Proulx, Jacques Cotting, Ronald Gottesman, Ari Joffe, Bendicht Wagner and Philippe Hubert contributed to the acquisition of the data. Stéphane Leteurtre, Alain Duhamel, Bruno Grandbastien, Jacques Lacroix, Francis Leclerc and Alain Martinot contributed to the analysis and interpretation of the data. Stéphane Leteurtre, Francis Leclerc, Jacques Lacroix and Alain Duhamel drafted the manuscript. All of the authors critically revised the manuscript for important intellectual content and approved the final version submitted for publication.
This article has been peer reviewed.
REFERENCES
- 1.Cengiz P, Zimmerman JJ. Prelude to pediatric multiple organ dysfunction syndrome: the golden hours concept revisited. Pediatr Crit Care Med. 2003;4:263–4. doi: 10.1097/01.PCC.0000059730.90082.75. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC. Modeling MODS: What can be learned from animal models of the multiple-organ dysfunction syndrome? Intensive Care Med. 2005;31:605–8. doi: 10.1007/s00134-005-2595-3. [DOI] [PubMed] [Google Scholar]
- 3.Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994;22:1025–31. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson JD, Pollack MM, Glass NL, et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987;111:324–8. doi: 10.1016/s0022-3476(87)80448-1. [DOI] [PubMed] [Google Scholar]
- 5.Tantalean JA, Leon RJ, Santos AA, et al. Multiple organ dysfunction syndrome in children. Pediatr Crit Care Med. 2003;4:181–5. doi: 10.1097/01.PCC.0000059421.13161.88. [DOI] [PubMed] [Google Scholar]
- 6.Proulx F, Joyal JS, Mariscalco MM, et al. The pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2009;10:12–22. doi: 10.1097/PCC.0b013e31819370a9. [DOI] [PubMed] [Google Scholar]
- 7.Proulx F, Fayon M, Farrell CA, et al. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–7. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 8.Graciano AL, Balko JA, Rahn DS, et al. The Pediatric Multiple Organ Dysfunction Score (P-MODS): development and validation of an objective scale to measure the severity of multiple organ dysfunction in critically ill children. Crit Care Med. 2005;33:1484–91. doi: 10.1097/01.ccm.0000170943.23633.47. [DOI] [PubMed] [Google Scholar]
- 9.Leteurtre S, Martinot A, Duhamel A, et al. Development of a pediatric multiple organ dysfunction score: use of two strategies. Med Decis Making. 1999;19:399–410. doi: 10.1177/0272989X9901900408. [DOI] [PubMed] [Google Scholar]
- 10.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–7. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 11.Marshall JC. Organ dysfunction as an outcome measure in clinical trials. Eur J Surg Suppl. 1999;584:62–7. doi: 10.1080/11024159950188583. [DOI] [PubMed] [Google Scholar]
- 12.Petros AJ, Marshall JC, van Saene HK. Should morbidity replace mortality as an endpoint for clinical trials in intensive care? Lancet. 1995;345:369–71. doi: 10.1016/s0140-6736(95)90347-x. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix J, Cotting J. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med. 2005;6:S126–34. doi: 10.1097/01.PCC.0000161287.61028.D4. [DOI] [PubMed] [Google Scholar]
- 14.Cappi SB, Sakr Y, Vincent JL. Daily evaluation of organ function during renal replacement therapy in intensive care unit patients with acute renal failure. J Crit Care. 2006;21:179–83. doi: 10.1016/j.jcrc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Cook R, Cook D, Tilley J, et al. Multiple organ dysfunction: baseline and serial component scores. Crit Care Med. 2001;29:2046–50. doi: 10.1097/00003246-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Doig CJ, Zygun DA, Fick GH, et al. Study of clinical course of organ dysfunction in intensive care. Crit Care Med. 2004;32:384–90. doi: 10.1097/01.CCM.0000108881.14082.10. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 18.Briskin A, Hladunewich M. What’s my mother’s SOFA today, doc? Commentary on: daily evaluation of organ function during renal replacement therapy in ICU patients with acute renal failure. J Crit Care. 2006;21:183–4. doi: 10.1016/j.jcrc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Kilic YA, Yorganci K, Sayek I. Visualizing multiple organ failure: a method for analyzing temporal and dynamic relations between failing systems and interventions. Crit Care. 2007;11:417. doi: 10.1186/cc5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno R, Vincent JL, Matos R, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25:686–96. doi: 10.1007/s001340050931. [DOI] [PubMed] [Google Scholar]
- 21.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 22.Rivera-Fernandez R, Nap R, Vazquez-Mata G, et al. Analysis of physiologic alterations in intensive care unit patients and their relationship with mortality. J Crit Care. 2007;22:120–8. doi: 10.1016/j.jcrc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 24.Timsit JF, Fosse JP, Troche G, et al. Calibration and discrimination by daily Logistic Organ Dysfunction scoring comparatively with daily Sequential Organ Failure Assessment scoring for predicting hospital mortality in critically ill patients. Crit Care Med. 2002;30:2003–13. doi: 10.1097/00003246-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Wagner DP, Knaus WA, Harrell FE, et al. Daily prognostic estimates for critically ill adults in intensive care units: results from a prospective, multicenter, inception cohort analysis. Crit Care Med. 1994;22:1359–72. doi: 10.1097/00003246-199409000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ferraris VA, Propp ME. Outcome in critical care patients: a multivariate study. Crit Care Med. 1992;20:967–76. doi: 10.1097/00003246-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Slater A, Shann F. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5:447–54. doi: 10.1097/01.PCC.0000138557.31831.65. [DOI] [PubMed] [Google Scholar]
- 29.Christian MD, Hawryluck L, Wax RS, et al. Development of a triage protocol for critical care during an influenza pandemic. CMAJ. 2006;175:1377–81. doi: 10.1503/cmaj.060911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent JL, Ferreira FL. Evaluation of organ failure: we are making progress. Intensive Care Med. 2000;26:1023–4. doi: 10.1007/s001340051313. [DOI] [PubMed] [Google Scholar]
- 31.Welke KF, Karamlou T, Diggs BS. Databases for assessing the outcomes of the treatment of patients with congenital and paediatric cardiac disease — a comparison of administrative and clinical data. Cardiol Young. 2008;18(Suppl 2):137–44. doi: 10.1017/S1047951108002837. [DOI] [PubMed] [Google Scholar]
- 32.Marshall JC. Measuring organ dysfunction in the intensive care unit: Why and how? Can J Anaesth. 2005;52:224–30. doi: 10.1007/BF03016054. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen TC, Han YY, Kiss JE, et al. Intensive plasma exchange increases a disintegrin and metalloprotease with thrombospondin motifs-13 activity and reverses organ dysfunction in children with thrombocytopenia-associated multiple organ failure. Crit Care Med. 2008;36:2878–87. doi: 10.1097/ccm.0b013e318186aa49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebert PC, MacDonald N. Preparing for pandemic (H1N1) 2009. CMAJ. 2009;181:E102–5. doi: 10.1503/cmaj.091545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanter RK. Post-intensive care unit pediatric hospital stay and estimated costs. Crit Care Med. 2000;28:220–3. doi: 10.1097/00003246-200001000-00036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.