Abstract
B lymphocytes play roles in many autoimmune diseases characterized by unresolved inflammation, and B cell ablation is proving to be a relatively safe, effective treatment for such diseases. B cells function, in part, as important sources of regulatory cytokines in autoimmune disease, but B cell cytokines also play roles in other non-autoimmune inflammatory diseases. B cell ablation may therefore benefit inflammatory disease patients in addition to its demonstrated efficacy in autoimmune disease. Current ablation drugs clear both pro- and anti-inflammatory B cell subsets, which may unexpectedly exacerbate some pathologies. This possibility argues that a more thorough understanding of B cell function in human inflammatory disease is required to safely harness the clinical promise of B cell ablation. Type 2 diabetes (T2D) and periodontal disease (PD) are two inflammatory diseases characterized by little autoimmunity. These diseases are linked by coincident presentation and alterations in Toll-like receptor (TLR)-dependent B cell cytokine production, which may identify B cell ablation as a new therapy for co-affected individuals. Further analysis of the role B cells and B cell cytokines play in T2D, PD and other inflammatory diseases is required to justify testing B cell depletion therapies on a broader range of patients.
Keywords: Human B cells, cytokines, chronic inflammation, periodontal disease, type 2 diabetes, B cell depletion, rituximab
1. B cell depletion as an important therapy for an array of inflammatory diseases
Inflammation is a common underlying condition in many chronic diseases including autoimmune diseases like multiple sclerosis (MS), Crohn’s disease (CD), and type 1 diabetes (T1D). Inflammation also plays important roles in diseases that lack significant autoimmune components, including diseases characterized by inappropriate innate immune responses, such as type 2 diabetes (T2D) and periodontal disease (PD). The dominance of B cells in some of these inflammatory diseases, including lupus and rheumatoid arthritis, has been clinically exploited due to the availability and general safety of the B cell depletion drug rituximab [1–3]. However, the ability of B cell depletion to induce remission of diseases more traditionally associated with T cell-mediated autoimmunity, such as MS [4, 5], suggests that even sub-dominant contributions of B cells can significantly alter disease pathogenesis. Although B cell depletion has promoted inflammatory disease in a small minority of case reports [6, 7], the overall acceptable safety profile of B cell depletion suggests that a wider array of inflammatory disease patients may benefit from this treatment [1] if timing and duration of treatment are properly controlled. Second generation B cell depletion drugs are under development that may benefit an even wider array of patients with B cell-influenced inflammatory disease [8]. The obvious first step towards more comprehensively identifying inflammatory disease patients that might benefit from B cell depletion therapy is to more broadly identify the role B cells play in human inflammatory disease. This review will focus on the role B cells play in two clinically linked inflammatory diseases, T2D and PD, towards exploring the use of B cell depletion in a broader range of pathologies.
2. Clinical linkage between type 2 diabetes and periodontal disease is associated with inflammation
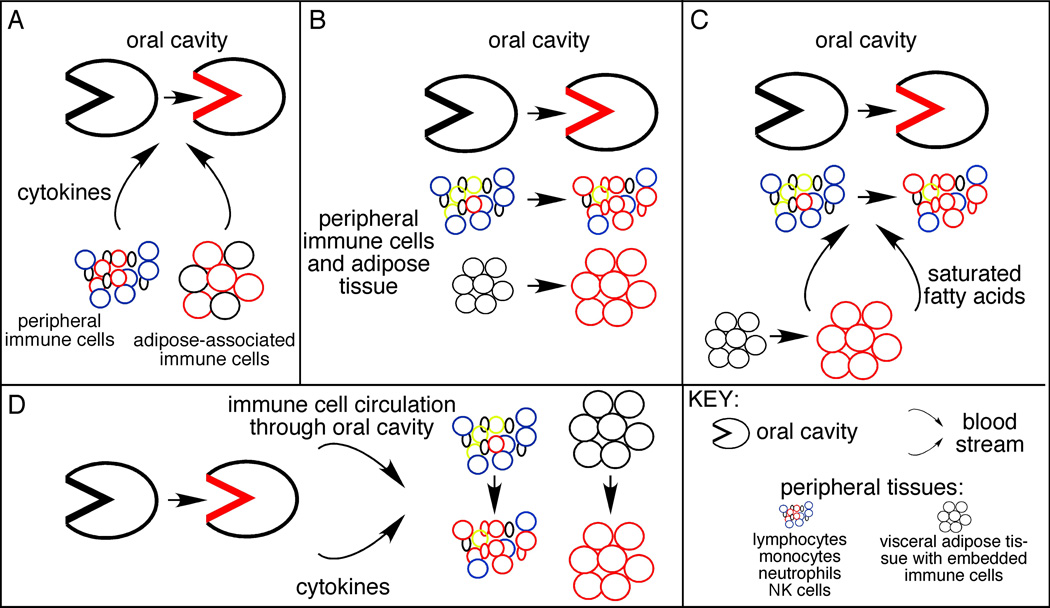
Unlike CD and other inflammatory diseases that occur in relative isolation, the link between T2D and PD is strong [9–11]. Foremost, the association of T2D and PD is supported by clinical evidence that shows about a 3-fold increased incidence of PD in T2D patients [12]. Multiple studies indicate that this linkage is largely due to the inflammation that precedes onset and promotes ongoing pathogenesis of both diseases. Several mechanisms may explain how inflammation in T2D patients promotes PD (Fig. 1). First, inflammation can originate from peripheral blood and adipose-associated monocytes or macrophages, respectively, from T2D patients that hyper-produce multiple pro-inflammatory cytokines [13–18] thus promote systemic inflammation (elevated serum IL-6, IL-8 and CRP; Fig. 1A). In turn, elevated systemic cytokine levels probably promote changes in the oral cavity that precipitate PD [19–22]. An alternative hypothesis from mouse T2D models is that T2D leads to suppression of pro-inflammatory innate immune receptor function. T2D thus blunts the immune response that normally prevents chronic PD in this model [23]. These findings remain to be supported by data from T2D patients. Second, the inflammatory response to challenge with PD-associated bacteria is elevated in a murine model of T2D, and involves hyper-production of proinflammatory cytokines [24]. These data suggest that an exaggerated oral inflammatory response that characterizes PD [25] may be a straight-forward parallel of the exaggerated systemic immune response that originates as a part of T2D pathology (Fig. 1B). Third, obesity independently promotes systemic inflammation in otherwise healthy individuals due to the generally pro-inflammatory action of altered lipolysis, adipose tissue expansion/inflammation and elevated saturated fatty acids in response to over nutrition [26–28]. Because obesity increases risk of both PD and T2D, the changes that originate due to dietary excesses may be the underpinning that promotes both diseases simultaneously despite temporal differences in diagnoses (Fig. 1C). More detailed analysis showed that insulin resistance in obesity patients further raised the odds that an individual had severe concomitant PD [29]. Taken together, these data suggest that underlying mechanisms explaining the relationship between T2D and PD are likely to involve inflammation, yet have additional components that fail to be explained by elevated serum pro-inflammatory cytokine levels or elevated BMI alone.
Figure 1.
Models explaining the clinically demonstrated association of T2D and PD. (A) Inflammation, mainly from immune system cells in the periphery and excess adipose tissue (in T2D patients) that secrete pro-inflammatory cytokines (and other mediators) to directly promote oral inflammation and the bone resorption characteristic of PD. The alternative, that T2D blunts the immune response to allow chronic infection with oral pathogens due to immune cell hypo-responsiveness, is not shown. Red indicates inflammatory cells/regions in all panels. (B) Metabolic and inflammatory changes occur concomitantly in the oral cavity, blood, and adipose tissue of T2D patients. Timing of T2D and PD diagnoses may differ either due to intrinsic differences in natural history of each disease, or due to differences in clinical diagnoses. Many endocrinologists and general practitioners who manage T2D patients do not focus on a PD diagnosis. (C) Alterations in adipose tissue metabolism elevates circulating pro-inflammatory molecules such as saturated fatty acids, which independently or interdependently activate inflammation in the oral cavity and periphery. (D) Unresolved bacterial infection in the oral cavity increases circulating levels of cytokines produced by resident oral cells, but also activates immune cells as they circulate through the gingiva. Because oral bacteria are also systemically elevated in PD patients (not shown), the bacteria may also activate immune cells at peripheral sites.
The ability of PD to predispose patients to T2D may also be at least partially rooted in elevated inflammation from the chronic oral infection. Inflammation in PD patients is not limited to the oral cavity (Fig. 1D), but instead corresponds to changes in systemic physiology [30–35] that may provide a pro-inflammatory milieu, for example a concomitant elevation of IL-1β and IL-6 that can predict the incidence of T2D [36]. Therefore PD inflammation, likely in the context of other less obvious disease-associated changes, also appears to promote T2D [37]. However, the ability of intensive PD management to moderate T2D severity, as measured by percentage glycated hemoglobin, is mixed [11]. This finding raises the possibility that T2D makes a dominant contribution in combination T2D/PD patients. Regardless, the relationship between inflammation from PD and T2D probably remains a “two-way street” with each disease confounding the other [21, 37]. Multiple lines of evidence suggest that lymphocytes, and especially B cells, may be important determinants of inflammatory diseases and may therefore moderate the complex relationship between T2D and PD. This review will focus predominantly on the role inflammation originating from the B cell compartment plays in T2D and PD towards identifying new ideas for treatments to alleviate both diseases in parallel.
3. Lymphocytes promote systemic inflammation through multiple mechanisms
Lymphocytes can contribute to systemic inflammation in T2D, PD and co-affected patients by multiple mechanisms. First, the uncontrolled oral flora in PD lesions locally activates lymphocytes [18, 38], which may then recirculate throughout the body, distributing inflammatory mediators along the way. Oral floral may also spread systemically [39, 40] to directly provide inflammatory signals to lymphocytes as they re-circulate through affected blood vessels.
Although there is no parallel chronic site of infection in T2D patients, inflammation is thought to initiate in the visceral adipose tissue due to changes in fat metabolism that occur in individuals with abnormally large fat depots due to, in most cases, over nutrition [41–43]. Interestingly, B cells, followed by closely by T cells, are the first immune system cells to infiltrate the expanding adipose tissue in response to high fat diet (HFD) in mice. In these experiments, the number of B cells is maximal 3 weeks following initiation of HFD, then falls as T cells then macrophages infiltrate [44]. These data are consistent with the possibility that B cells are activated by products of altered lipolysis in the expanding adipose tissue, then leave the adipose tissue to recirculate throughout the body. However, the possibility that B cells die by apoptosis in the expanding fat tissue has not been rigorously excluded. The second mechanism by which lymphocytes may contribute to systemic inflammation in PD and T2D may be direct secretion of soluble products such as cytokines and antibodies into the circulation regardless of the site of lymphocyte activation. The current literature does not distinguish between these two scenarios that could transition local inflammatory responses to systemic inflammation: cell migration vs. systemic distribution of inflammatory products. However, the strong link between T2D and PD suggests oral infection and systemic disease are joined by a positive feedback loop hinging on soluble products that are systemically distributed regardless of the location of the producing cell.
4. Roles for B cells in inflammatory disease
4.1: B cell antibodies are implicated in inflammatory disease
B cells are activated by a variety of ligands that engage an array of surface receptors to trigger B cell responses. Naïve B cells require a combination of ligands to achieve an activated phenotype. These ligands must engage a combination of surface immunoglobulin, the co-activator CD40, and a third signal, often provided by toll-like receptor (TLR) engagement [45]. Memory B cells, a subset set aside for rapid immune responses to subsequent antigen exposure, are more amenable to activation and can respond, at least in part, to any one of the three signals in isolation. Of these, the most intensively characterized B cell receptor is surface immunoglobulin. Immunoglobulins are highly specific receptors that trigger B cell proliferation only in response to specific ligands, known as “cognate antigens”. B cells also secrete soluble antibodies that bind cognate antigens independent of cell contact, thus facilitate soluble antigen clearance. Antibodies can also promote pathogen clearance by binding cognate antigen located on the pathogen surface. Antigen interacts selectively with a single immunoglobulin species in a “lock and key”-like mechanism that is responsible for the adaptive immune properties of B cells. Many inflammatory diseases, including PD, are characterized by the development of autoimmune antibodies, albeit at modest levels in many cases. Such antibodies interact with antigens in host molecules and thus trigger an immune response that destroys healthy tissue. Antibody-driven pathogenic responses can occur through ligation of Fc receptors on myeloid cells [46, 47]. Alternatively, immunoglobulin/antigen complexes activate complement that engages complement receptors on myeloid cells. Either scenario triggers myeloid cell activation thus cytokine release, inflammation, and tissue destruction [48]. Antibodies play roles in many autoimmune inflammatory diseases, including lupus and type 1 diabetes. Roles in non-autoimmune inflammatory diseases are not well defined, probably due to the overall lack of appreciation that B cells are key players in diseases initially defined by dominance of either the T cell or myeloid compartment.
4.2: The multiple roles of B cells in PD
PD is characterized by initial infiltration of the periodontal tissue with neutrophils, followed by monocytes, then lymphocytes, such that the vast majority of cells in periodontal lesions (>65%) are B lineage cells, including antibody-producing plasma cells [49–51]. The order of immune cell infiltration into the gingiva during PD etiology therefore differs from the order identified in adipose tissue from the diet-induced obese (DIO) mouse model of insulin resistance and T2D (see above). The percentage of B cells in chronic PD lesions positively correlates with disease severity [50, 52–54], suggesting B cells promote rather than initiate PD. The B cells in human PD lesions have a transitional or activated phenotype, as indicated by surface expression of the B cell activation marker CD5 [55–59]. More definitive evidence for a role of B cells in PD are studies in a rat model, which demonstrated that B cells are important for osteoclast formation and periodontal bone loss in response to a common periodontal pathogen, A. actinomycetemcomitans (A.a.) [60]. However, these results required in vivo priming of donor B cells with A.a., transfer of donor B cells to a recipient rat, then PD induction in the recipient rat via direct injection of formalin-killed A.a. into the gingival tissue. This approach therefore did not address whether in situ B cells play similarly important roles in PD.
B cells may promote PD through multiple mechanisms, including antibody production [61–63]. Auto-reactive antibodies in PD tissues and blood recognize collagen and other extra-cellular matrix proteins [64, 65], and may play roles in oral tissue destruction and the chronic systemic inflammation associated with PD. Alternatively, antibodies may play a protective role in PD, as indicated by experiments showing B cells decrease dissemination of anaerobic oral infections in mice, probably through protective immunoglobulin production [66, 67]. The role of B cell-derived antibodies in PD is therefore likely to be complex.
Based on the role cytokines play in PD, B cells are also likely to regulate PD through pro- and anti-inflammatory cytokine production. Human B cells constitutively and inducibly secrete PD-promoting cytokines such as TNF-α and IL-1β [68], and B cells from PD patients hyper-produce these and other cytokines in response to TLR engagement. Due to the numerical predominance of B cells in chronic PD lesions, it is likely that B cell cytokines are major sources of unresolved local, and perhaps systemic inflammation in PD. The roles of individual B cell cytokines in PD are detailed below.
B cells may also promote PD through multiple indirect cytokine- and antibody-independent mechanisms. For example, B cells within PD lesions express elevated levels of the T cell co-stimulatory molecule CD86 [38] suggesting that B cells function in vivo, at least in part, by activating T cells. B cells may also activate T cells through a unique role in antigen presentation and/or up regulation of surface CD83 [38, 53]. However, data from SCID mice predict that B cells are not absolutely essential for T cell-mediated PD initiation and progression [69], although the presence of residual B cells in these mice cannot formally rule out this possibility. B cells also secrete RANKL, a cytokine that promotes osteoclastogenesis [70]. B cell RANKL production is activated by A.a. and, importantly, RANKL-positive B cells induce osteoclast formation on periodontal bone and bone destruction. Because the recipient rats used in the studies supporting these conclusions were athymic, bone resorption in the presence of RANKL-expressing B cells did not require T cell function [60]. Despite all the possible mechanisms that probably link B cells with severe PD, additional model organism studies will be required to definitively show that in situ B cells are critical modulators of PD pathogenesis.
4.3: The role of B cells in T2D
Many cell types produce inflammatory cytokines [71–73], but multiple lines of evidence show hematopoietic cells are mainly responsible for the inflammation that links obesity with systemic insulin resistance and T2D. Cytokine-producing hematopoietic cells are necessary and sufficient for obese mice to become insulin resistant in response to DIO [74]. Additional studies have specifically implicated myeloid cell cytokine production in insulin resistance in mouse studies [75], which is further supported by demonstrations that monocytes from T2D patients secrete elevated levels of key pro-inflammatory cytokines such as IL-1β and TNF-α [15].
In contrast to the strong association between exaggerated myeloid cell function and T2D, the role of B cells and soluble B cell products in T2D has been addressed in very few studies. Recent studies in DIO mice demonstrated that B cells are the first immune cell type to infiltrate the adipose tissue in response to DIO, followed by T cells, then macrophages [44]. This order of cellular infiltration differs substantially compared to infiltration of immune cells in PD. Mice lacking both B and T cells (RAG-null mice) have an elevated number of macrophages in the adipose tissue late in DIO compared to the numbers in wild-type (lymphocyte-sufficient) controls, indicating cellular infiltration in fat is a highly regulated process perhaps orchestrated by initial B cell infiltration [44]. These and other studies concluded that lymphocyte infiltration is a protective response that moderates adipose tissue inflammation [44, 76, 77]. However, subsequent studies showed lymphocytes can alternatively promote inflammation and insulin resistance, in part based on the CD4/CD8 and T effector/T regulatory cell (Treg) cell balance [76–78]. In these studies, RAG-null (lymphocyte-free) mice responded to DIO by increased weight gain, accumulation of more epididymal fat (a leading measure of altered lipid metabolism), and larger diameter adipocytes (a second measure of changes in lipid metabolism) than RAG-sufficient mice [76]. Follow-up analyses showed CD4+ T cells/Tregs play a role in DIO disease protection, while CD8+ T cells instead promote insulin resistance in response to DIO [76–78]. The function of B cells and regulatory B cells (Bregs; Refs. [79–81]) in these mice was not reported.
The lack of attention on roles B cells play in T2D may be based on the traditional categorization of B cells as sources of pro-inflammatory auto-antibodies. Although auto-antibodies are thought to play major roles in disease pathogenesis of auto-immune type 1 diabetes, the presence of auto-antibodies in type 2 diabetes patients has traditionally been considered a signal that the etiology of glucose dysregulation/metabolic imbalance has been misdiagnosed, or that the patient is immunologically responding to palliative insulin used in some T2D patients. More recent findings support the idea that auto antibodies to important cellular modulators, such as G coupled receptors and Rho GTPases, may associate with vascular complications common in T2D [82–84]. However, despite studies linking auto antibodies with processes related to T2D disease pathogenesis [83, 85], a definitive link between auto antibodies and T2D remains equivocal. This general lack of appreciation that B cells may play important antibody-independent roles in T2D has perhaps limited enthusiasm for studies aimed at understanding the role these cells play in T2D pathogenesis. New studies raising the possibility that B cell cytokines play a role in T2D are outlined below.
5. Roles of B cell cytokines in chronic inflammatory disease
5.1: IL-10 is a protective B cell cytokine in inflammatory disease
B cells are demonstrated sources of cytokines both in healthy individuals and those with chronic, unresolved inflammatory disease. IL-10 is arguably the B cell cytokine most commonly implicated in inflammatory disease. IL-10 is generally considered anti-inflammatory, although it can have pro-inflammatory functions in some circumstances (i.e. up-regulation of surface TLR2; Ref. 86). IL-10 producing human B cells arise upon stimulation through surface immunoglobulin alone or in combination with CD40 [87], or upon stimulation through TLRs [88]. IL-10 producing B cells have been identified in mice as a separate B cell subpopulation, designated B10 cells, or as a less restricted subset designated “regulatory B cells” (Bregs) [79–81, 89–91]. Evidence for a human equivalent of Bregs was first uncovered based on a population of transitional B cells might protect against inflammatory disease in humans [92]. These transitional B cells may be similar to the CD27-IL-10-producing “naïve” B cells that repopulate multiple sclerosis (MS) patients following B cell depletion [93], or the transitional B cells that repopulate B cell-depleted lupus and rheumatoid arthritis patients [92, 94–96]. B cell IL-10 has also been shown to prevent the Th1 differentiation critical for arthritis and EAE, the latter a model for multiple sclerosis (MS) [98, 99]. In the EAE model, B cells secrete IL-10 in response to TLR engagement [100], and both TLRs and the MyD88 adaptor protein are necessary for B cells to produce the IL-10 that blocks T cell-mediated inflammation. Thus B cell pattern recognition receptors (TLRs) play important roles in resolving a three Th1-mediated diseases: lupus, EAE and collagen-induced arthritis.
Parallel analysis on B cells from MS patients has also suggested a role for B cell IL-10 in human inflammatory disease. B cells from active MS patients secrete significantly lower levels of IL-10 than B cells from healthy donors, and IL-10 secretion increases upon treatment with the palliative drug mitoxantrane and concomitant disease remission. [93]. TLR-mediated B cell activation may also have important protective functions in other inflammatory diseases, but a specific requirement of B cells for IL-10-mediated protection remains to be demonstrated [81, 97]. Finally, genetic studies have linked elevated IL-10 levels to protection from metabolic syndrome and T2D in humans [101]. Again, B cells could not be named as the specific source of IL-10 in these human subjects studies. However, very recent work has identified a population of IL-10 producing CD24hi CD38hi B cells that block Th1 differentiation in healthy donor samples. Evidence for a role of these human “Breg” cells in human disease includes the demonstration that these cells secretes less IL-10 and fail to suppress Th1 cells in lupus patients [97].Taken together however, multiple lines of evidence including mouse model and patient analyses indicate that IL-10, and specifically B cell IL-10 produced in response to TLR ligand, can protect against a wide array of chronic inflammatory diseases.
Although IL-10 also acts as an anti-inflammatory cytokine in PD, the precise source and function of IL-10 in PD patients or model organism studies has not been elucidated. Our recent data indicate that circulating B cells from PD patients constitutively secrete only low (‘healthy”) levels of IL-10. However, B cells from PD patients, but not healthy donors, respond to TLR2 ligand by up regulating IL-10 production. Surprisingly, PD B cell co-stimulation through TLR2 and TLR4 leads to lower levels of IL-10 production as compared to B cell stimulation with TLR2 ligand alone [88]. These results indicated that the increased percentage of TLR4-positive B cells identified in PD patients in our earlier work [102] may be a harbinger of unexpected changes in TLR4 function: moderation of anti-inflammatory IL-10 production. Importantly, these data show that TLR4 can trigger a response that dominates an IL-10 activating response triggered through TLR2 [104]. Parallel studies on B cells from T2D patients show that these B cells, in contrast to B cells from PD patients or healthy donors, fail to secrete IL-10 in response to stimulation through TLR2, TLR4, or TLR9 (Jagannathan et al., in press). Thus the altered IL-10 levels highlighted by genetic studies in T2D patients [101] may trace its origins to B cells, making the lack of B cell IL-10 physiologically dominant in inflammation as demonstrated for other chronic diseases.
5.2: B cells as sources of IL-6 in inflammatory disease
IL-6 is a second prototypic B cell cytokine implicated in inflammatory diseases, including PD and T2D. IL-6 is a plieotropic cytokine with multiple functions in T2D. Human IL-6 polymorphisms have been implicated in the transition from glucose intolerance to frank type 2 diabetes [103]. In addition to its proinflammatory roles, elevated serum IL-6 probably plays roles in glucose metabolism through stimulation of skeletal muscle [104, 105]. Our preliminary data indicate that B cells from T2D patients produce “healthy” amounts of IL-6 both constitutively and in response to TLR stimulation (Jagannathan et al., in press). These data suggest that B cell IL-6 may play an insignificant role in T2D, and that elevated levels of IL-6 in serum of T2D patients originate from non-B cell sources. In contrast, B cells from non-diabetic PD patients secrete significantly more IL-6 than B cells from T2D patients (Jagannathan et al, in press). Thus B cells are likely contributors to the elevated levels of IL-6 in the serum and gingival crevicular fluid of PD patients [106, 107]. In contrast to its assumed pro-inflammatory roles in chronic inflammatory disease, the contribution of IL-6 to PD may be less pathogenic, with some studies concluding that IL-6 instead partially protects against periodontal tissue destruction through its ability to induce IL-1 receptor antagonist, a natural anti-inflammatory competitive inhibitor of the IL-1 receptor [108, 109]. The mixed contribution of IL-6 to PD is perhaps echoed by studies in IL-6-null mice, which spontaneously develop insulin resistance [110]. These results indicate that the simplistic model of IL-6 as a pro-inflammatory cytokine that promotes inflammatory disease ignores the more complex biological functions of IL-6. The ability of Th2 cells to also secrete IL-6 and the presence of these cells in some periodontal lesions [18, 111] suggests that non-B cells contribute to overall levels of IL-6 in PD (in addition to T2D), thus IL-6 levels may or may not be substantially altered upon B cell depletion.
5.3: B cells as sources of IL-8 in inflammatory disease
We have recently identified IL-8 as unexpected pro-inflammatory chemokine hyper-produced by B cells from chronic inflammatory disease patients. Our first studies showed IL-8 is constitutively produced by B cells from Crohn’s disease (CD) patients. The positive correlation between constitutive blood B cell IL-8 production and disease severity in CD patients further suggested an association between B cell IL-8 and inflammatory disease. B cells from both CD and ulcerative colitis (UC) patients also responded to TLR2 ligand by secreting (additional) IL-8, probably based at least in part in the elevated percentage of surface TLR2-positive cells in patient blood and tissue. These data suggest that altered TLR function, probably due at least partially to elevated TLR2 expression, plays an important role in inflammatory bowel disease [112]. Interestingly, the correlation between the percentage of TLR2-positive B cells and indices of CD severity is positive, while the correlation between the percentage of TLR2-positive B cells and clinical UC severity is negative, despite the ability of TLR2 ligands to activate IL-8 production to similar levels in B cells from both patient cohorts [112]. Although these results do not present a unified conclusion for a role of B cells and B cell TLRs in these inflammatory diseases, they nonetheless suggest that disease-associated changes in B cell responses play important roles in final outcome of TLR engagement and subsequent cytokine production.
Our most recent data also shows that blood and gingival B cells from PD patients constitutively produce IL-8, and that IL-8 production is further increased in response to TLR2 or TLR4 engagement [88]. Because IL-8 gradients are important for the maintenance of healthy gingiva, we speculate that IL-8 production by B cells disrupts healthy IL-8 gradients in PD. IL-8 gradient disruption presumably hampers restorative neutrophil influx into diseased periodontal tissue [113, 114] and instead promotes the massive, prolonged neutrophil accumulation characteristic of PD. The relative contributions of B cells compared to macrophages in overall IL-8 production is unknown, but the absolute concentrations of IL-8 secreted by the two cells types in ex vivo experiments is comparable [88, 112]. Taken together these findings indicate significantly elevated B cell IL-8 production associates with multiple mucosal inflammatory diseases: PD, CD and UC.
Circulating B cells from T2D patients also constitutively secrete IL-8 (Jagannathan et al., in press). These data suggest that unexpected B cell IL-8 production may be a common characteristic of chronic inflammation beyond mucosal inflammatory diseases. Further evidence of B cell IL-8 secretion in inflammatory disease is the demonstration that B cells from inflamed appendix are intracellular IL-8 positive [112]. The general increase in IL-8 secretion in B cells from CD, UC, PD and T2D patients in response to TLR2 and TLR4 ligand (Ref. 112; Jagannathan et al, in press), demonstrate that TLR-mediated B cell IL-8 secretion is a common feature of B cells from inflammatory disease patients regardless of the downstream effects of IL-8 function. In contrast to significant surface TLR2 up regulation on B cells from CD patients, B cells from T2D patients express little surface TLR2 [112], despite their ability to respond to TLR2 ligand via elevated IL-8 secretion. These data suggest that although TLR hyper-responsiveness associates with a range of chronic inflammatory diseases, changes in TLR expression associate with only a subset of such patients.
5.4: B cells as sources of a broad array of cytokines in inflammatory disease
In addition to IL-10, IL-6 and now, IL-8, the list of B cell-generated cytokines includes additional cytokines elevated in the gingival crevicular fluid of PD patients, specifically IL-1β and TNF-α [106, 107, 115]. These cytokines are associated with oral bacterial load, and are themselves a measure of PD severity [107]. Although macrophages are prototypic sources of IL-1β, new data show that B cells from PD patients constitutively secrete elevated levels of IL-1β, and that these levels are further increased in response to TLR2 ligand [88]. B cells from PD patients also inducibly hyper-produce TNF-α in response to either TLR2 or TLR4 ligand, strongly suggesting that the copious numbers of B cells in the inflamed gingival are promoting inflammation and concomitant bone loss through elevated osteoclastogenic cytokine production [88]. In contrast, TLR-stimulated B cells from T2D patients fail to secrete higher levels of IL-1β and TNF-α as compared to B cells from non-diabetic donors (Jagannathan et al., in press).
The ability of human B cells to recapitulate the murine “B effector 1” and “B effector 2” subsets that mimic cytokine production by Th1 or Th2 effector T cell subsets, respectively [62], have been partially investigated outside the context of disease. IL-12 induces B cells from healthy donors to secrete IFN-γ, a characteristic of mouse B effector 1 (and Th1) cells [61]. IL-12 is variably detected in the gingival crevicular fluid of PD patients [116, 117], suggesting that the B effector 1 subset may be variably significant in PD inflammation. Additional supporting data for this conclusion show that circulating B cells from PD patients secrete relatively low concentrations of a second B effector 1 cytokine, IFN-γ, which approximate levels produced by healthy B cells [88]. Similarly, B cells from T2D patients secrete little IFN-γ (Jagannathan et al., in press), consistent with demonstrations that IFN-γ is not elevated in T2D and therefore putative B effector 1 cells are minor players in T2D pathogenesis [118]. Regardless, the IL-1β and TNF-α data highlight specific differences in B cell function between inflammatory diseases (T2D and PD; summarized in Table 1), despite the general conclusion that elevated proinflammatory cytokine production, coupled with decreased IL-10 production, define B cells as pro-inflammatory regulators of systemic inflammation.
Table 1.
comparison of B cells from PD and T2D patients
| PD | Role in PD1 | T2D | Role in T2D1 | References | |
|---|---|---|---|---|---|
| tissue infiltration | late | fuel chronic inflammation |
early | establish tissue inflammation |
44, 76–78 |
| surface TLR2 expression |
+2 | increase cytokine production |
− | increase cytokine production |
88, 112 |
| surface TLR4 expression |
++ | regulate cytokine production |
+ | unknown | 1023 |
| IL-1β secretion | +/++4 | pro-inflammatory and pro- osteoclastogenic |
−/− | promotes inflammation and insulin resistance |
68,88 |
| IL-6 secretion | −/+ | complex5 | −/− | complex | unpublished3 |
| IL-8 secretion | ++/++ + |
restorative neutrophil infiltration |
++/++ + |
unknown | 883 |
| IL-10 secretion | −/+ | anti-inflammatory | 0/06 | N/A7 | 883 |
| TNF-α secretion | −/++ | pro-inflammatory and pro- osteoclastogenic |
0/−6 | pro-inflammatory | 68,883 |
| IFN-γ secretion | −/− | N/A | −/− | N/A | 883 |
Includes general and disease-specific roles; in some cases roles are inferred from evidence cited in the main text.
Expression levels range from “−“ (indicating levels are indistinguishable from B cells from healthy donors) to “+++” (hyper-expressed at highly significant levels above samples from healthy donors).
Indicates data also appear in Jagannathan et al., in press.
For all cytokine data, left symbol indicates constitutive expression; right symbol indicates TLR-inducible expression.
IL-6 has both pro- and anti-inflammatory activities and has been shown to promote or inhibit PD and T2D by independent studies.
”0” indicates that secretion is significantly lower than in B cells from healthy donors.
Not applicable.
5.5: Relative contributions of B cells compared to monocytes in chronic inflammatory disease
The cytokines produced by B cells are thought to be functionally indistinguishable from cytokines secreted by other cellular sources in vivo, raising questions about the relative contribution of B cells to inflammatory disease. Early studies indicated B cells and monocytes can secrete comparable levels of cytokines including IL-1 and GM-CSF [119, 120]. However, new techniques will allow more accurate quantitation of B cell vs. monocyte cytokine production by highly purified cellular populations, as we used to show B cells from CD patients secrete monocyte-like levels of IL-8 [112]. Regardless, in PD patients, the level of inflammation (i.e. proinflammatory cytokines) in the oral cavity is thought to surpass some critical pathogenic “tipping point” at which homeostasis cannot be re-established without clinical intervention [68]. Although B cells may produce quantitatively lower levels of some pro-inflammatory cytokines than, for example, monocytes, this threshold hypothesis indicates that every source of inflammatory cytokines may additively, or even synergistically contribute to the overall inflammatory environment. Alternatively, localization of cytokine-producing B cells may play important roles in either establishing or maintaining chronic inflammation. The demonstration that B cells infiltrate epididymal fat pads as early as three weeks after mice are switched to an obesity-inducing high fat diet (i.e. several weeks prior to significant macrophage infiltration) suggests that B cells may prime the region for subsequent infiltration first by T cells, then finally by macrophages [44].
Whether the proposed tissue “priming” by B cell also occurs in the generally protective subcutaneous fat depots [121] in the DIO model or whether these early infiltrating B cells secrete cytokines, is untested. In contrast to early B cell infiltration in the DIO model, PD is characterized by late B cell infiltration. Eventually B lineage cells represent the majority cell type in PD lesions. These data are consistent with the likelihood that B cells are major sources of the pro-inflammatory cytokines that drive periodontal bone loss that characterizes later stages of PD. Whether B cells affect entry or exit of more classical cytokine-producing (myeloid lineage) cells is untested, but the dominant presence of B cells may allow them to serve as non-redundant anchor cells for chronic inflammation in PD.
6. The promise of B cell depletion as a therapy for PD and T2D
Given the important direct (antibody and cytokine production) and indirect (T cell activation) roles B cells play in inflammatory diseases, it may be surprising that B cell depletion is generally safe, and has beneficial outcomes for multiple auto-immune inflammatory diseases [2, 3, 122, 123]. Perhaps even more unexpected is the absence of increased serious infections with Rituximab treatment, at least in rheumatoid arthritis patients [1]. These data suggest that levels of cytokines produced by non-B cell sources are sufficient for maintaining homeostasis and preventing rampant systemic infection. However, B cell depletion is not without risk. In one limited study on lupus patients, 4 of 32 patients refractory to standard treatment had serious side effects, some of which were not characteristic of lupus, following B cell depletion [3]. Rutuximab treatment has also preceded reactivation of hepatitis B and C, cytomegalovirus, and varicella with fatal consequences in case study reports [124–128]. However, in some cases, complications from concomitant treatment with other chemotherapeutic agents could not be ruled out [128]. Regardless, the development of highly fatal progressive multifocal leucoencephalopathy (PML) has been reported in multiple rituximab-treated patients seeking treatment for unrelated pathologies [129–135], although most PML reports involved lymphoma and lupus patients. Interestingly, rituximab-induced PML is extremely rare in patients afflicted with other lupusrelated rheumatic diseases. Although the occurrence of serious complications including PML is a rare complication of B cell depletion, further work is needed to define whether B cell depletion or other more standard treatments administered along with rituximab prime patients for short-term remission followed by serious treatment-induced consequences. Unexpected outcomes of combining rituximab with standard PD or T2D treatments cannot be ruled out at present, even if ongoing studies predict a very low incidence of serious side effects.
7. Future Directions
The roles of B cells in autoimmune and non-autoimmune inflammatory diseases have focused mainly on the ability of B cells to secrete auto antibodies. More recently, B cells have been identified as important sources of pro-inflammatory and anti-inflammatory cytokines. B cells can either provide a quantitatively or functionally dominant source of cytokines. Alternatively, B cells may additively contribute to inflammation by skewing the overall pro-to-anti-inflammatory cytokine ratio past some critical threshold that differentiates chronic inflammation characterizing diseases such as T2D and PD from healthy inflammatory response. Antibody-mediated inflammation may further define the contribution of B cells to some diseases. Recent demonstrations that B cell ablation is a generally safe, effective treatment for autoimmune inflammatory disease such as multiple sclerosis, lupus and rheumatoid arthritis [1, 2, 4] raise the possibility that a greater understanding of B cell function in non-autoimmune inflammatory disease may identify B cell depletion as a fundamentally new therapy for breaking the chronic inflammatory cycle. However, the role of B cells as anti-inflammatory cell types in some scenarios and rare but serious side effects of rituximab suggest caution must be exercised by investigators designing B cell ablation trials. Pro-inflammatory receptors in the TLR family are altered on B cells from inflammatory disease patients and can drive B cell cytokine production. However, because B cell TLR expression and function 1. differs significantly between model organisms (mouse) and human; 2. differs among human patient cohorts grouped by diagnosis; and 3. can result in both pro- and anti-inflammatory cytokine production, more detailed analyses are required to predict the outcome of B cell ablation prior to initiation of clinical trials. B cell responses to TLRs must also be considerations of studies aimed at exploiting the power of TLRs for regulating the immune system in new pharmaceuticals and vaccine adjuvants based on TLR function in cells from healthy individuals.
Acknowledgements
Supported by NIH R01 AI54611 and a Research Grant from the American Diabetes Association. The author thanks Madhumita Jagannathan, Ann Marshak-Rothstein, Rachel Ettinger, and Marie McDonnell for valuable conversations related to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomized placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–2677. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 3.Ng KP, Cambridge G, Leandro MJ, Edwards JC, Ehrenstein M, Isenberg DA. B cell depletion therapy in systemic lupus erythematosus: long-term follow-up and predictors of response. Ann Rheum Dis. 2007;66:1259–1262. doi: 10.1136/ard.2006.067124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 5.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedus L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves' disease. Gut. 2008;57:714–715. doi: 10.1136/gut.2007.138305. [DOI] [PubMed] [Google Scholar]
- 7.Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–2718. doi: 10.1002/art.22811. [DOI] [PubMed] [Google Scholar]
- 8.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–289. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 9.D'Aiuto F, Graziani F, Tete S, Gabriele M, Tonetti MS. Periodontitis: from local infection to systemic diseases. Int J Immunopathol Pharmacol. 2005;18:1–12. [PubMed] [Google Scholar]
- 10.Stump CS, Clark SE, Sowers JR. Oxidative stress in insulin-resistant conditions: cardiovascular implications. Treat Endocrinol. 2005;4:343–351. doi: 10.2165/00024677-200504060-00003. [DOI] [PubMed] [Google Scholar]
- 11.Preshaw PM, Foster N, Taylor JJ. Cross-susceptibility between periodontal disease and type 2 diabetes mellitus: an immunobiological perspective. Periodontol. 2000;45:138–157. doi: 10.1111/j.1600-0757.2007.00221.x. 2007. [DOI] [PubMed] [Google Scholar]
- 12.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 13.Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Ann Periodontol. 1998;3:40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- 14.Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147:2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- 15.Giulietti A, Stoffels K, Decallonne B, Overbergh L, Mathieu C. Monocytic expression behavior of cytokines in diabetic patients upon inflammatory stimulation. Ann N Y Acad Sci. 2004;1037:74–78. doi: 10.1196/annals.1337.011. [DOI] [PubMed] [Google Scholar]
- 16.Foss-Freitas MC, Foss NT, Donadi EA, Foss MC. In vitro TNF-alpha and IL-6 production by adherent peripheral blood mononuclear cells obtained from type 1 and type 2 diabetic patients evaluated according to the metabolic control. Ann N Y Acad Sci. 2006;1079:177–180. doi: 10.1196/annals.1375.027. [DOI] [PubMed] [Google Scholar]
- 17.Sherry CL, O'Connor JC, Kramer JM, Freund GG. Augmented lipopolysaccharide-induced TNF-alpha production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 MAPK. J Immunol. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- 18.Takeichi O, Haber J, Kawai T, Smith DJ, Moro I, Taubman MA. Cytokine profiles of T-lymphocytes from gingival tissues with pathological pocketing. J Dent Res. 2000;79:1548–1555. doi: 10.1177/00220345000790080401. [DOI] [PubMed] [Google Scholar]
- 19.Stephens JW, Hurel SJ, Lowe GD, Rumley A, Humphries SE. Association between plasma IL-6, the IL6 −174G>C gene variant and the metabolic syndrome in type 2 diabetes mellitus. Mol Genet Metab. 2006 doi: 10.1016/j.ymgme.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Wara-Aswapati N, Boch JA, Auron PE. Activation of interleukin 1beta gene transcription by human cytomegalovirus: molecular mechanisms and relevance to periodontitis. Oral Microbiol Immunol. 2003;18:67–71. doi: 10.1034/j.1399-302x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 21.Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 22.Engebretson S, Chertog R, Nichols A, Hey-Hadavi J, Celenti R, Grbic J. Plasma levels of tumour necrosis factor-alpha in patients with chronic periodontitis and type 2 diabetes. J Clin Periodontol. 2007;34:18–24. doi: 10.1111/j.1600-051X.2006.01017.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A. 2009;106:10740–10745. doi: 10.1073/pnas.0904412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naguib G, Al-Mashat H, Desta T, Graves DT. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. 2004;123:87–92. doi: 10.1111/j.0022-202X.2004.22711.x. [DOI] [PubMed] [Google Scholar]
- 25.Kantarci A, Oyaizu K, Van Dyke TE. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alabdulkarim M, Bissada N, Al-Zahrani M, Ficara A, Siegel B. Alveolar bone loss in obese subjects. J Int Acad Periodontol. 2005;7:34–38. [PubMed] [Google Scholar]
- 28.Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids. 2005;73:17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- 30.Iacopino AM. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- 31.Loos BG, Craandijk J, Hoek FJ, Wertheim-van Dillen PM, van der Velden U. Elevation of systemic markers related to cardiovascular diseases in the peripheral blood of periodontitis patients. J Periodontol. 2000;71:1528–1534. doi: 10.1902/jop.2000.71.10.1528. [DOI] [PubMed] [Google Scholar]
- 32.Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221–1227. doi: 10.1902/jop.2000.72.9.1221. [DOI] [PubMed] [Google Scholar]
- 33.Offenbacher S, Beck JD. A perspective on the potential cardioprotective benefits of periodontal therapy. Am Heart J. 2005;149:950–954. doi: 10.1016/j.ahj.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 34.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 35.Ford PJ, Yamazaki K, Seymour GJ. Cardiovascular and Oral Disease Interactions: What is the Evidence? Prim Dent Care. 2007;14:59–66. doi: 10.1308/135576107780556806. [DOI] [PubMed] [Google Scholar]
- 36.Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 37.Mealey BL, Rethman MP. Periodontal disease and diabetes mellitus. Bidirectional relationship. Dent Today. 2003;22:107–113. [PubMed] [Google Scholar]
- 38.Mahanonda R, Sa-Ard-Iam N, Yongvanitchit K, Wisetchang M, Ishikawa I, Nagasawa T, Walsh DS, Pichyangkul S. Upregulation of co-stimulatory molecule expression and dendritic cell marker (CD83) on B cells in periodontal disease. J Periodontal Res. 2002;37:177–183. doi: 10.1034/j.1600-0765.2002.00664.x. [DOI] [PubMed] [Google Scholar]
- 39.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 41.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 42.Soyka LF, Haessler HA, Crawford JD. Altered composition and lipolysis of adipose tissue from gold thioglucose obese mice. Am J Physiol. 1969;217:1088–1093. doi: 10.1152/ajplegacy.1969.217.4.1088. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzawa Y, Shimomura I, Nakamura T, Keno Y, Tokunaga K. Pathophysiology and pathogenesis of visceral fat obesity. Diabetes Res Clin Pract. 1994;24 Suppl:S111–S116. doi: 10.1016/0168-8227(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 44.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 46.Rhodes J. Receptor for monomeric IgM on guinea-pig splenic macrophages. Nature. 1973;243:527–528. doi: 10.1038/243527a0. [DOI] [PubMed] [Google Scholar]
- 47.Messner RP, Jelinek J. Receptors for human gamma G globulin on human neutrophils. J Clin Invest. 1970;49:2165–2171. doi: 10.1172/JCI106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein IM, Roos D, Kaplan HB, Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975;56:1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–249. [PubMed] [Google Scholar]
- 50.Reinhardt RA, Bolton RW, McDonald TL, DuBois LM, Kaldahl WB. In situ lymphocyte subpopulations from active versus stable periodontal sites. J Periodontol. 1988;59:656–670. doi: 10.1902/jop.1988.59.10.656. [DOI] [PubMed] [Google Scholar]
- 51.Berglundh T, Donati M, Zitzmann N. B cells in periodontitis: friends or enemies? Periodontol. 2000;45:51–66. doi: 10.1111/j.1600-0757.2007.00223.x. 2007. [DOI] [PubMed] [Google Scholar]
- 52.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–374. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berglundh T, Donati M. Aspects of adaptive host response in periodontitis. J Clin Periodontol. 2005;32 Suppl 6:87–107. doi: 10.1111/j.1600-051X.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi K, Moughal NA, Mooney J, Kinane DF. Kappa light chain mRNA bearing plasma cells are predominant in periodontitis lesions. J Periodontal Res. 1996;31:256–259. doi: 10.1111/j.1600-0765.1996.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 55.Freedman AS, Freeman G, Whitman J, Segil J, Daley J, Levine H, Nadler LM. Expression and regulation of CD5 on in vitro activated human B cells. Eur J Immunol. 1989;19:849–855. doi: 10.1002/eji.1830190511. [DOI] [PubMed] [Google Scholar]
- 56.Freedman AS, Freeman G, Whitman J, Segil J, Daley J, Nadler LM. Studies of in vitro activated CD5+ B cells. Blood. 1989;73:202–208. [PubMed] [Google Scholar]
- 57.Berglundh T, Liljenberg B, Tarkowski A, Lindhe J. The presence of local and circulating autoreactive B cells in patients with advanced periodontitis. J Clin Periodontol. 2002;29:281–286. doi: 10.1034/j.1600-051x.2002.290402.x. [DOI] [PubMed] [Google Scholar]
- 58.Donati M, Liljenberg B, Zitzmann NU, Berglundh T. B-1a cells and plasma cells in periodontitis lesions. J Periodontal Res. 2009 doi: 10.1111/j.1600-0765.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 59.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han X, Kawai T, Eastcott JW, Taubman MA. Bacterial-responsive B lymphocytes induce periodontal bone resorption. J Immunol. 2006;176:625–631. doi: 10.4049/jimmunol.176.1.625. [DOI] [PubMed] [Google Scholar]
- 61.Durali D, de Goer de Herve MG, Giron-Michel J, Azzarone B, Delfraissy JF, Taoufik Y. In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood. 2003;102:4084–4089. doi: 10.1182/blood-2003-02-0518. [DOI] [PubMed] [Google Scholar]
- 62.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 63.Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- 64.Anusaksathien O, Singh G, Matthews N, Dolby AE. Autoimmunity to collagen in adult periodontal disease: immunoglobulin classes in sera and tissue. J Periodontal Res. 1992;27:55–61. doi: 10.1111/j.1600-0765.1992.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 65.De-Gennaro LA, Lopes JD, Mariano M. Autoantibodies directed to extracellular matrix components in patients with different clinical forms of periodontitis. J Periodontol. 2006;77:2025–2030. doi: 10.1902/jop.2006.060104. [DOI] [PubMed] [Google Scholar]
- 66.Mouton C, Hammond PG, Slots J, Genco RJ. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981;31:182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou L, Sasakj H, Stashenko P. B-Cell deficiency predisposes mice to disseminating anaerobic infections: protection by passive antibody transfer. Infect Immun. 2000;68:5645–5651. doi: 10.1128/iai.68.10.5645-5651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graves DT, Cochran D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J Periodontol. 2003;74:391–401. doi: 10.1902/jop.2003.74.3.391. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi M, Ukai T, Kaneko T, Yoshinaga M, Yokoyama M, Ozaki Y, Hara Y. T cells are able to promote lipopolysaccharide-induced bone resorption in mice in the absence of B cells. J Periodontal Res. 2008;43:549–555. doi: 10.1111/j.1600-0765.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- 70.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 73.Laharrague P, Fontanilles AM, Tkaczuk J, Corberand JX, Penicaud L, Casteilla L. Inflammatory/haematopoietic cytokine production by human bone marrow adipocytes. Eur Cytokine Netw. 2000;11:634–639. [PubMed] [Google Scholar]
- 74.Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in Hematopoietically Derived Cells Contributes to Diet-Induced Inflammation and Insulin Resistance without Affecting Obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 75.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 76.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker DJ, Engleman E, Winer D, Dosch HM. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 79.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 80.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 81.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fredrikson GN, Anand DV, Hopkins D, Corder R, Alm R, Bengtsson E, Shah PK, Lahiri A, Nilsson J. Associations between autoantibodies against apolipoprotein B-100 peptides and vascular complications in patients with type 2 diabetes. Diabetologia. 2009;52:1426–1433. doi: 10.1007/s00125-009-1377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zimering MB, Pan Z. Autoantibodies in type 2 diabetes induce stress fiber formation and apoptosis in endothelial cells. J Clin Endocrinol Metab. 2009;94:2171–2177. doi: 10.1210/jc.2008-2354. [DOI] [PubMed] [Google Scholar]
- 84.Hempel P, Karczewski P, Kohnert KD, Raabe J, Lemke B, Kunze R, Bimmler M. Sera from patients with type 2 diabetes contain agonistic autoantibodies against G protein-coupled receptors. Scand J Immunol. 2009;70:159–160. doi: 10.1111/j.1365-3083.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 85.Wenzel K, Haase H, Wallukat G, Derer W, Bartel S, Homuth V, Herse F, Hubner N, Schulz H, Janczikowski M, Lindschau C, Schroeder C, Verlohren S, Morano I, Muller DN, Luft FC, Dietz R, Dechend R, Karczewski P. Potential relevance of alpha(1)-adrenergic receptor autoantibodies in refractory hypertension. PLoS One. 2008;3:e3742. doi: 10.1371/journal.pone.0003742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ganley-Leal LM, Liu X, Wetzler LM. Toll-like receptor 2-mediated human B cell differentiation. Clin Immunol. 2006;120:272–284. doi: 10.1016/j.clim.2006.04.571. [DOI] [PubMed] [Google Scholar]
- 87.Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 88.Jagannathan M, Hasturk H, Liang Y, Shin H, Hetzel JT, Kantarci A, Rubin D, McDonnell ME, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. TLR Cross-Talk Specifically Regulates Cytokine Production by B Cells from Chronic Inflammatory Disease Patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 90.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–1089. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 91.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 92.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res. 2009 doi: 10.1007/s12026-009-8096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 94.Roll P, Palanichamy A, Kneitz C, Dorner T, Tony HP. Regeneration of B cell subsets after transient B cell depletion using anti-CD20 antibodies in rheumatoid arthritis. Arthritis Rheum. 2006;54:2377–2386. doi: 10.1002/art.22019. [DOI] [PubMed] [Google Scholar]
- 95.Anolik JH, Barnard J, Owen T, Zheng B, Kemshetti S, Looney RJ, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis Rheum. 2007;56:3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 96.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner EC, Fisher RI, Sanz I. B cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B Cells Exhibit Regulatory Capacity in Healthy Individuals but Are Functionally Impaired in Systemic Lupus Erythematosus Patients. Immunity. 2010 doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 99.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, Fillatreau S. TLR-activated B cells suppress T cell-mediated autoimmunity. J Immunol. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 101.van Exel E, Gussekloo J, de Craen AJ, Frolich M, Bootsma-Van Der Wiel A, Westendorp RG. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes : the Leiden 85-Plus Study. Diabetes. 2002;51:1088–1092. doi: 10.2337/diabetes.51.4.1088. [DOI] [PubMed] [Google Scholar]
- 102.Shin H, Zhang Y, Jagannathan M, Hasturk H, Kantarci A, Liu H, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. B cells from periodontal disease patients express surface Toll-like receptor 4. J Leukoc Biol. 2009;85:648–655. doi: 10.1189/jlb.0708428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M, Laakso M. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2003;52:1872–1876. doi: 10.2337/diabetes.52.7.1872. [DOI] [PubMed] [Google Scholar]
- 104.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54 Suppl 2:S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 105.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 106.Lee HJ, Kang IK, Chung CP, Choi SM. The subgingival microflora and gingival crevicular fluid cytokines in refractory periodontitis. J Clin Periodontol. 1995;22:885–890. doi: 10.1111/j.1600-051x.1995.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 107.Hirose K, Isogai E, Miura H, Ueda I. Levels of Porphyromonas gingivalis Fimbriae and inflammatory cytokines in gingival crevicular fluid from adult human subjects. Microbiol Immunol. 1997;41:21–26. doi: 10.1111/j.1348-0421.1997.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 108.Irwin CR, Myrillas TT. The role of IL-6 in the pathogenesis of periodontal disease. Oral Dis. 1998;4:43–47. doi: 10.1111/j.1601-0825.1998.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 109.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an antiinflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 110.Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 111.Yamazaki K, Nakajima T, Gemmell E, Polak B, Seymour GJ, Hara K. IL-4- and IL-6-producing cells in human periodontal disease tissue. J Oral Pathol Med. 1994;23:347–353. doi: 10.1111/j.1600-0714.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 112.Noronha AM, Liang Y, Hetzel JT, Hasturk H, Kantarci A, Stucchi A, Zhang Y, Nikolajczyk BS, Farraye FA, Ganley-Leal LM. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009 doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tonetti MS, Imboden MA, Lang NP. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol. 1998;69:1139–1147. doi: 10.1902/jop.1998.69.10.1139. [DOI] [PubMed] [Google Scholar]
- 115.Ishihara Y, Nishihara T, Kuroyanagi T, Shirozu N, Yamagishi E, Ohguchi M, Koide M, Ueda N, Amano K, Noguchi T. Gingival crevicular interleukin-1 and interleukin-1 receptor antagonist levels in periodontally healthy and diseased sites. J Periodontal Res. 1997;32:524–529. doi: 10.1111/j.1600-0765.1997.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 116.Yucel OO, Berker E, Gariboglu S, Otlu H. Interleukin-11, interleukin-1beta, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J Clin Periodontol. 2008;35:365–370. doi: 10.1111/j.1600-051X.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 117.Orozco A, Gemmell E, Bickel M, Seymour GJ. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 2006;21:256–260. doi: 10.1111/j.1399-302X.2006.00292.x. [DOI] [PubMed] [Google Scholar]
- 118.Tsiavou A, Degiannis D, Hatziagelaki E, Koniavitou K, Raptis SA. Intracellular IFN-gamma production and IL-12 serum levels in latent autoimmune diabetes of adults (LADA) and in type 2 diabetes. J Interferon Cytokine Res. 2004;24:381–387. doi: 10.1089/1079990041535665. [DOI] [PubMed] [Google Scholar]
- 119.Pistoia V, Cozzolino F, Rubartelli A, Torcia M, Roncella S, Ferrarini M. In vitro production of interleukin 1 by normal and malignant human B lymphocytes. J Immunol. 1986;136:1688–1692. [PubMed] [Google Scholar]
- 120.Zupo S, Perussia B, Baldi L, Corcione A, Dono M, Ferrarini M, Pistoia V. Production of granulocyte-macrophage colony-stimulating factor but not IL-3 by normal and neoplastic human B lymphocytes. J Immunol. 1992;148:1423–1430. [PubMed] [Google Scholar]
- 121.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 123.Fleischmann RM. Safety of biologic therapy in rheumatoid arthritis and other autoimmune diseases: focus on rituximab. Semin Arthritis Rheum. 2009;38:265–280. doi: 10.1016/j.semarthrit.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 124.Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769–774. doi: 10.1007/s00277-004-0899-y. [DOI] [PubMed] [Google Scholar]
- 125.Tsutsumi Y, Kanamori H, Mori A, Tanaka J, Asaka M, Imamura M, Masauzi N. Reactivation of hepatitis B virus with rituximab. Expert Opin Drug Saf. 2005;4:599–608. doi: 10.1517/14740338.4.3.599. [DOI] [PubMed] [Google Scholar]
- 126.Suzan F, Ammor M, Ribrag V. Fatal reactivation of cytomegalovirus infection after use of rituximab for a post-transplantation lymphoproliferative disorder. N Engl J Med. 2001;345:1000. doi: 10.1056/NEJM200109273451315. [DOI] [PubMed] [Google Scholar]
- 127.Bermudez A, Marco F, Conde E, Mazo E, Recio M, Zubizarreta A. Fatal visceral varicella-zoster infection following rituximab and chemotherapy treatment in a patient with follicular lymphoma. Haematologica. 2000;85:894–895. [PubMed] [Google Scholar]
- 128.Ennishi D, Yokoyama M, Terui Y, Takeuchi K, Ikeda K, Tanimoto M, Hatake K. Does rituximab really induce hepatitis C virus reactivation? J Clin Oncol. 2008;26:4695–4696. doi: 10.1200/JCO.2008.18.7609. author reply 4696. [DOI] [PubMed] [Google Scholar]
- 129.Bonavita S, Conforti R, Russo A, Sacco R, Tessitore A, Gallo A, Della Corte M, Monsurro MR, Tedeschi G. Infratentorial progressive multifocal leukoencephalopathy in a patient treated with fludarabine and rituximab. Neurol Sci. 2008;29:37–39. doi: 10.1007/s10072-008-0857-x. [DOI] [PubMed] [Google Scholar]
- 130.Harris HE. Progressive multifocal leucoencephalopathy in a patient with systemic lupus erythematosus treated with rituximab. Rheumatology (Oxford) 2008;47:224–225. doi: 10.1093/rheumatology/kem299. [DOI] [PubMed] [Google Scholar]
- 131.Hopfinger G, Plessl A, Grisold W, Klimpfinger M, Hoftberger R, Bernt R, Mostl M, Waldner R, Pittermann-Hocker E. Progressive multifocal leukoencephalopathy after rituximab in a patient with relapsed follicular lymphoma and low IgG levels and a low CD4+lymphocyte count. Leuk Lymphoma. 2008;49:2367–2369. doi: 10.1080/10428190802404048. [DOI] [PubMed] [Google Scholar]
- 132.Kranick SM, Mowry EM, Rosenfeld MR. Progressive multifocal leukoencephalopathy after rituximab in a case of non-Hodgkin lymphoma. Neurology. 2007;69:704–706. doi: 10.1212/01.wnl.0000267325.06000.d9. [DOI] [PubMed] [Google Scholar]
- 133.Steurer M, Clausen J, Gotwald T, Gunsilius E, Stockhammer G, Gastl G, Nachbaur D. Progressive multifocal leukoencephalopathy after allogeneic stem cell transplantation and posttransplantation rituximab. Transplantation. 2003;76:435–436. doi: 10.1097/01.TP.0000078897.11633.5F. [DOI] [PubMed] [Google Scholar]
- 134.Yokoyama H, Watanabe T, Maruyama D, Kim SW, Kobayashi Y, Tobinai K. Progressive multifocal leukoencephalopathy in a patient with B-cell lymphoma during rituximab-containing chemotherapy: case report and review of the literature. Int J Hematol. 2008;88:443–447. doi: 10.1007/s12185-008-0168-2. [DOI] [PubMed] [Google Scholar]
- 135.Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis Rheum. 2009;60:3225–3228. doi: 10.1002/art.24906. [DOI] [PubMed] [Google Scholar]