Abstract
The objectives of this study were to determine whether PlGF exerts a vasodilatory effect on rat uterine vessels (arcuate arteries and veins) and to examine regional differences in reactivity by comparing these responses to those of comparably-sized mesenteric vessels. We also sought to examine and compare its effects on human uterine and subcutaneous vessels. All vessels were studied in vitro, under pressurized (rat) or isometric wire-mounted (human) conditions, and exposed to a range of PlGF concentrations. Inhibitors of nitric oxide and prostaglandin synthesis were included in an effort to understand the causal mechanism(s). In rat uterine arteries, the effects of receptor inhibition and activation using selective ligands for VEGFR-1 (PlGF) vs. VEGFR-2 (VEGF-E) were determined, and real-time RT-PCR was performed to evaluate the effect of pregnancy on relative abundance of VEGFR-1 and VEGFR-2 message in the vascular wall. PlGF was a potent vasodilator of all vessels studied, with greatest sensitivity observed in rat uterine arteries. Pregnancy significantly augmented dilator sensitivity to PlGF, and this effect was associated with selective upregulation of VEGFR-1 message in the pregnant state. The contribution of nitric oxide was appreciable in rat and human uterine arteries, with lesser effects in rat uterine veins and mesenteric arteries, and with no observable effect in human subcutaneous vessels. Based on these results, we conclude that PlGF is a potent vasodilator of several vessel types in both humans and rats. Its potency and mechanism varies with physiological state and vessel location, and is mediated solely by the VEGFR-1 receptor subtype. Gestational changes in the uterine circulation suggest that this factor may play a role in modulating uterine vascular remodeling and blood flow during the pregnant state.
Keywords: VEGF, nitric oxide, uterine circulation, rat, human resistance arteries, VEGFR-1, Flt-1
INTRODUCTION
Earlier studies from our laboratory have shown that rat uterine resistance arteries are highly sensitive to dilation to vascular endothelial growth factor (VEGF), and that this response is further enhanced during pregnancy through a mechanism linked to estrogen [10, 11].
PlGF is a member of the VEGF growth factor family that was discovered in 1991. Unlike some VEGF isoforms, which bind to VEGFR-1 (Flt) and/or VEGFR-2 (KDR or Flk), PlGF binds specifically to the VEGFR-1 receptor. Thus far, four studies have examined the effects of placental growth factor (PlGF) on vascular reactivity, with reports of vasodilatory action in isolated renal [8], placental [12], pulmonary [5] and mammary vessels [15]. The mechanism of dilation has not been determined. Vasodilation to VEGF has been more widely reported and is associated with signaling through the VEGFR-2 receptor via a mechanism that involves activation of phosphoinositide 3-kinase (PI3K) and Akt-dependent phosphorylation of eNOS, resulting in increased NO production [e.g. 13]. In contrast, one report concluded that the vasodilator response may involve both types of receptor [5].
The physiologic role of PlGF is not known, however, studies have shown that its circulating concentrations exceed those of VEGF by >40-fold during normal gestation, although its affinity for the VEGFR-1 receptor is only 1/10th of the affinity of VEGF [1]. During preeclampsia, a disease associated with hypertension and uteroplacental underperfusion, free PlGF concentrations are significantly reduced due to elevations of a soluble form of the VEGFR-1 receptor (sFlt-1) [8,14]. Adenoviral overexpression of sFlt-1 in rats results in a preeclampsia-like syndrome characterized by hypertension, proteinuria, and renal endothelial glomerular endotheliosis [8]. Recently, it has been suggested that a ratio of sFlt-1 to PlGF may be a useful predictive marker for the development of preeclampsia [14].
Based on these observations, we hypothesized that PlGF, like VEGF, may exert a vasodilatory effect on the peripheral vasculature, and thus modulate regional blood flow and resistance. In view of previous results of enhanced uterine artery dilation to VEGF in pregnancy [2], we also measured uterine artery reactivity to PlGF as a function of concentration, as well as the expression of both the VEGF-1 and VEGF-2 receptor message in uterine arteries from pregnant vs. nonpregnant animals to determine whether changes occur during pregnancy.
Pregnancy-induced, species-related, and regional differences in the vasodilatory actions of PlGF were evaluated by extending reactivity studies to mesenteric arteries in rats, and to myometrial and subcutaneous vessels in humans with and without inhibitors of endothelial dilators (nitric oxide, prostaglandins). We also examined its effect on the rat uterine veins that drain the placenta. Due to placental production and secretion of this growth factor, uterine veins are likely to contain the highest concentrations of PlGF prior to systemic dilution.
METHODS
Isolated rat uterine and mesenteric artery reactivity
Adult (12–14 week-old) pregnant and nonpregnant Sprague-Dawley rats were purchased from Charles River Canada, and shipped to the University of Vermont. All procedures were approved by the Institutional Animal Care and Use Committee. Vessels were obtained from late pregnant (Day 20/22 of gestation; n=23) and age-matched nonpregnant animals (n=13) following euthanasia with an injection of methohexital sodium (Brevital, 50 mg/kg i.p) and decapitation in a small animal guillotine. Vessels from pregnant animals were used for reactivity experiments; in addition, those from nonpregnant animals were used to test for the effects of pregnancy on PlGF reactivity (n=5), and for determination of receptor message (n=8).
The uterus and a section of the gut 5–10 cm distal to the pylorus were removed and placed in separate petri dishes containing cold (4°C) HEPES-buffered PSS. HEPES-PSS was composed of (mM): 141.8 NaCl, 4.7 KCl, 1.7 MgSO4, 0.5 EDTA, 2.8 CaCl2, 1.2 KH2PO4, 10.0 HEPES, and 5.0 glucose (pH = 7.4).
Segments (1–2mm long) of uterine arcuate arteries, uterine arcuate veins, and third-order mesenteric arteries were dissected free from connective and adipose tissue and transferred to the chamber of a small vessel arteriograph. One end of the vessel was tied onto a glass cannula and flushed of any luminal contents by increasing the pressure prior to securing the distal end onto a second cannula using a servo-null pressure system (Living Systems Instrumentation). All vessels were initially pressurized (arteries: 50 mmHg; veins: 10 mmHg), and equilibrated for 45–60 min at 37°C prior to the beginning of experimentation. Lumen diameter was measured by trans-illuminating each vessel segment and using a video dimension analyzer (Living Systems Instrumentation) in conjunction with data acquisition software (Ionoptix) to continuously record lumen diameter.
Following equilibration, all vessels were preconstricted with phenylephrine to produce a 50–70% reduction in baseline diameter. Once constriction was achieved, PlGF-2 (mouse, R&D Systems) was added in increasing concentrations (0.1 pM – 10 nM), and the resulting dilation recorded. Separate vessel segments were preincubated with L-NNA (0.2 mM) to inhibit nitric oxide prior to the administration of PlGF.
To determine the receptor subtype responsible for PlGF-induced dilation in the uterine circulation, vessels were incubated with inhibitory antibodies to either VEGFR-1 (R&D Systems; 10 µg/ml) or VEGFR-2 (R&D Systems; 1 µg/ml) for 15 minutes prior to constriction with phenylephrine. These inhibiting antibodies were utilized at the maximal effective concentration specified by the manufacturer, and confirmed experimentally in preliminary tests in our lab.
Once the concentration-response characteristics were defined, a separate group of vessels was prepared as described, and incubated with VEGF-E, an isoform of VEGF that is selective for VEGFR-2 activation alone (1 nM arteries, 10 nM veins; Research Diagnostics Inc.), or in combination with PlGF (0.1 nM in arteries, 5 nM in veins).
Vascular reactivity of human subcutaneous and myometrial arteries
These studies were carried out in collaboration with the Karolinska Institute, Sweden, by two co-authors (K.K. and L.L.). The investigation was undertaken with the approval of the local research ethics committee. Biopsies of subcutaneous fat and myometrium were obtained from healthy pregnant women with a median age of 26 years (range 24–34) and at a median gestational age of 38 weeks (range 37–39) undergoing elective cesarean section due to breach presentation (n=4) or psychological reasons (n=3). Myometrial arterial segments were obtained from the upper edge of the transverse incision in the lower uterine segment during C-section. Biopsies were immediately placed in cold physiological salt solution (PSS, mM: NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.026, glucose 5.5) and utilized for isolation of small arteries, which were mounted in the organ baths of a four-channel wire myograph (Multimyograph, model 610, Danish Myo Technology; Aarhus, Denmark), as previously described [7]. Each organ bath contained warmed (37°C) physiological saline solution (PSS) that was continuously bubbled with 5% CO2 carbon dioxide/95% O2 oxygen. Following a 30 minute equilibration period, a passive circumference–tension curve was created for each segment in order to set optimum resting tension. Endothelium-dependent vasodilatation was assessed by the addition of a single concentration of bradykinin (BK, 1 µmol/L) to each chamber after pre-constriction with norepinephrine (NE, 1 µmol/L). Arteries that did not achieve at least 70% vasorelaxation to BK were excluded from the study.
Both myometrial and subcutaneous arteries were pre-constricted with norepinephrine (3 µM), and concentration-response curves to PlGF (0.01–10 nM) were generated before and after a 30 minute incubation with a solution containing a combination of L-NAME (300 µM) and indomethacin (Indo, 10 µM) to inhibit production of endothelial NO.
Measurement of message for VEGFR-1 and VEGFR-2 by RT-PCR
Main uterine arteries were isolated and flash-frozen in liquid nitrogen for subsequent extraction and purification of mRNA, followed by real-time RT-PCR Taqman assay to evaluate the mRNA expression of VEGFR-1 and VEGFR-2. Proprietary sequences were purchased from ABI. Total RNA was extracted using Trizol (Invitrogen) as described by the manufacturer. RNA concentrations were determined by measuring the absorbance at a wavelength of 260 and 280 nm. RNA was then converted to cDNA using random hexamer primers and the Superscript TM First-strand Synthesis System, and stored at −70°C until ready for use. Real-time quantification of target mRNA transcripts was performed using an ABI Prism 7000 Sequence Detection System. All samples were quantified in a multiplex reaction with 18s ribosomal RNA to control for differences in sample preparation. Control (Ribosomal VIC RNA control; ABI) and targeted cDNA were amplified in a single reaction tube. The efficiency of amplification of each target transcript was evaluated by plotting cycle threshold to log (sample concentration) for each standard curve. Reactions were carried out using Universal Master Mix (ABI). Amplification was performed over 50 cycles of denaturation at 95°C for 15 sec, and annealing/extension at 60°C for 60sec. Cycle threshold values were determined for each reaction using real-time plots of fluorescence versus cycle. Relative quantification was determined using the standard curve method. Target cDNA expression was normalized to 18s ribosomal RNA, and each sample was run in triplicate to obtain an average value.
Drugs and solutions
All chemicals were purchased from Sigma Chemical (St. Louis, MO), including: salts for buffer preparation, L-NNA, indomethacin, phenylephrine, diltiazem, papaverine. PlGF-2 (mouse) and antibodies to VEGF receptors were purchased from R&D Systems; VEGF-E was obtained from Research Diagnostics Inc.
Statistical analysis
Data are expressed as mean values ± standard error of the mean (SEM), where n is the number of arterial segments studied. Paired or unpaired Student’s t-tests were used as appropriate to determine the significance of differences between sets of data, and differences considered significant at p ≤0.05. When more than two treatment groups were evaluated, as in the VEGF-E and antibody studies, differences in responses between groups were determined with ANOVA followed by a multiple comparisons test (Tukey’s) to evaluate the significance of differences between treatment means.
In wire-mounted human vessels, relaxation to PlGF was expressed as percent inhibition of the contraction induced by NE. Differences in responses between groups were determined by comparing concentration-response curves using a two-way repeated-measures ANOVA, using PLGF concentration as a within-subject factor and artery type or incubational conditions as a between-subject factors. All data are presented as mean values ± SEM; n represents the number of vessels (one vessel per woman).
Calculations
Pharmacologic sensitivity to vasodilation by PlGF (IC50) was calculated for each vessel by normalizing the fractional response to a particular concentration of PlGF to the maximal response obtained at the highest concentration of drug tested. Efficacy was defined as the % of maximal dilation, which was determined at the end of each experiment by the addition of diltiazem (10 µM) and papaverine (100 µM), a relaxing solution that induces complete vascular smooth muscle relaxation. In wire-mounted vessels efficacy was defined as the % of maximal dilation relative to pre-constrictor level.
RESULTS
PlGF induced significant dilation in all arterial types studied, although there were significant differences in sensitivity, efficacy, and relative contribution of NO to the observed effects.
Vasodilatory effects of PlGF on rat uterine vessels; effects of gestation; expression of message for VEGFR-1 vs. VEGFR-2
Arcuate arteries and veins obtained from the uterine circulation dilated readily to PlGF. As previously observed with VEGF [10], arteries obtained from pregnant animals were significantly (1000x) more sensitive than those from age-matched nonpregnant controls (IC50 values: NP = 2.7 ± 1.4 nM; LP = 0.003 ± 0.0015 nM; p<0.01), although the extent of maximal dilation (efficacy) was comparable (NP= 86 ± 5.8% ; LP= 78 ± 11; p>0.05).
Moreover, the sensitivity of LP uterine arteries to the vasodilator effects of PlGF was significantly (200x) greater than that of veins (IC50 value: 0.6 ± 0.17 nM in veins; Figure 1A) and dilated to a greater extent than veins (efficacy averaged 78% and 39% of maximal dilation, respectively, at maximal concentrations; Figure 1B).
Figure 1.
A. Concentration-response curve showing dilation of pressurized rat uterine arcuate arteries (50 mmHg; n=5; open circles) and veins (10 mmHg; n=3; filled circles) to PlGF. Efficacy (maximal dilation) of arteries was twice that of veins (78% vs. 39%; p<0.01). B. Same data shown as sensitivity (normalized to maximal dilation obtained in each vessel to PlGF). Note approximately 200x greater sensitivity in arteries vs. veins (IC50 values in text).
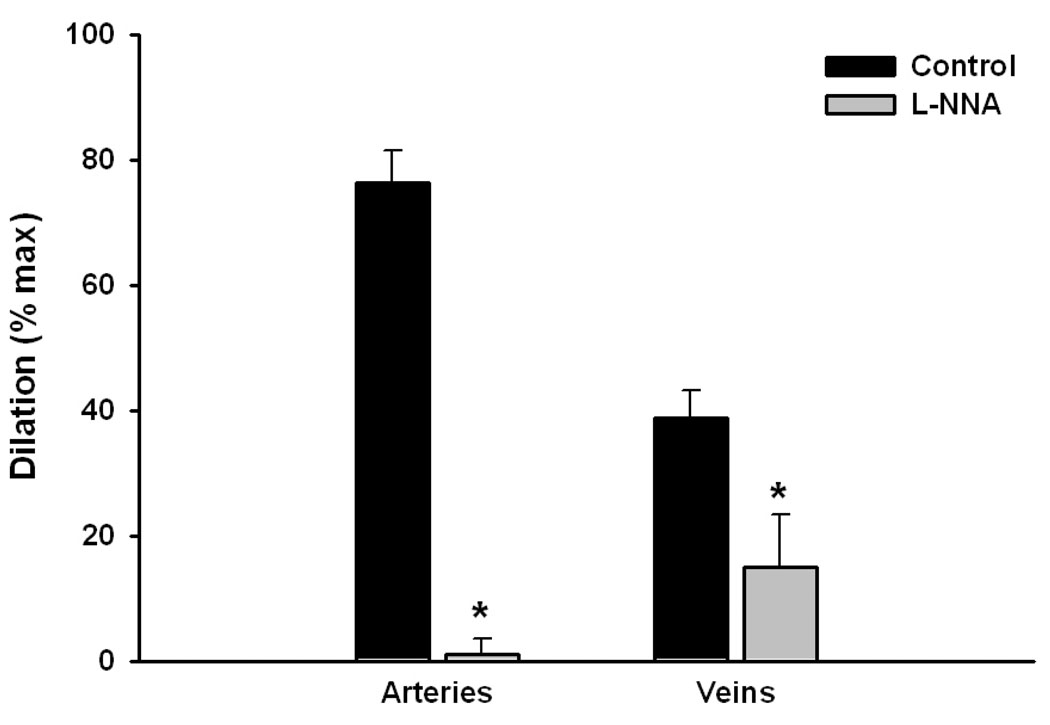
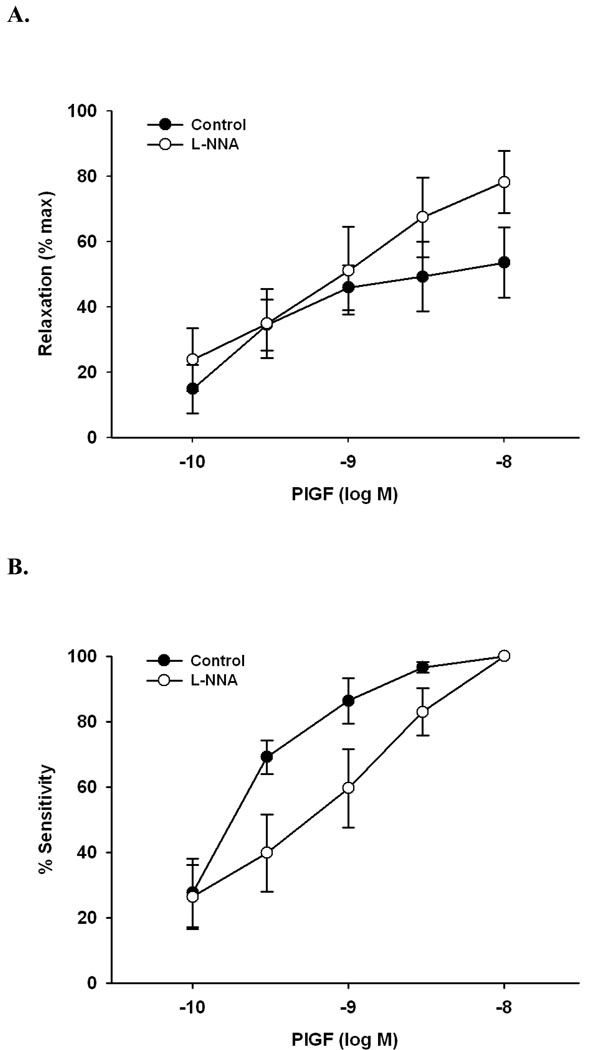
In rat uterine arcuate arteries, the vasodilatory actions of PlGF were completely eliminated by preincubation with L-NNA (0.2 mM), an inhibitor of nitric oxide synthase. Conversely, in veins, approximately 30% of the dilation remained (Figure 2).
Figure 2.
Nitric oxide synthase inhibition (L-NNA, 0.2 mM) eliminated and significantly (>60%) diminished vasodilation to PlGF in rat uterine arteries (n=5) vs. veins (n=3), respectively.
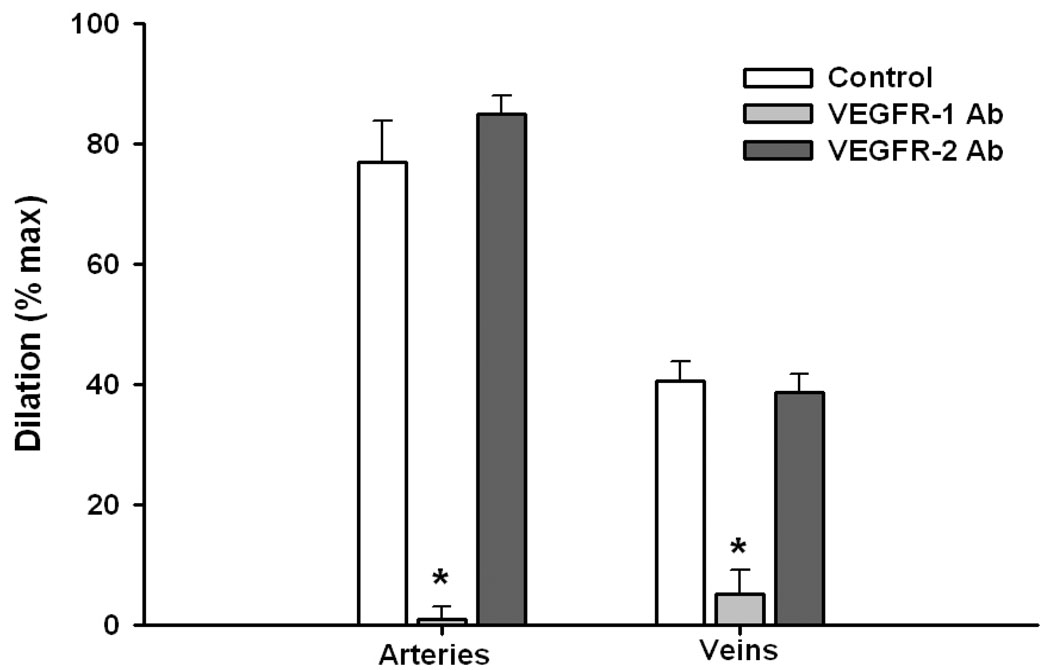
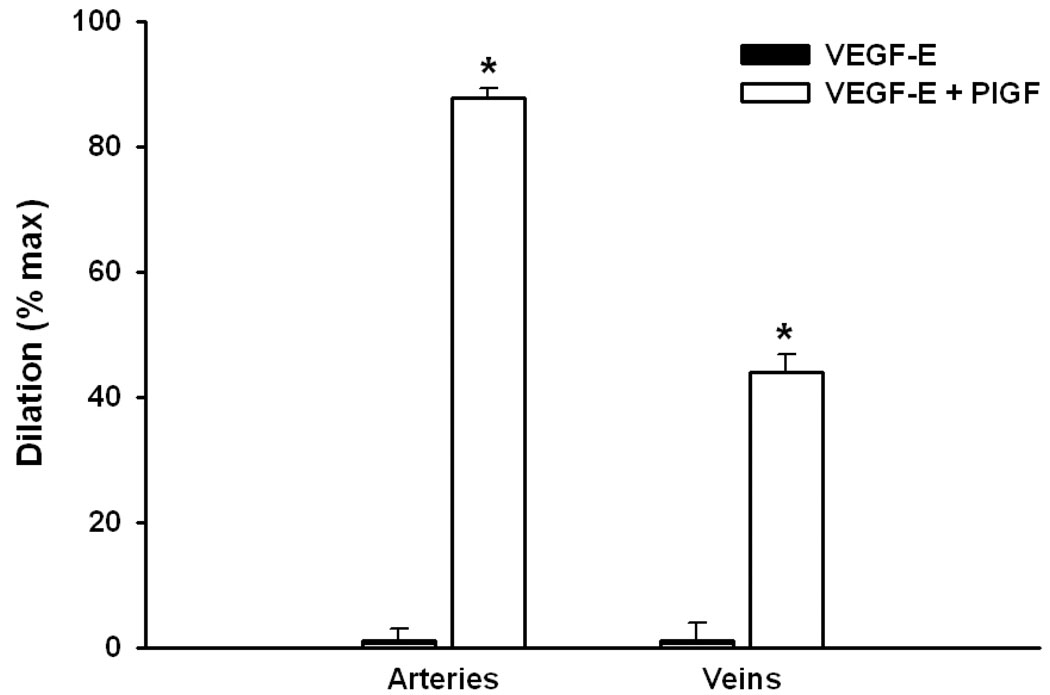
This vasodilatory effect was due to activation of the VEGFR-1 receptor, as preincubation of vessels with an antibody to the R1 receptor eliminated >90% of the observed d ilation, while pre-exposure to an anti-R2 antibody was completely without effect (Figure 3). The addition of a selective agonist for the VEGFR-2 receptor (VEGF-E) was also without effect, and there was no amplification of the response to PlGF + VEGF-E vs. PlGF alone (Figure 4).
Figure 3.
Effects of preincubation in a solution containing antibodies to VEGFR-1 (10 mg/ml) and VEGFR-2 (1 mg/ml) on vascular dilation to PlGF. Nearly complete (>90%; p<0.001) inhibition of dilation was observed in response to the VEGFR-1 antibody; while there was no observable effect of VEGFR-2 inhibition (p>0.05; n=4 for each set of data).
Figure 4.
Uterine arcuate arteries (n=4) and veins (n=3) failed to dilate to VEGF-E (10 nM), an agonist that is selective for VEGFR-2; subsequent addition of PlGF (a selective VEGFR-1 agonist; 1 nM - arteries; 10 nM - veins) produced significant dilation of both types of vessels.
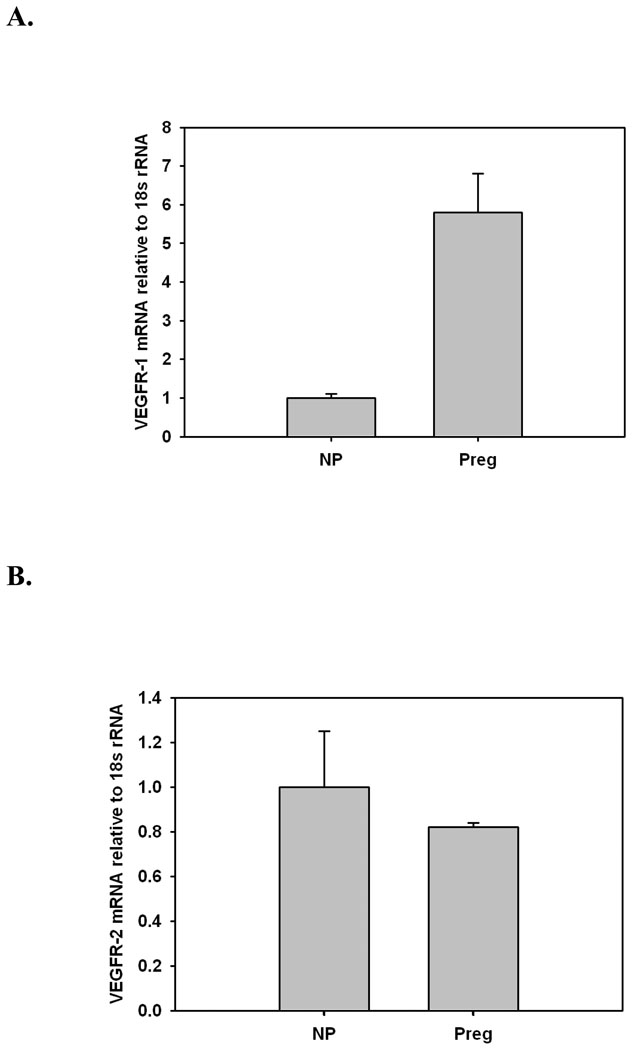
Real-time RT-PCR measurement of mRNA showed that message for both receptor subtypes was present in the uterine artery wall. VEGFR-1 but not VEGFR-2 message was upregulated during gestation (Figures 5A and B).
Figure 5.
Message for VEGFR-1 (A) and VEGFR-2 (B) as determined by real-time RT-PCR Taqman assay in uterine arteries from nonpregnant (NP, n=8) and pregnant (Preg) rats. Gestation resulted in a significant (p<0.05) upregulation of VEGFR-1 message (A); in contrast, no changes were observed in VEGFR-2 message expression (B).
Effects of PlGF on reactivity of rat mesenteric arteries
Third-order mesenteric arteries dilated to PlGF (≈ 50% of maximal dilation at the highest concentration, 10 nM) to a lesser extent than comparably-sized uterine arteries (compare Figure 6A vs. 1A); dilation was also considerably slower than that observed in uterine vessels, taking 15–45 instead of 5–15 minutes to stabilize. Preincubation in L-NNA (0.2 mM) did not affect efficacy, but decreased sensitivity (IC50 values: Control: 0.22 ± 0.04 nM; L-NNA: 1.20 ± 0.47 nM; p < 0.05; Figures 6A and 6B).
Figure 6.
Graphs showing concentration-dependent dilation of isolated third order rat mesenteric arteries in response to PlGF in the absence (control; n=5) and presence (n=4) of nitric oxide synthase inhibition with L-NNA (0.2 mM). A. There was no observable effect of L-NNA on efficacy, with slightly greater dilation observed in its presence (p>0.05) at the highest concentration of PlGF (10 nM). B. Based on IC50 values (p<0.05; see text), sensitivity to dilation was decreased approximately five-fold in the presence of L-NNA.
Effects of PlGF on human myometrial and subcutaneous arteries
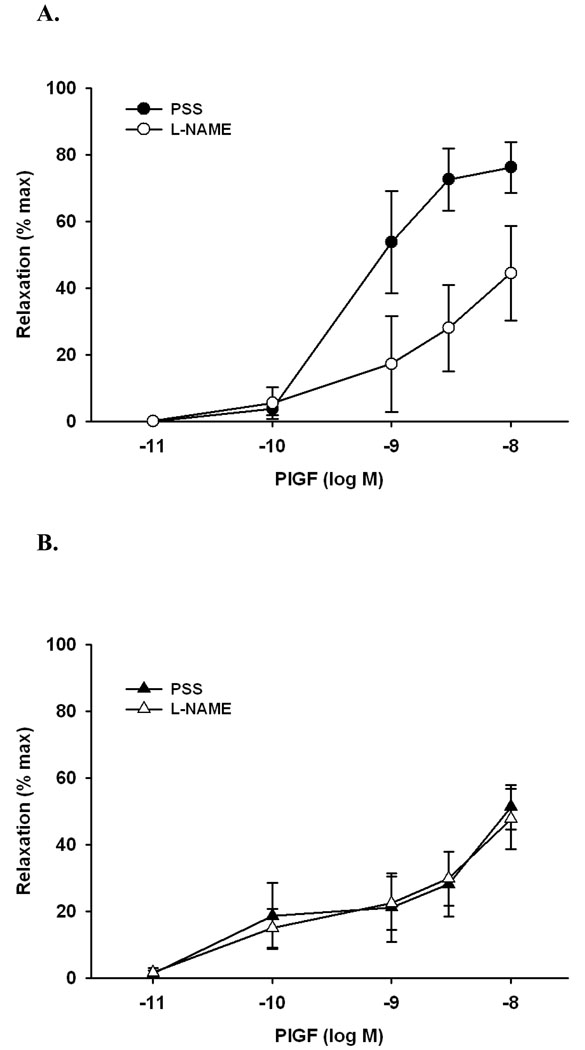
Both types of human vessels relaxed to PlGF in a concentration-dependent manner (Figure 7). The extent of dilatation to the maximal concentration of PlGF (10 nM) was almost twice as large in myometrial vs. subcutaneous arteries ((79 ± 7 % vs. 45 ± 8 %; p<0.05), Figure 7), which were also more sensitive to PlGF-induced vasodilation (IC50: 1.1 ± 0.38 nM vs. 3.0 ± 0.45 nM; p<0.05, Figure 7 and 8).
Figure 7.
Concentration-response curves showing the efficacy of PlGF in isometric (wire-mounted) myometrial (A, n=5) and subcutaneous (B, n=6) resistance arteries from pregnant women before and after incubation with a solution containing Nw-nitro-L-arginine methyl ester (L-NAME; 300 µM) plus indomethacin (Indo; 10µM). Note proportionately greater relaxation of myometrial vs. subcutaneous vessels. At the highest concentration (10 nM), preincubation with L-NAME and indomethacin eliminated approximately 50% of the relaxation response to PlGF in myometrial arteries, however, it had no effect on efficacy in subcutaneous arteries.
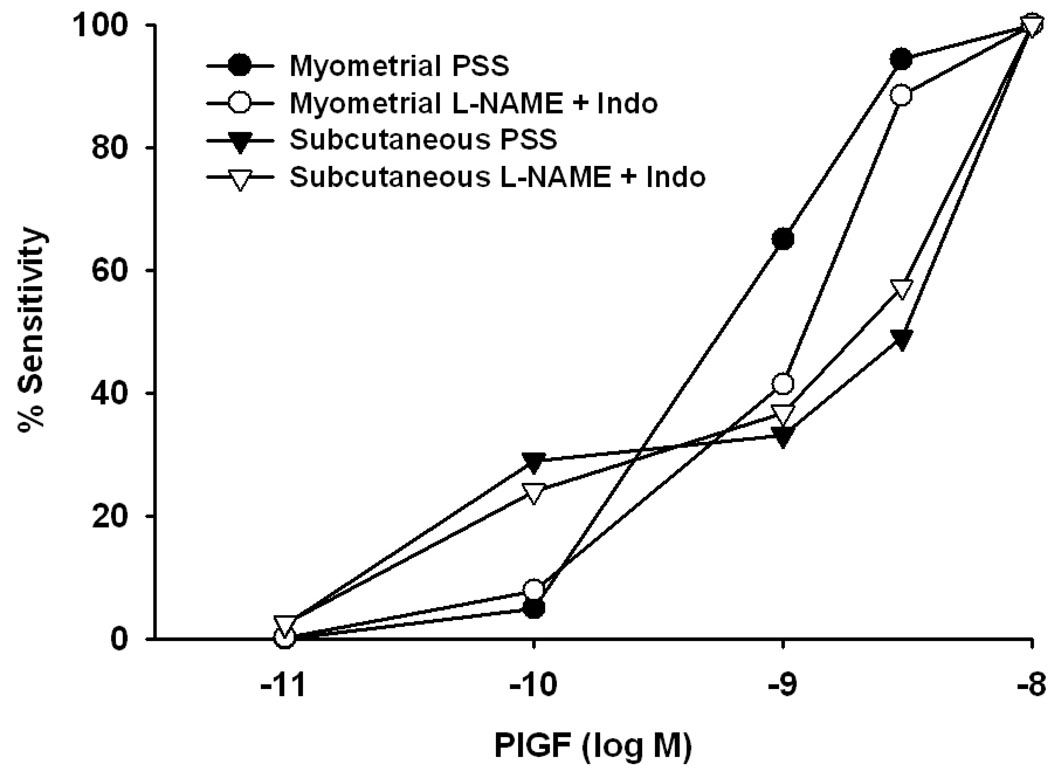
Figure 8.
Graph summarizing relative sensitivity of human uterine myometrial and subcutaneous vessels with and without pre-exposure to nitric oxide synthase and cyclooxygenase inhibition. There was a difference in sensitivity to PlGF between subcutaneous and myometrial arteries (p<0.05, ANOVA), however after pre-exposure to these inhibitors the sensitivity of myometrial and subcutaneous arteries was comparable (p>0.05, ANOVA).
Preincubation with L-NAME (300 µM) and indomethacin (Indo, 10 µM) eliminated approximately 50% of the relaxation response to PlGF in myometrial arteries (e.g., at 10 nM: 79±7 % PSS vs. 44±14% L-NAME + Indo, n=5, p<0.05, ANOVA), however, it had no effect on efficacy in subcutaneous arteries (51±7% PSS vs. 48±9% in L-NAME + Indo, n=6).
The data shown in Figure 7 indicate the extent of dilation under each condition relative to pre-constriction level (efficacy). Calculation of pharmacologic sensitivity requires the normalization of this parameter to the maximal effect observed in response to the compound in question (PlGF) for each vessel. Hence, the data in Figure 7 were normalized in this manner to assess sensitivity, and are re-plotted in Figure 8. As indicated above, there was a difference in sensitivity to PlGF between subcutaneous and myometrial arteries (p<0.05, ANOVA), however, after inhibition of NOS and COX products, the sensitivity of myometrial arteries was comparable to that obtained in subcutaneous arteries before and after L-NAME + Indo, (p>0.05, ANOVA). After preincubation with L-NAME + Indo, IC50 values were: 2.2 ± 0.65 nM (myometrial) to 2.4 ± 0.5 nM (subcutaneous), and were no longer statistically different (p> 0.05).
DISCUSSION
The main findings of this study are that: (1) PlGF is a potent vasodilator of resistance arteries and veins from the rat uterine circulation, with arteries displaying significantly greater sensitivity and efficacy than veins; (2) in rat uterine arteries, pregnancy significantly enhanced sensitivity to PlGF without altering its efficacy; (3) PlGF dilation is principally mediated by the release of NO in rat uterine arteries, while other, still-unidentified factors contribute to the vasodilation of uterine veins. (4) In rat tissues, message for both VEGF receptor subtypes is present in the uterine artery wall, and VEGFR-1 (but not VEGFR-2) message is significantly increased in pregnancy. (5) Human uterine (myometrial) arteries also dilate in response to PlGF, although the contribution of nitric oxide to the vasorelaxation, while significant, is proportionately less in human vs. rat uterine arteries. Finally, (6) both rat mesenteric and human subcutaneous arteries dilate to PlGF in an NO-independent manner. Together, these observations demonstrate the broad and mechanistically-diverse actions of PlGF on the vascular wall of resistance arteries and veins. They also support the importance of PlGF/VEGFR-1 signaling in uterine blood flow regulation during pregnancy, and raise the potential of an influence on blood pressure and peripheral resistance, as well as venous capacitance.
In this study, we were interested in probing the role of the tissue (non-soluble) VEGFR-1 receptor in uterine circulatory function during pregnancy. An in vitro approach using isolated vessels allowed us to quantitate the vasodilatory effects of PlGF, a selective agonist for VEGFR-1. As the results indicate, PlGF is an extremely potent vasodilator, with significant effects documented at sub-nanomolar concentrations, particularly in uterine arteries. The observation of a venodilatory effect is novel, and the fact that PlGF levels would be highest in the veins that drain the placenta suggests that it may play a role in regulating venous tone. Further, uterine venorelaxation is associated with an increased permeability of the vascular wall [2] and, in view of the well-documented proximity of arteries to veins in the uterine circulation of several animal species, as well as in the human, its permeability-enhancing action may facilitate signal transfer from vein to artery, and thereby potentially alter uterine resistance artery tone, placental blood flow, and vascular structure [3].
Earlier [10], we reported a significant effect on uterine artery sensitivity to VEGF and, in a subsequent study, determined this to be secondary to elevated circulating levels of estrogen by studying oophorectomized animals in combination with selective hormone replacement [ ]. These findings did not distinguish the receptor subtype involved in VEGF vasodilation since VEGF activates both VEGFR-1 and VEGFR-2 receptors. In this study, we demonstrate a similar change in reactivity to PlGF, a selective agonist for the VEGFR-1 receptor subtype, and implicate a transcriptional mechanism as the underlying cause since: (1) VEGFR-1 but not VEGFR-2 message was significantly upregulated in pregnancy and, as shown by the experiments that utilized receptor blocking antibodies, (2) only the VEGFR-1 receptor appears to be involved in the vasodilatory process, at least in the vessels studied herein. This finding does not preclude altered post-receptor signaling as an additional, or complementary mechanism for enhanced vasodilator sensitivity.
The important question from a physiological standpoint is whether PlGF signaling contributes to the profound increase in uterine blood flow that characteristically occurs in the pregnant state. In this regard, it was recently noted [6] that fetal and placental weights were significantly reduced in a rat model in which PlGF/VEGF signaling was attenuated by adenoviral overexpression of sFlt). Although uterine blood flow was not measured in that study, there is a long-standing association between reduced uteroplacental perfusion and reduced fetal and placental weights in studies that utilize a surgical approach to impose a state of reduced uteroplacental perfusion in rats (the RUPP model [e.g. 16,17]. More directly pertinent to this study, it was recently shown that, in addition to the hypertension that is associated with RUPP, there is also a significant (~800%) increase in plasma sFlt-1a concentration. As might be predicted, this excess of soluble receptor was associated with significant reductions in the circulating free concentrations of both PlGF and VEGF [18].
The actions of PlGF extend to the human, as it was a potent vasodilator of human myometrial resistance arteries, with IC50 values in the sub-nanomolar range. As in rat uterine arteries, in which NO accounted for >90% of the dilation to PlGF, NO contributed significantly to human uterine artery vasodilation, since its inhibition eliminated >40% of the vasodilation. In contrast, eNOS inhibition was of little consequence in PlGF-induced dilation of rat mesenteric and human subcutaneous arteries, demonstrating regional differences in its mechanism of action.
The experiments with anti-VEGFR-1 and anti-VEGFR-2 antibodies, and the complete absence of a vasodilatory effect of VEGF-E - at least in rat uterine vessels - confirm that dilation is mediated entirely through VEGFR-1 receptor. Although this is not surprising in view of the specificity of PlGF for VEGFR-1 signaling, it is noteworthy in that other reports have associated vasodilation and the production of nitric oxide most closely with VEGFR-2 signaling through mechanisms involving phospholipase C and Akt-1 [6,13].
It is also interesting to note that only VEGFR-1 and not VEGFR-2 message was upregulated in gestation, pointing to an unrecognized role for this receptor, and of signal transduction pathways subsequent to its activation in the adaptation of the maternal uterine circulation to pregnancy. Combined with two earlier reports that showed an increased sensitivity to VEGF dilation in rat uterine arteries from pregnant rats [10], and of its linkage to estrogen [11], the current findings support the hypothesis that enhanced vasodilation may result from an estrogen-induced increase in VEGFR-1 receptors in the vascular wall. Changes in other signaling pathways downstream to the receptor (e.g. production of NO or other vasodilators) cannot be ruled out, however, and will be evaluated in subsequent studies.
ACKNOWLEDGMENTS
The authors would like to acknowledge the excellent technical assistance of Ms. Kristen VanWoert with carrying out small artery reactivity experiments, and of Dr. Huiling Li (Brown University) with real-time RT-PCR measurement of message for VEGFR-1 and VEGFR-2. This study was supported by NIH RO1 HL-079253 [GO]
REFERENCES
- 1.Autiero M, Luttun A, Tjwa M, Carmeliet P. PlGF and its receptor VEGFR-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thrombosis Haemostasis. 2003;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 2.Celia G, Osol G. Uterine venous permeability in the rat is altered in response to pregnancy, vascular endothelial growth factor, and venous constriction. Endothelium. 2005;12:81–88. doi: 10.1080/10623320590933879. [DOI] [PubMed] [Google Scholar]
- 3.Celia G, Osol G. Mechanism of VEGF-induced uterine venous permeability. J Vas Res. 2005;42:47–54. doi: 10.1159/000082976. [DOI] [PubMed] [Google Scholar]
- 4.Gelinas DS, Bernatchez PN, Rollin S, Bazan NG, Sirois MG. Immediate and delayed VEGF-mediated NO synthesis in endothelial cells: role of PI3K, PKC and PLC pathways. doi: 10.1038/sj.bjp.0704956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janvier A, Nadeau S, Baribeau J, Perreault T. Role of VEGFR-1 and VEGFR-2 in the vasodilator response to VEGF in the neonatal piglet lung. Crit Care Med. 2005;33:860–866. doi: 10.1097/01.ccm.0000159563.97092.a7. [DOI] [PubMed] [Google Scholar]
- 6.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, Hankins GD, Saade GR. The effect of overexpression of s-Flt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstetr Gynecol. 2007 doi: 10.1016/j.ajog.2006.12.024. 196.396.e1-e7. [DOI] [PubMed] [Google Scholar]
- 7.Lukscha Nisell H, Kublickiene K. The mechanism of EDHF-mediated responses in subcutaneous small arteries from pregnant women. Am. J Physiol. 2004:R1102–R1109. doi: 10.1152/ajpregu.00550.2003. [DOI] [PubMed] [Google Scholar]
- 8.Maynard SE, Min J, Merchan J, Lim K, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FG, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt-1) may contribute to endothelial dysfunction, hypertension and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neagoe PE, Lemieux C, Sirois MG. VEGF)-A165 -induced prostacyclin synthesis requires the activation of VEGFR Receptor-1 and -2 heterodimer. J Biol Chem. 2005;11:9904–9912. doi: 10.1074/jbc.M412017200. [DOI] [PubMed] [Google Scholar]
- 10.Ni Y, May V, Braas K, Osol G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Physiol. 1997;273:H938–H944. doi: 10.1152/ajpheart.1997.273.2.H938. [DOI] [PubMed] [Google Scholar]
- 11.Storment JM, Meyer M, Osol M. Estrogen augments the vasodilatory effects of vascular endothelial growth factor in the uterine circulation of the rat. Am J Obstet Gynecol. 2000;183:449–453. doi: 10.1067/mob.2000.105910. [DOI] [PubMed] [Google Scholar]
- 12.Szukiewicz D, Szewczyk G, Watroba M, Kurowska E, Maslinski S. Isolated placental vessel response to vascular endothelial growth factor and placenta growth factor in normal and growth-restricted pregnancy. Gynecol Obstet Invest. 2005;59:102–107. doi: 10.1159/000082622. [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto T, Jin ZG, Berk BC. Transactivation of VEGF receptor Flk-1/KDR is involved in sphingosine 1-phosphate -stimulated phosphorylation of Akt and eNOS. J Biol Chem. 2002;45:42997–423001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- 14.Tjoa ML, Levine RF, Karumanchi SA. Angiogenic factors and preeclampsia. Front Biosci. 2007;12:2395–2402. doi: 10.2741/2241. [DOI] [PubMed] [Google Scholar]
- 15.Wei W, Jin H, Chen ZW, Zioncheck TF, Yim AP, He GW. VEGF-induced nitric oxide- and PGI2-dependent relaxation in human internal mammary arteries: a comparative study with KDR and Flt-1 selective mutants. Cardiovasc Pharmacol. 2004;44:615–621. doi: 10.1097/00005344-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Wigglesworth JS. Fetal growth retardation. Animal model: uterine vessel ligation in the pregnant rat. Am J Patholo. 1972;77:347–350. [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert JS, Dukes M, LaMarca BB, Cockrell K, Babcock SA, Granger JP. Effects of reduced uterine perfusion pressure on blood pressure and metabolic factors in the pregnant rat. Am J Hypertens. 2007;20:686–691. doi: 10.1016/j.amjhyper.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert JS, Babcodk SA, Granger JP. Hypertension producec by reduced uterine perfusion in pregnant rats is associated with increased soluble Fms-like Tyrosine Kinase-1 expression. Hypertension. 2007;50:1142–1147. doi: 10.1161/HYPERTENSIONAHA.107.096594. [DOI] [PubMed] [Google Scholar]