Abstract
Spina bifida myelomeningocele (SBM) is a specific type of neural tube defect whereby the open neural tube at the level of the spinal cord alters brain development during early stages of gestation. Some structural anomalies are virtually unique to individuals with SBM, including a complex pattern of cerebellar dysplasia known as the Chiari II malformation. Other structural anomalies are not necessarily unique to SBM, including altered development of the corpus callosum and posterior fossa. Within SBM, tremendous heterogeneity is reflected in the degree to which brain structures are atypical in qualitative appearance and quantitative measures of morphometry. Hallmark structural features of SBM include overall reductions in posterior fossa and cerebellum appearance, size, and volume. Studies of the corpus callosum have shown complex patterns of agenesis or hypoplasia along its rostral-caudal axis, with rostrum and splenium regions particularly susceptible to agenesis. Studies of cortical regions have demonstrated complex patterns of thickening, thinning, and gyrification. Diffusion tensor imaging studies have reported compromised integrity of some specific white matter pathways. Given equally complex ocular motor, motor, and cognitive phenotypes consisting of relative strengths and weaknesses that seem to align with altered structural development, studies of SBM provide new insights to our current understanding of brain structure-function associations.
Keywords: Chiari II malformation, cerebellum, neuroimaging, structure, function, cortex, corpus callosum
Introduction
Spina bifida myelomeningocele (SBM) is a special type of neural tube defect whereby a failure in programmed fetal development (e.g. neural tube closure) at the level of the spine translates into substantially altered brain development, in both structural and functional domains. Both qualitative and quantitative studies have documented a remarkable degree of heterogeneity among individuals with SBM in terms of size, shape, and appearance of the cerebellum, corpus callosum, and cerebral cortex. Similarly, a wide range of cognitive strengths and relative weaknesses among individuals with SBM are also documented in the published literature. As highlighted below in the present paper, qualitative and quantitative investigations of brain regions hypothesized to be associated with cognitive strengths and weaknesses in individuals with SBM provide new insights into current models of brain-behavior associations in general. Although hydrocephalus commonly occurs in individuals with SBM, it will not be discussed in detail here, and readers are referred to Del Bigio [in press].
Overview of structural features associated with SBM
Common structural characteristics associated with SBM include anomalous development of the skull, as well as infra- and supra-tentorial regions of the brain [McLone and Dias 2003]. Chiari II malformation (CII) is a complex congenital anomaly that involves the midbrain and hindbrain (i.e. pons, medulla, and the cerebellum) and cervical spinal cord [Barkovich 2005; Harding and Copp 2002] which occurs universally and exclusively in SBM [Wagner et al. 2002]. Prominent features of CII include a significantly smaller posterior fossa with its contents crowded and distorted in appearance [Barkovich 2005]. Additional features of SBM can include tectal beaking, hydrocephalus, and corpus callosum hypoplasia and dysgenesis, but these features are variable. Although less studied, an enlarged massa intermedia and gyral malformations of the neocortex also commonly occur in SBM with CII.
The main features of CII are largely based on qualitative descriptions of gross anomalies (Table 1), particularly midline brain structures [McLone and Dias 2003]. The mesencephalic tectum is often distorted and stretched posteriorly and inferiorly (see Figure 1), which is frequently described on magnetic resonance imaging (MRI) scans as “beaking” of the tectum. Tectal beaking is reported to occur in 75% of CII cases, being particularly prominent in older patients with an extensive myelomeningocele [Fletcher et al. 2005]. Typically in CII, the brainstem is stretched down to the level of the foramen magnum or cervical spine with narrowing in its anterior-posterior diameter. A cervico-medullary kink may be seen at the junction between the cervical spinal cord and the medulla oblongata [Barkovich 2005] which may lead to symptoms of spinal cord compression [Oaks and Gaskill 1992]. While the inferior vermis frequently herniates down to the upper cervical vertebrae (C), at C2 or C4 level, the superior part of the cerebellar vermis herniates upwards (Figure 1). As described by Barkovich (2005) and shown in Figure 2, the cerebellum wraps around the brainstem. In some cases, low insertion of the tentorium cerebelli, cerebellar dysplasia, agenesis, or hypoplasia of the lateral cerebellar hemispheres also occur [Barkovich 2005; Gilbert et al. 1986; Harding and Copp 2002].
Table 1.
Structural changes within the posterior fossa in Chiari II malformation
| Small posterior fossa |
| Small cerebellar volume (especially the cerebellar hemispheres) |
| Reduced cerebellar weight |
| Dysplastic looking cerebellum |
| Expanded midsagittal vermis area |
| Vermis (± tonsils) herniation below foramen magnum |
| Upward herniation of the superior vermis |
| Wrapping of the cerebellar hemispheres around the brainstem |
| Enlarged tentorium incisura |
| Dysplastic tentorium cerebelli with abnormally low insertion |
| Inferior displacement of the lower brainstem |
| Tectal beaking |
| Medullary-cervical kink |
| Aqueduct stenosis |
| Small and inferiorly displaced fourth ventricle |
| Enlarged foramen magnum |
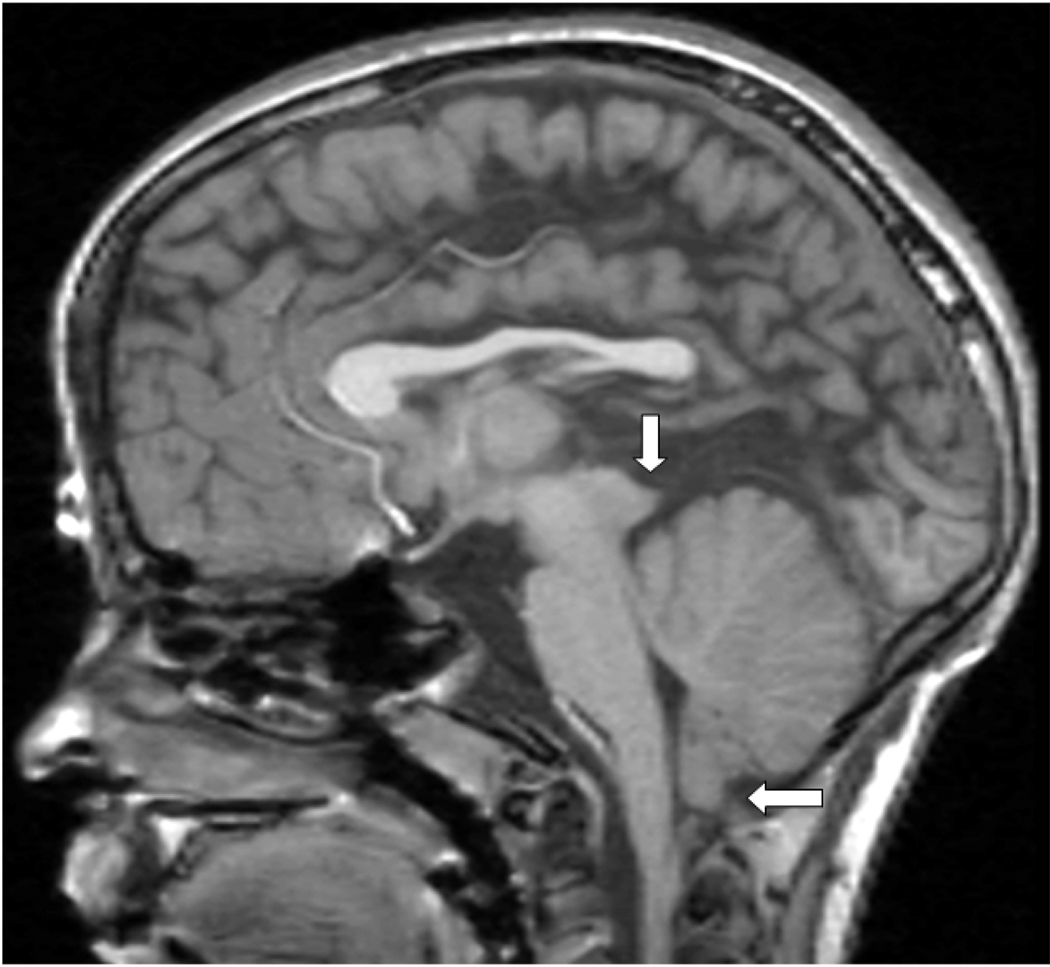
Figure 1.
T1-weighted midsagittal MRI in a child with Chiari type II malformation illustrating herniation of the inferior cerebellar vermis (horizontal arrow) and tectal beaking (vertical arrow). The fourth ventricle is small in size.
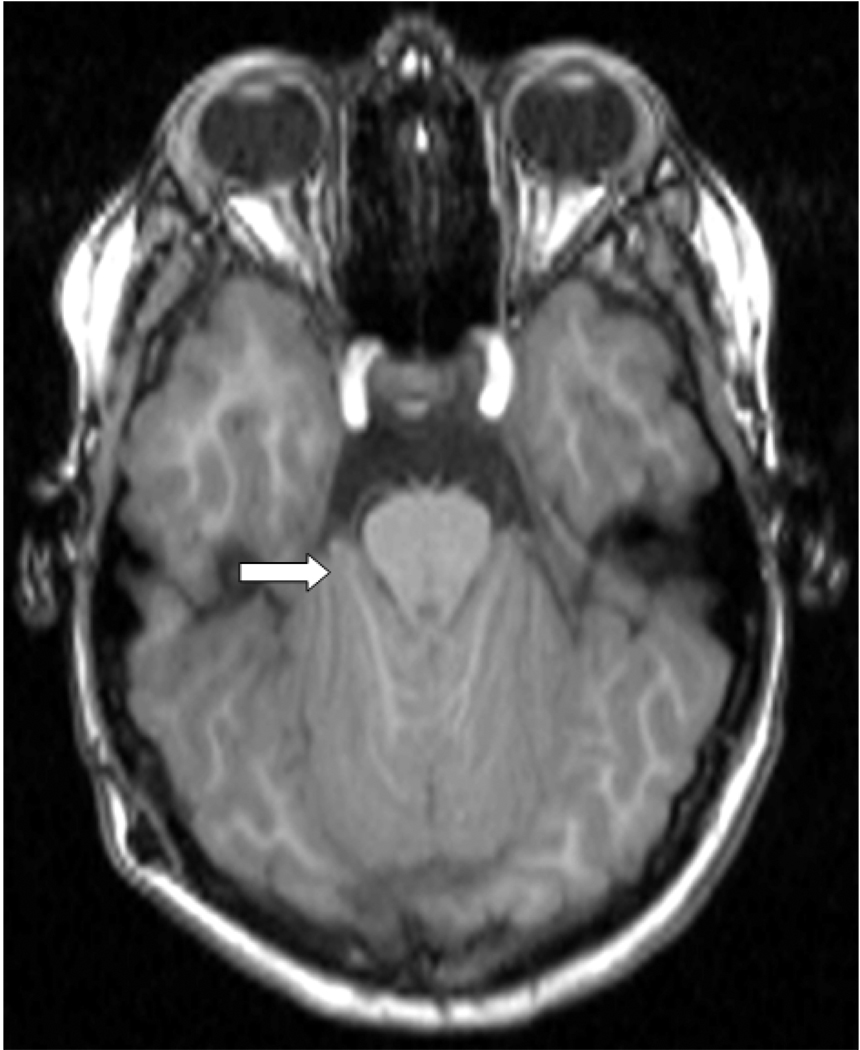
Figure 2.
T1-weighted axial MRI at the level of the pons in a child with Chiari type II malformation illustrating wrapping of the cerebellar hemispheres around the brainstem (arrow).
Morphology of the corpus callosum (CC) is also compromised during early embryogenesis in SBM [Barkovich 2005] as the CC’s programmed pattern of development usually occurs in utero between weeks 7 and 20 of gestation [Barkovich and Norman 1988]. Complex patterns of CC dysmorphology occur along its rostrocaudal axis in SBM [Hannay et al. 2009]. While partial agenesis or dysgenesis and hypoplasia (e.g. underdevelopment) are descriptive terms frequently used to communicate type of dysmorphology, the genu, body, splenium and rostrum are subdivisions of the CC used to communicate where the dysmorphology has occurred [Fletcher et al. 2005; Miller et al. 2008]. An association has been reported between spatial location of CC agenesis and level of the vertebral column where the myelomeningocele occurs (e.g. thoracic vs lumbar/sacral), with thoracic lesions being twice as likely to have agenesis of the splenium as lumbar/sacral level lesions [Hannay et al. 2009]. Thin regions of the CC are generally thought to develop secondary to increased intracranial pressure and hydrocephalus which develop in 80–90% of children born with SBM [Reigel and Rotenstein 1994].
Theories and mechanisms of Chiari type II malformation
Over the years, several theories have been proposed to explain CII. Although no single theory fully accounts for all features of CII [Stevenson 2004; Tubbs et al. 2008], the currently most widely accepted theory is the “unified theory” [McLone and Knepper 1989]. According to this theory, cerebrospinal fluid (CSF) leaks through the spinal defect into the intrauterine environment. Failure of neural tube closure hinders normal distension of the embryonic ventricular system through the pressure generated from CSF build-up during the temporary period of spinal neurocele occlusion [Desmond 1982]. Lack of ventricular distension in utero limits the normal growth of the bony elements of the posterior fossa which significantly reduces posterior fossa size [McLone and Dias 2003; McLone and Knepper 1989]. The contents of the posterior fossa, which proliferate rapidly later [Griffiths et al. 2004], are therefore constrained by a smaller than normal posterior fossa, thereby lending the cerebellum to compression and subsequent volumetric reduction as well as displacement and herniation along its vertical and anterior-posterior axes.
In light of the unified theory, several testable hypotheses to prevent CII from developing have emerged. The basic rationale is that preventing CII will also prevent downstream consequences of CII [Walsh et al. 2001]. Specifically, one might predict that fetal surgical techniques designed to repair the spinal defect in utero would stop CSF leakage through the meningomyelocele, thereby preserving normal distension of the ventricular system, and thus facilitating normal bone growth [see Bowman and McLone. in press]. Reports of reduced or prenatal resolution of hindbrain herniation following surgical repair of spinal meningomyelocele in both animal model systems [Meuli et al. 1995] and human patients [Adzick et al. 1998; Bruner et al. 1999; Sutton et al. 1999; Tulipan et al. 1999] are consistent with the unified theory. Additionally, infants with SBM who had fetal surgery to close their spinal defect have decreased symptoms and signs of brainstem compression [Danzer et al. 2008]. Currently, an NIH-funded multi-center study is conducting a prospective randomized clinical trial (e.g. Management of Myelomeningocele Study) to compare efficacy of prenatal fetal surgery with standard postnatal repair on improving neurological outcome and reducing anomalous brain development in SBM [Bowman and McLone in press; Bowman et al. 2009; Mitchell et al. 2004; Sutton and Adzick 2004].
Additional theories proposed to explain CII that have not been widely supported include: hindbrain dysgenesis theory, which postulated that the hindbrain was underdeveloped in SBM; the traction theory in which downward traction of the lower spinal cord was thought to pull down the hindbrain into the spinal canal; the overgrowth theory which suggested there is overgrowth of the neural tissue prior to closure of the neural tube; and the hydrodynamic theory in which there is downward herniation of the hindbrain caused by the raised intracranial pressure from the hydrocephalus [Gardner et al. 1975; Gilbert et al. 1986; Harding and Copp 2002; McLone and Knepper 1989].
Recent investigations have tried to bridge both “ends” of the myelomeningocele problem: the open neural tube defect in the spinal cord and reduced posterior fossa size in the cranium. As suggested by Williams [2008], both genetic and environmental factors are likely to have key roles in the etiology of posterior fossa hypoplasia in CII, which together with low spinal pressure, contribute to the development of CII [Williams 2008]. In Sarnat’s molecular-genetic explanation for CII abnormalities, he suggested that abnormal rhombomere formation and ectopic expression of homeobox genes of the rostrocaudal axis of the neural tube may explain CII features [Sarnat 2000]. Clearly, additional research is needed to clarify the mechanism(s) mediating the robust association between the formation of the myelomeningocele in the spine and the CII in the cranium.
As enthusiasm for genotype-phenotype studies focused on the cerebellum continues to spread, our knowledge of molecular cascades underlying neural tube closure and posterior fossa development will expand accordingly [see Au et al. in press]. Recent reports suggest a transcription factor expressed in the head mesenchyme, FOXC1, is required for normal skull development [Rice et al. 2003] and normal cerebellar development [Aldinger et al. 2009b]. Variations in the amino acid sequence of FOXC1 can result in a spectrum of phenotypes in cerebellar morphology via defective mesenchymal signaling [Aldinger et al. 2009a]. FOXC1 is a reasonable candidate for further study as it is expressed near the time of neural tube closure and is localized to the mesenchyme where skull development, brainstem, and cerebellar development could all be influenced by variability in its expression levels [Aldinger et al. 2009b]. However, definitive mechanisms underlying CII development still remain to be elucidated.
The cerebellum: quantitative studies
Studies of the cerebellum have consistently reported reduced cerebellar size in SBM, which is even evident early in fetal development. In a 16-week old fetus with SBM, the neural tissue in the posterior fossa was found to be half the size of the neural tissue in the posterior fossa in a control fetus [Brocklehurst 1969]. In young neonates, significant reduction in cerebellar weight was found in 100 children with SBM, who died within four weeks of birth, in comparison to cerebellar weight of 60 children with normal vermis who died from other causes [Variend and Emery 1973]. Subsequent degeneration of the cerebellum in CII, also referred to as ‘vanishing cerebellum’, in CII has also been reported (e.g., in Sener’s [1995] study of four children, age 1 month to 4.5 years, using magnetic resonance imaging (MRI) of the brain). Microscopic studies have described reduced cell numbers and DNA content, particularly in the internal granular layer of the inferior vermis, in 100 children with SBM compared with 120 controls [Emery and Gadsdon 1975]. More recently, microscopic analyses of herniated cerebellar tissue have documented granule and Purkinje cell loss, reduction and gliosis of the folia, and myelin depletion [Harding and Copp 2002].
Distance measurements, planimetry (e.g. area measurements), and biometric (e.g. shape) analyses of the posterior fossa and its contents have been performed in MRI studies of individuals with SBM to quantify abnormalities, document variability, and predict function or prognosis. In SBM, the longest transverse and longitudinal distances across the midsagittal vermis indicated expansion, not reduction, relative to age-matched controls [Salman et al. 2006a]. In the same study, mean area measurements of the midsagittal vermis (anterior, posterior, and inferior subdivisions) were significantly increased by 25% in SBM compared to normal controls despite a 9% reduction in posterior fossa area measurements in SBM [Salman et al. 2006a]. Using shape analyses, Tsai and colleagues [Tsai et al. 2002] quantitatively documented the tremendous variability across individuals with SBM in the degree to which the hindbrain is malformed. According to their findings, shape changes in bone and vermian descent co-occur in a relational fashion. However, herniation of the cervico-medullary junction appears to be independent of vermian herniation, thereby suggesting different etiologies for herniation of the vermis and herniation of the cervico-medullary junction.
Early volumetric studies of the cerebellum in a group of individuals with SBM used non-invasive MRI methods to quantitatively assess group differences between 52 children with SBM and16 age-matched controls [Dennis et al. 2004]. While this study found significantly reduced cerebellar volumes in the SBM group, it also described a significant effect of lesion level on cerebellar gray matter (GM) and white matter (WM) volumes. Consistent with neurological outcomes, where individuals with lower level spinal lesions are less impaired than those with thoracic level lesions, higher spinal lesion levels exhibited greater reductions in cerebellar volumes of GM and WM (~37%) than those with lower level lesions (~22%) [Dennis et al. 2004]. Another study of children with SBM [Fletcher et al. 2005] reported reduced cerebrum and cerebellar volumes in children with upper (n = 25) in comparison with lower (n = 61) spinal lesion levels. Together, these studies demonstrate that upper level spinal lesions in SBM are a marker of more severe brain dysmorphology, and are associated with poorer cognitive and behavioral outcomes [Dennis et al. 2004; Fletcher et al. 2005].
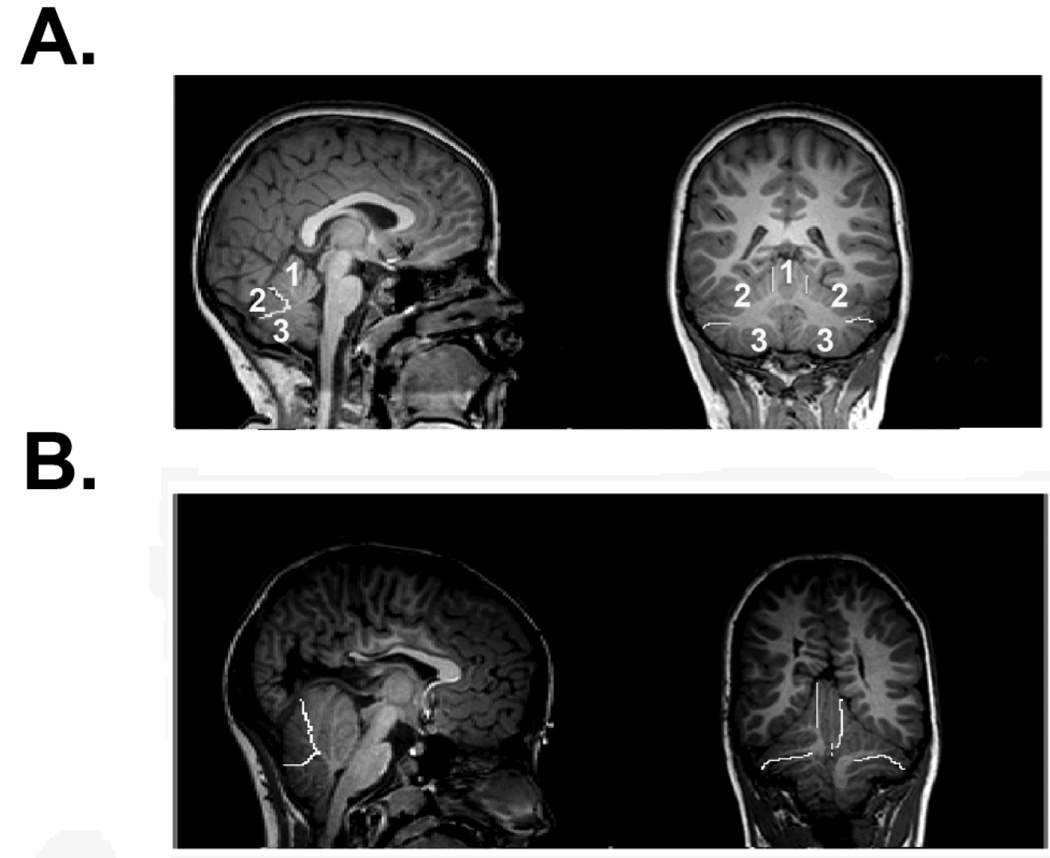
A recent volumetric MRI study used a simple approach for parcellating the cerebellum into meaningful subunits: the corpus medullare, the anterior lobe, and the posterior-superior and posterior-inferior subdivisions [Juranek et al. 2010]. Despite the overall reduction in cerebellar volume in the SBM group, absolute and relative volume of the anterior lobe was significantly increased relative to a typically developing comparison group. Although the absolute volume of the corpus medullare was significantly reduced in the SBM group, the relative volume of the corpus medullare was not significantly different from the typically-developing group when corrected for total cerebellar volume. As shown in Figure 3, the smaller cerebellum observed in individuals with SBM is not simply linearly reduced equally across all regions, but rather disproportionately enlarged in the anterior lobe, reduced in the posterior-inferior subdivision, and not significantly different in the corpus medullare or the posterior-superior subdivision.
Figure 3.
The cerebellum has been parcellated into three primarily gray matter compartments as shown in sagittal (left panels) and coronal (right panels) views. Boundary demarcations between compartments are indicated by bright white lines (anterior lobe, superior-posterior, and inferior-posterior subdivisions) in three representative subjects (A–B). A: Typically-developing child with cerebellar subdivisions numbered as 1=anterior lobe, 2=superior-posterior, 3=inferior-posterior. B: Child with SBM. The central corpus medullare was also parceled separate from the other three parcels for quantitative analyses. The smaller cerebellum observed in individuals with SBM is not simply linearly reduced equally across all regions, but rather disproportionately enlarged in the anterior lobe, reduced in the posterior-inferior subdivision, and not significantly different in the corpus medullare or the posterior-superior subdivision.
The functional significance of regionally-specific patterns of volumetric enlargement and reduction in cerebellar subdivisions remains to be established. While the anterior lobe is generally associated with motor function, it is not yet clear whether a larger anterior lobe confers advantages or disadvantages in the motor function domain. Individuals with SBM demonstrate a wide range of deficits in motor skills requiring strength or speed [Hetherington and Dennis 1999] or dynamic regulatory control [Jewell et al. 2009; Sandler et al. 1993]. In contrast, they perform pretty well on motor skills requiring learning and adaptation [Colvin et al. 2003; Dennis et al. 2006a; Edelstein et al. 2004]. Thus, ongoing studies investigating these types of brain structure-function associations are ideally-suited to provide new knowledge and insight for continued progress in hypothesis-driven research.
Consequences of Chiari type II malformation on hindbrain motor and visceral functions
Intrinsic brainstem abnormalities and pressure on the brainstem from a crowded posterior fossa and hydrocephalus give rise to a diverse range of symptoms such as headaches, apnea, bradycardia, dysphagia, torticollis, spasticity, and impaired auditory evoked potentials [Bowman and McLone in press; Henriques Filho and Pratesi 2006; Wagner et al. 2002]. Brainstem compression occurs in a third of patients with CII, and may be fatal in about a third of these patients [Stevenson 2004]. Surgical decompression of the posterior fossa for symptomatic CII is required in 8 to 17% of subjects with SBM [Wagner et al. 2002] and usually leads to clinical improvement. Maternal fetal surgery for SBM may also reduce the incidence and severity of brainstem dysfunction [Danzer et al. 2008].
Several eye movement abnormalities occur in CII including impaired smooth ocular pursuit, saccadic (i.e., fast eye movements) dysmetria, impaired performance of the vestibular-ocular reflex, various forms of pathological nystagmus (abnormal ocular oscillations), strabismus, and internuclear ophthalmoplegia (a type of gaze palsy that causes double vision) [Leigh and Zee 2006]. Recording different classes of eye movements in CII has shed new light on motor function of the hindbrain in CII in relation to the anatomical changes in this malformation. Midsagittal vermis expansion, including the significant expansion of vermis lobules VI–VII area and preserved medial cerebellar volume corresponded to sparing of eye movements processed by the vermis, despite the overall reduction in cerebellar size that accompanies the deformity of CII [Salman et al. 2009]. These eye movement systems include saccadic accuracy, saccadic adaptation (a form of ocular motor learning), and smooth ocular pursuit [Salman et al. 2005; Salman et al. 2006b; Salman et al. 2007] In contrast, CII patients with abnormal ocular motor functions did not have a significantly expanded midsagittal vermis area. Furthermore, their midsagittal vermis area occupied a smaller midsagittal posterior fossa area, and their cerebellar volumes were smaller than in patients with CII, who had normal eye movements [Salman et al. 2009].
Therefore, the overall reduction in posterior fossa and cerebellar sizes in CII does not affect cerebellar structures or functions uniformly, because there is evidence for functional ocular motor circuits within the deformed hindbrain structures in many patients with CII. In addition, saccadic adaptation still occurred in CII despite the small and dysplastic cerebellum and was evident even in patients with eye movement abnormalities [Salman et al. 2006b; Salman et al. 2009]. Other types of motor learning have also been reported to be preserved in patients with CII including adaptation on reaching or ballistic movements, mirror drawing performance, and weight judgments [Colvin et al. 2003; Dennis et al. 2006a; Edelstein et al. 2004].
The corpus callosum
Even before sophisticated imaging methods became available for quantitative analyses of WM in SBM, aberrant development of the corpus callosum in 70–90% of SBM cases [Barkovich 2005] made it a key focus of early qualitative imaging investigations. Dysgenesis of the corpus callosum (CC) is not a unique feature to SBM, as it also occurs in other neurogenetic syndromes [Ettlinger 1977]. Yet, along with hydrocephalus, CC dysmorphology was one of the earliest visible signs that incomplete neural tube closure at the level of the spinal cord had serious consequences extending beyond the tentorium.
As reported in qualitative studies of the CC, morphological variability is remarkable across individuals with SBM [Reigel and Rotenstein 1994]. Although some of this variability may be related to lesion level, lesion level does not account for all of the heterogeneity [Fletcher et al. 2005]. Recently, 26 regional patterns of CC morphology have been carefully documented in a sample of 193 children with SBM based on qualitative assessment of CC appearance across four subdivisions: rostrum, genu, body, and splenium [Hannay et al. 2009]. While more than 50% of the study sample exhibited partial dysgenesis in either the rostrum (~21%) or splenium (~10%) or both regions (~22%), only 4% of the study sample had normal-appearing CC morphology across all four subdivisions. The remainder of the study sample did not exhibit any dysgenesis, but rather displayed various regional patterns of hypoplastic and normal appearing subdivisions of the CC.
In early quantitative MRI investigations of the corpus callosum in SBM, cross-sectional area measurements were obtained from a single mid-sagittal slice in each participant [Fletcher et al. 1992; 1996;Hommet et al. 2002; Kawamura et al. 2002]. On average, the area measurement of the CC was small in children with SBM compared to controls. In all these studies, a diverse spectrum of corpus callosum morphology across SBM cases was reported; a minority of cases appearing to have normal corpus callosum size and morphology, and the majority of cases appearing abnormal with either hypoplastic features and/or partial agenesis. Consistent with a rostral-caudal developmental course of the corpus callosum [Barkovich and Norman 1988], the genu was reported to be relatively well-preserved in individuals with SBM, with hypoplastic features occurring in the body, and agenesis most prominent in the isthmus/splenium sub-region [Kawamura et al. 2002].
Given the high incidence of posterior corpus callosum dysmorphology in individuals with SBM, it is not too surprising that significant deficits commonly occur in performance of cognitive tasks requiring recruitment of posterior networks, interhemispheric transfer, and transfer of language information [Dennis et al. 2006b; Hannay et al. 2008; Huber-Okrainec et al. 2005]. Thus, processing of semantic information is performed relatively easily, presumably due to a well-preserved genu as suggested by results of split-brain surgical patient studies [Sidtis et al. 1981]. However, idiom comprehension is negatively impacted by hypoplastic CC morphology [Huber-Okrainec et al. 2005]. Even interhemispheric processing of simple auditory signals, as used in a dichotic listening task, is influenced by splenium dysgenesis, but not CC hypoplasia [Hannay et al. 2008].
The cortex
Originally described nearly thirty years ago using pneumoencephalography and cerebral tomography imaging, posterior thinning of the cortical mantle in SBM was an important discovery that seemed to explain behavioral deficits in posterior network function [Dennis et al. 1981]. Using en bloc regional definitions in their volumetric analyses of structural MRIs, Fletcher et al. [2005] described significant reductions in both GM and WM volumes posterior to the genu of the corpus callosum in SBM. More recently, high-resolution surface-based cortical reconstruction analysis methods developed by Fischl and colleagues [Fischl and Dale 2000] have been employed. This study reported significantly reduced cortical thickness in posterior regions and significantly increased cortical thickness in frontal regions based on group comparisons between an SBM group and healthy controls matched on age and gender [Juranek et al. 2008]. These findings of altered spatial patterns of cortical thickening and thinning suggest a potential structural correlates of cognitive strengths and weaknesses generally exhibited by individuals with SBM.
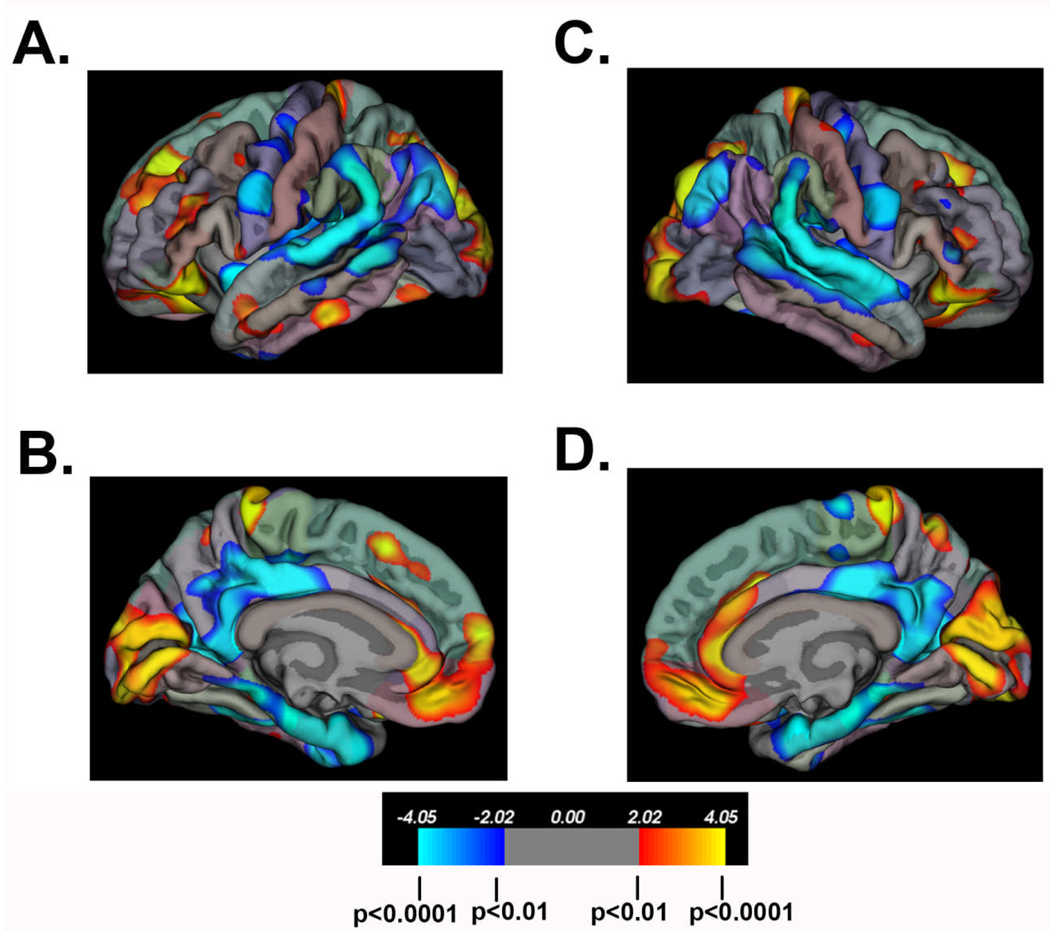
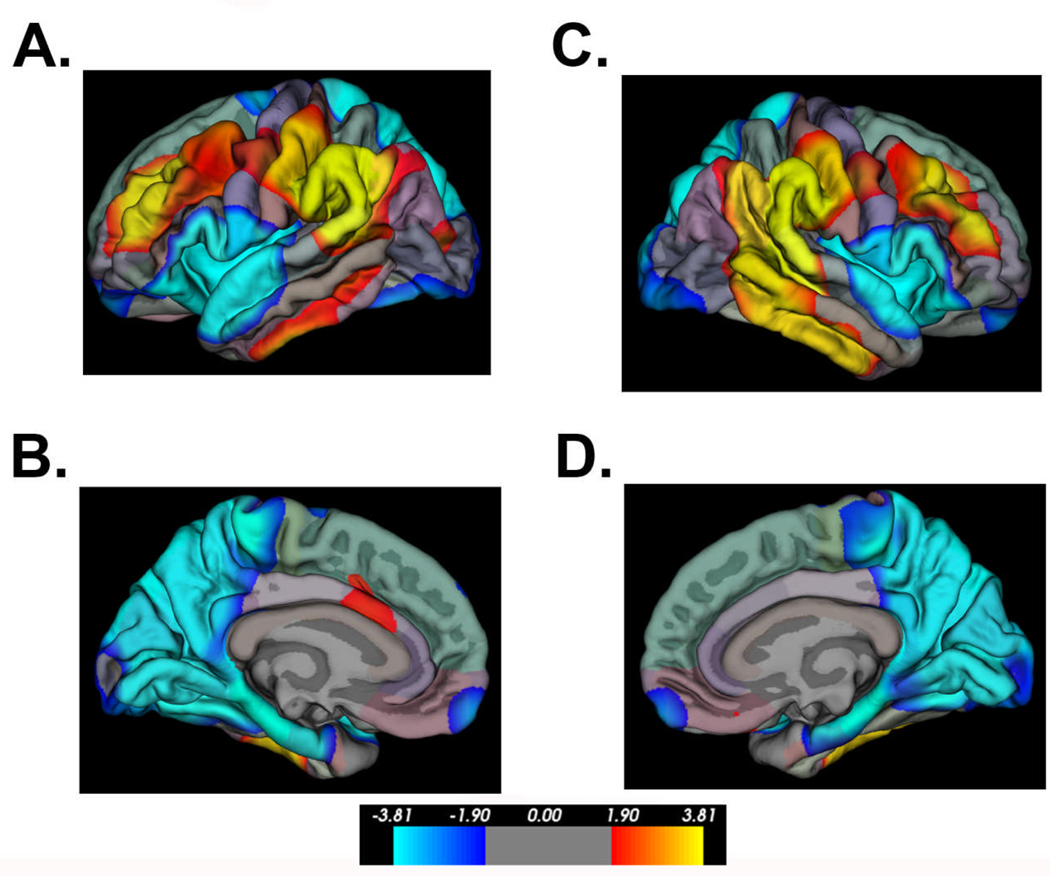
Additional morphometry measures derived from surface-based analyses of the neocortex in individuals with SBM have included cortical GM volume and cortical complexity (e.g. gyrification). Using a 3D local gyrification index (LGI) measure of cortical complexity developed by Schaer et al. [2008], spatial patterns of cortical gyrification have been contrasted with patterns of cortical thickness between a group of individuals with SBM (n=74) and a comparison group of typically-developing study participants (n=31). As shown in Figures 4–5, inferior parietal and posterior temporal areas exhibiting cortical thinning are also characterized by increased cortical complexity. In frontal regions, SBM exhibits increased cortical thickness bilaterally in the following regions: inferior frontal, middle frontal, medial orbitofrontal, rostral anterior cingulate, and superior frontal. While the thickened inferior frontal and orbitofrontal regions have reduced cortical complexity, the thickened middle frontal regions concurrently have increased cortical complexity.
Figure 4.
Significant group differences in cortical thickness between individuals with SBM (n=74) and a comparison group of typically-developing (TD) study participants (n=31). “Hot” colors indicate cortical thickness is greater in the SBM group relative to the TD group; “cold” colors indicate cortical thickness is reduced in the SBM group relative to the TD group. Color scale bar indicates significance values (corrected for multiple comparisons) ranging from p<0.0001 to p<0.01. In frontal regions, the SBM group exhibits increased cortical thickness bilaterally in the following regions: inferior frontal, middle frontal, medial orbitofrontal, rostral anterior cingulate, and superior frontal. In bilateral inferior parietal and posterior temporal areas, the SBM group displays significant cortical thinning.
Figure 5.
Significant group differences in cortical complexity between individuals with SBM (n=74) and a comparison group of typically-developing (TD) study participants (n=31). “Hot” colors indicate cortical complexity is greater in the SBM group relative to the TD group; “cold” colors indicate cortical complexity is reduced in the SBM group relative to the TD group. In bilateral inferior parietal and posterior temporal areas, the SBM group displays significantly increased cortical complexity. In bilateral inferior frontal and orbitofrontal regions, the SBM group shows significantly reduced cortical complexity.
White matter pathways: Diffusion Tensor Imaging (DTI) studies
DTI provides a non-invasive measure of structural integrity of WM tracts. This MR modality capitalizes on the inherent properties of the motion of water. The averaged rate of diffusion and directionality of water movement can be quantified as mean diffusivity and fractional anisotropy, respectively [Basser et al. 1994]. Disruptions in WM integrity are reflected in mean diffusivity (MD) coefficients and fractional anisotropy (FA) measures despite the normal appearance of WM on routine MRIs. Due to the cylindrical geometry of neuronal fibers in WM, anisotropy is higher than in GM where the cellular geometry is more globular. Thus, lower anisotropy values in WM are indicative of poor structural organization of WM tracts because highly organized fiber bundles constrain motion of water molecules in a preferred direction parallel to the orientation of the fiber tracts. Higher mean diffusivity values reflect poor structural integrity of WM since motion of water molecules is poorly constrained when cellular architecture of neuronal fibers is compromised [Beaulieu 2002].
Using DTI acquisition and analysis methods, Vachha et al. [2006] investigated limbic tract anomalies in 13 children with SBM. The majority of children with SBM had atypical tractography results for the fornix (9/13) and cingulum (10/13). While the fimbria of the hippocampus and the columns of the fornix were consistently intact in the SBM cases, the crura and the body of the fornix exhibited defects, frequently including thinning. Reconstruction of the cingulum was particularly challenging in SBM cases for the fronto-parietal, temporal, and pre-septal segments which were either absent or markedly hypoplastic [Vachha et al. 2006].
Hasan et al. [2008b] investigated DTI metrics of WM in several regions of interest, including the CC, arcuate fasiculus, and the anterior and posterior limbs of the internal capsule in 29 SBM children. In addition, the inferior longitudinal, inferior fronto-occipital, and uncinate fasiculi were evaluated in 38 SBM children [Hasan et al. 2008a]. Similar to investigations of other brain regions, a complex pattern of white matter (WM) differences between SBM and healthy controls has begun to emerge. In Hasan’s studies, group comparisons of some WM pathways exhibited no significant differences in fractional anisotropy (FA) measures (e.g. anterior and posterior limbs of the internal capsule, and the arcuate fasiculus), while other WM pathways were reported to have significantly lower FA values (e.g. genu of the CC, inferior longitudinal, inferior fronto-occipital, uncinate, and arcuate fasiculi). Thus, less-organized WM in SBM primarily occurs in the long association pathways linking posterior brain regions with anterior regions (e.g. inferior longitudinal and inferior fronto-occipital) which prove to be particularly difficult to track completely in SBM. However, the genu of the CC also exhibited significantly reduced FA values in SBM, indicating that commissural fibers between frontal regions are also compromised in SBM.
In another recent DTI study, Herweh and colleagues [Herweh et al. 2009] evaluated FA values from the CC and the anterior commissure in 6 SBM cases relative to a comparison group (n=6) of age- and gender-matched healthy controls. similar to Hasan et al. [2008], these investigators also reported significantly reduced FA values of the genu in the SBM group. Additionally, the midsagittal area of the genu was significantly reduced in the SBM group. In their analyses of the anterior commissure, significantly increased midsagittal area was observed in the SBM group, accompanied by a non-significant increase in FA. These contrasting differences between the genu of the CC and the anterior commissure in SBM are particularly enlightening since the anterior commissure develops in utero before the CC [Barkovich and Norman 1988].
While it is not yet clear whether hydrocephalus contributes to aberrant development of the corpus callosum [Gadsdon et al. 1979] or other WM pathways in SBM, it is quite clear that WM development is a protracted process that starts early in gestation, with changes in macro- and micro-structure continuing throughout the lifespan. The vulnerability of WM to multiple insults at multiple developmental stages makes this particular tissue class a common candidate for investigations of neurological disorders. Due to tightly-coupled developmental relationships between WM and GM, neither tissue class is altered in isolation; similarly, the cerebellum does not develop in isolation of the cerebrum. In SBM, posterior cortex and the hindbrain exhibit complex structural differences in GM and WM properties relative to age-matched healthy controls; anterior cortex, genu of the corpus callosum, and the anterior commissure are structurally well-suited for preservation of anterior network cognitive function.
Conclusions
As multi-modal imaging studies continue to provide additional insight into the neurophysiological, structural, functional, and cognitive profiles of specific brain-behavior associations in SBM, accounts of strengths and weaknesses continue to provide essential clues for a better understanding of normal neurodevelopment and function. In addition to targeting specific structures (either GM regions or WM pathways) in hypothesis-driven research, network-level analyses will advance our understanding of neural circuit structure and function to account for connections between multiple structures underlying behavior.
ACKNOWLEDGEMENT
Preparation of this paper was supported in part by grants P01-HD35946 and R01 HD046609 awarded from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the National Institutes of Health.
Grant Sponsor: NIH
Grant Numbers: P01-HD35946 and R01-HD046609
Footnotes
Callouts:
Prominent features of CII include a significantly smaller posterior fossa with its contents crowded and distorted in appearance
Recording different classes of eye movements in CII has shed new light on motor function of the hindbrain in CII in relation to the anatomical changes in this malformation.
Along with hydrocephalus, CC dysmorphology was one of the earliest visible signs that incomplete neural tube closure at the level of the spinal cord had serious consequences extending beyond the tentorium.
Contributor Information
Jenifer Juranek, Department of Pediatrics, Children’s Learning Institute, University of Texas Health Science Center at Houston, Houston, TX, USA.
Michael S. Salman, Section of Pediatric Neurology, Children’s Hospital, Faculty of Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
REFERENCES
- Adzick NS, Sutton LN, Crombleholme TM, et al. Successful fetal surgery for spina bifida. The Lancet. 1998;352(9141):1675–1676. doi: 10.1016/S0140-6736(98)00070-1. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Elsen GE, Prince VE, et al. Model Organisms Inform the Search for the Genes and Developmental Pathology Underlying Malformations of the Human Hindbrain. Seminars in Pediatric Neurology. 2009a;16(3):155–163. doi: 10.1016/j.spen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Lehmann OJ, Hudgins L, et al. FOXC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009b;41(9):1037–1042. doi: 10.1038/ng.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au P, Ashley-Koch A, Northrup N. Spina bifida: Epidemiologic and genetic aspects. Dev Dis Res Rev. doi: 10.1002/ddrr.93. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Norman D. Anomalies of the corpus callosum: correlation with further anomalies of the brain. Am J Roentgenol. 1988;151(1):171–179. doi: 10.2214/ajr.151.1.171. [DOI] [PubMed] [Google Scholar]
- Barkovich J. Pediatric neuroimaging. Philadelphia, PA: Lippincott, Williams & Wilkens; 2005. [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical Journal. 1994;66(1):259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: a review from the Children's Memorial Hospital. Childs Nerv Syst. 2009;25(7):801–806. doi: 10.1007/s00381-009-0865-z. [DOI] [PubMed] [Google Scholar]
- Bowman R, McLone D. Neurosurgical management of spina bifida: Research issues. Dev Dis Res Rev. doi: 10.1002/ddrr.100. in press. [DOI] [PubMed] [Google Scholar]
- Brocklehurst G. A quantitative study of a spina bifida foetus. J Pathology. 1969;99(3):205–211. doi: 10.1002/path.1710990304. [DOI] [PubMed] [Google Scholar]
- Bruner JP, Tulipan N, Paschall RL, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282(19):1819–1825. doi: 10.1001/jama.282.19.1819. [DOI] [PubMed] [Google Scholar]
- Colvin AN, Yeates KO, Enrile BG, et al. Motor adaptation in children with myelomeningocele: Comparison to children with ADHD and healthy siblings. Journal of the International Neuropsychological Society. 2003;9(04):642–652. doi: 10.1017/S1355617703940045. [DOI] [PubMed] [Google Scholar]
- Danzer E, Finkel RS, Rintoul NE, et al. Reversal of hindbrain herniation after maternal-fetal surgery for myelomeningocele subsequently impacts on brain stem function. Neuropediatrics. 2008;39(06):359–362. doi: 10.1055/s-0029-1202835. [DOI] [PubMed] [Google Scholar]
- Del Bigio M. Neuropathology and structural changes in hydrocephalus. Dev Dis Res Rev. doi: 10.1002/ddrr.94. in press. [DOI] [PubMed] [Google Scholar]
- Dennis M, Edelstein K, Hetherington R, et al. Neurobiology of perceptual and motor timing in children with spina bifida in relation to cerebellar volume. Brain. 2004;127(6):1292–1301. doi: 10.1093/brain/awh154. [DOI] [PubMed] [Google Scholar]
- Dennis M, Fitz C, Netley C, et al. The intelligence of hydrocephalic children. Archives of Neurology. 1981;38(10):607–615. doi: 10.1001/archneur.1981.00510100035004. [DOI] [PubMed] [Google Scholar]
- Dennis M, Jewell D, Edelstein KIM, et al. Motor learning in children with spina bifida: Intact learning and performance on a ballistic task. Journal of the International Neuropsychological Society. 2006a;12(05):598–608. doi: 10.1017/S1355617706060772. [DOI] [PubMed] [Google Scholar]
- Dennis M, Landry SH, Barnes M, et al. A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society. 2006b;12(02):285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- Desmond M. Description of the occlusion of the spinal cord lumen in early human embryos. The Anatomical Record. 1982;204:89–93. doi: 10.1002/ar.1092040112. [DOI] [PubMed] [Google Scholar]
- Edelstein K, Dennis M, Copeland K, et al. Motor learning in children with spina bifida: Dissociation between performance level and acquisition rate. Journal of the International Neuropsychological Society. 2004;10(06):877–887. doi: 10.1017/s1355617704106085. [DOI] [PubMed] [Google Scholar]
- Emery J, Gadsdon D. A quantitative study of the cell population of the cerebellum in children with myelomeningocele. Developmental Medicine and Child Neurology. 1975;35:20–25. doi: 10.1111/j.1469-8749.1975.tb03575.x. [DOI] [PubMed] [Google Scholar]
- Ettlinger G. Agenesis of the corpus callosum. In: Vinken V, Bruyn G, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier/North-Holland Biomedical Press; 1977. pp. 285–297. [Google Scholar]
- Fischl B, Dale A. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Science, USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, et al. Cerebral white matter and cognition in hydrocephalic children. Arch of Neuro. 1992;49:818–824. doi: 10.1001/archneur.1992.00530320042010. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Bohan TP, Brandt ME, et al. Morphometric evaluation of the hydrocephalic brain: Relationships with cognitive abilities. Ch Nerv Sys. 1996;12:192–199. doi: 10.1007/BF00301250. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Copeland K, Frederick JA, et al. Spinal lesion level in spina bifida: a source of neural and cognitive heterogeneity. Journal of Neurosurgery: Pediatrics. 2005;102(3):268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- Gadsdon D, Variend S, Emery J. Myelination of the corpus callosum. II. The effect of relief of hydrocephalus upon the processes of myelination. Zeitschrift fur Kinderchirurgie und Grenzgebiete. 1979;28(4):314–321. [PubMed] [Google Scholar]
- Gardner E, O'Rahilly R, Prolo D. The Dandy-Walker and Arnold-Chiari malformations. Clinical, developmental, and teratological considerations. Archives in Neurology. 1975;32(6):393–407. doi: 10.1001/archneur.1975.00490480059007. [DOI] [PubMed] [Google Scholar]
- Gilbert J, Jones K, Rorke L, et al. Central nervous system anomalies associated with menigomyocele, hydrocephalus, and the Arnold-Chiari malformation: reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery. 1986;18:559–564. doi: 10.1227/00006123-198605000-00008. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Wilkinson I, Variend S, et al. Differential growth rates of the cerebellum and posterior fossa assessed by post mortem magnetic resonance imaging of the fetus: implications for the pathogenesis of the chiari 2 deformity. Acta Radiologica. 2004;45(2):236–242. doi: 10.1080/02841850410003572. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Dennis M, Kramer L, et al. Partial agenesis of the corpus callosum in spina bifida meningomyelocele and potential compensatory mechanisms. Journal of Clinical and Experimental Neuropsychology. 2009;31(2):180–194. doi: 10.1080/13803390802209954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannay HJ, Walker AMY, Dennis M, et al. Auditory interhemispheric transfer in relation to patterns of partial agenesis and hypoplasia of the corpus callosum in spina bifida meningomyelocele. Journal of the International Neuropsychological Society. 2008;14(05):771–781. doi: 10.1017/S1355617708080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B, Copp A. Malformations. In: Graham D, Lantos P, editors. Greenfield's Neuropathology. London: Edward Arnold; 2002. pp. 376–386. [Google Scholar]
- Hasan K, Eluvathingal T, Kramer L, et al. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: A diffusion tensor tractography study of the association pathways. Journal of Magnetic Resonance Imaging. 2008a;27(4):700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Sankar A, Halphen C, et al. Quantitative diffusion tensor imaging and intellectual outcomes in spina bifida. Journal of Neurosurgery: Pediatrics. 2008b;2(1):75–82. doi: 10.3171/PED/2008/2/7/075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques Filho P, Pratesi R. Abnormalities in auditory evoked potentials of 75 patients with Arnold-Chiari malformations types I and II. Arquivos de neuro-psiquiatria. 2006;64(3A):619–623. doi: 10.1590/s0004-282x2006000400019. [DOI] [PubMed] [Google Scholar]
- Herweh C, Akbar M, Wengenroth M, et al. DTI of commissural fibers in patients with Chiari II-malformation. NeuroImage. 2009;44(2):306–311. doi: 10.1016/j.neuroimage.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Hetherington R, Dennis M. Motor function profile in children with early onset hydrocephalus. Developmental Neuropsychology. 1999;15(1):25–51. [Google Scholar]
- Hommet C, Cottier JP, Billard C, et al. MRI morphometric study and correlation with cognitive functions in young adults shunted for congenital hydrocephalus related to spina bifida. European Neurology. 2002;47(3):169–174. doi: 10.1159/000047977. [DOI] [PubMed] [Google Scholar]
- Huber-Okrainec J, Blaser SE, Dennis M. Idiom comprehension deficits in relation to corpus callosum agenesis and hypoplasia in children with spina bifida meningomyelocele. Brain and Language. 2005;93(3):349–368. doi: 10.1016/j.bandl.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Jewell D, Fletcher J, Mahy C, et al. Upper limb cerebellar motor function in children with spina bifida. Child's Nervous System. 2009;26(1):67–73. doi: 10.1007/s00381-009-0991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Dennis M, Cirino PT, et al. The cerebellum in children with spina bifida and Chiari II malformation: Quantitative volumetrics by region. The Cerebellum. 2010 doi: 10.1007/s12311-010-0157-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Fletcher JM, Hasan KM, et al. Neocortical reorganization in spina bifida. NeuroImage. 2008;40(4):1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Nishio S, Morioka T, et al. Callosal anomalies in patients with spinal dysraphism: Correlation of clinical and neuroimaging features with hemispheric abnormalities. Neurological Research. 2002;24:463–467. doi: 10.1179/016164102101200348. [DOI] [PubMed] [Google Scholar]
- Leigh R, Zee D. The neurology of eye movements. New York: Oxford University Press; 2006. [Google Scholar]
- McLone DG, Dias MS. The Chiari II malformation: cause and impact. Child's Nervous System. 2003;19(7):540–550. doi: 10.1007/s00381-003-0792-3. [DOI] [PubMed] [Google Scholar]
- McLone DG, Knepper PA. The cause of Chiari II malformation: a unified theory. Pediatr Neurosci. 1989;15:1–12. doi: 10.1159/000120432. [DOI] [PubMed] [Google Scholar]
- Meuli M, Meuli-Simmen C, Hutchins GM, et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med. 1995;1(4):342–347. doi: 10.1038/nm0495-342. [DOI] [PubMed] [Google Scholar]
- Miller E, Widjaja E, Blaser S, et al. The old and the new: supratentorial MR findings in Chiari II malformation. Child's Nervous System. 2008;24(5):563–575. doi: 10.1007/s00381-007-0528-x. [DOI] [PubMed] [Google Scholar]
- Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. The Lancet. 2004;364(9448):1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- Oaks W, Gaskill S. Symptomatic Chiari malformations in childhood. In: Park T, editor. Spinal dysraphism. Boston, MA: Blackwell Scientific Publications; 1992. pp. 104–125. [Google Scholar]
- Reigel D, Rotenstein D. Spina bifida. In: Cheek W, editor. Pediatric neurosurgery. 3rd ed. Philadelphia: WB Saunders; 1994. pp. 51–76. [Google Scholar]
- Rice R, Rice DPC, Olsen BR, et al. Progression of calvarial bone development requires Foxc1 regulation of Msx2 and Alx4. Developmental Biology. 2003;262(1):75–87. doi: 10.1016/s0012-1606(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Salman MS, Blaser S, Sharpe J, et al. Cerebellar vermis morphology in children with spina bifida and Chiari type II malformation. Child's Nervous System. 2006a;22(4):385–393. doi: 10.1007/s00381-005-1180-y. [DOI] [PubMed] [Google Scholar]
- Salman MS, Dennis M, Sharpe JA. The cerebellar dysplasia of Chiari II malformation as revealed by eye movements. Canadian Journal of Neurological Sciences. 2009;36(6):713–724. doi: 10.1017/s0317167100008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, et al. Saccades in children with spina bifida and Chiari type II malformation. Neurology. 2005;64(12):2098–2101. doi: 10.1212/01.WNL.0000166034.71337.5E. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Eizenman M, et al. Saccadic adaptation in Chiari type II malformation. Canadian Journal of Neurological Sciences. 2006b;33(4):372–378. doi: 10.1017/s0317167100005321. [DOI] [PubMed] [Google Scholar]
- Salman MS, Sharpe JA, Lillakas L, et al. Smooth ocular pursuit in Chiari type II malformation. Developmental Medicine & Child Neurology. 2007;49(4):289–293. doi: 10.1111/j.1469-8749.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- Sandler A, Macias M, Brown T. The drawings of children with spina bifida: developmental correlations and interpretations. European Jouranl of Pediatric Surgery. 1993;3(S1):25–27. [PubMed] [Google Scholar]
- Sarnat HB. Molecular Genetic Classification of Central Nervous System Malformations. J Child Neurol. 2000;15(10):675–687. doi: 10.1177/088307380001501007. [DOI] [PubMed] [Google Scholar]
- Schaer M, Cuadra M, Tamarit L, et al. A surface-based approach to quantify local cortical gyrification. IEEE Transactions on Medical Imaging. 2008;27(2):161–170. doi: 10.1109/TMI.2007.903576. [DOI] [PubMed] [Google Scholar]
- Sener RN. Cerebellar agenesis versus vanishing cerebellum in Chiari II malformation. Computerized Medical Imaging and Graphics. 1995;19(6):491–494. doi: 10.1016/0895-6111(96)00002-x. [DOI] [PubMed] [Google Scholar]
- Sidtis JJ, Volpe BT, Holtzman JD, et al. Cognitive Interaction after Staged Callosal Section: Evidence for Transfer of Semantic Activation. Science. 1981;212(4492):344–346. doi: 10.1126/science.6782673. [DOI] [PubMed] [Google Scholar]
- Stevenson K. Chiari Type II malformation: past, present, and future. Neurosurgical Focus. 2004;16(2):E5. doi: 10.3171/foc.2004.16.2.6. [DOI] [PubMed] [Google Scholar]
- Sutton LN, Adzick NS. Fetal surgery for myelomeningocele. Clinical Neurosurgery. 2004;51:155–162. [PubMed] [Google Scholar]
- Sutton LN, Adzick NS, Bilaniuk LT, et al. Improvement in Hindbrain Herniation Demonstrated by Serial Fetal Magnetic Resonance Imaging Following Fetal Surgery for Myelomeningocele. JAMA. 1999;282(19):1826–1831. doi: 10.1001/jama.282.19.1826. [DOI] [PubMed] [Google Scholar]
- Tsai T, Bookstein FL, Levey E, et al. Chiari-II Malformation: A Biometric Analysis. Eur J Pediatr Surg. 2002;12(S 1):12–18. doi: 10.1055/s-2002-36865. [DOI] [PubMed] [Google Scholar]
- Tubbs R, Shoja M, Ardalan M, et al. Hindbrain herniation: A review of embryological theories. Italian Jouranl of Anatomy and Embryology. 2008;113(1):37–46. [PubMed] [Google Scholar]
- Tulipan N, Hernanz-Schulman M, Lowe LH, et al. Intrauterine Myelomeningocele Repair Reverses Preexisting Hindbrain Herniation. Pediatric Neurosurgery. 1999;31(3):137–142. doi: 10.1159/000028849. [DOI] [PubMed] [Google Scholar]
- Vachha B, Adams RC, Rollins NK. Limbic Tract Anomalies in Pediatric Myelomeningocele and Chiari II Malformation: Anatomic Correlations with Memory and Learning -- Initial Investigation. Radiology. 2006;240(1):194–202. doi: 10.1148/radiol.2401050674. [DOI] [PubMed] [Google Scholar]
- Variend S, Emery J. The weight of the cerebellum in children with myelomeningocele. Developmental Medicine & Child Neurology. 1973;29:77–83. doi: 10.1111/j.1469-8749.1973.tb04944.x. [DOI] [PubMed] [Google Scholar]
- Wagner Wa, Schwarz Ma, Perneczky Ab. Primary myelomeningocele closure and consequences. Current Opinion in Urology. 2002;12(6):465–468. doi: 10.1097/00042307-200211000-00003. [DOI] [PubMed] [Google Scholar]
- Walsh DS, Adzick NS, Sutton LN, et al. The Rationale for in utero Repair of Myelomeningocele. Fetal Diagnosis and Therapy. 2001;16(5):312–322. doi: 10.1159/000053934. [DOI] [PubMed] [Google Scholar]
- Williams H. A unifying hypothesis for hydrocephalus, Chiari malformation, syringomyelia, anencephaly and spina bifida. Cerebrospinal Fluid Research. 2008;5(1):7. doi: 10.1186/1743-8454-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]