Abstract
Quiescent pulmonary endothelium establishes an anti-thrombotic, anti-inflammatory surface that promotes blood flow. However, the endothelium rapidly responds to injury and inflammation by promoting thrombosis and enabling the directed transmigration of inflammatory cells, such as neutrophils, into the alveolar airspace. While the endothelial cell signals responsible for establishing a pro-thrombotic surface are distinct from those responsible for recognizing circulating neutrophils, these processes are highly inter-related. Von Willebrand factor stimulated secretion plays an important role in thrombus formation, while P-selectin surface expression plays a key role in neutrophil binding necessary for transmigration. Both von Willebrand factor and P-selectin are located within Weibel-Palade bodies in pulmonary arteries and arterioles, yet Weibel-Palade bodies are absent in capillaries. Despite the absence of the Weibel-Palade bodies, pulmonary capillaries express both von Willebrand factor and P-selectin. The physiological and pathophysiological significance of these observations is unclear. In this review, we address some anatomic and physiologic features that distinguish pulmonary artery, capillary, and vein endothelium. In addition, we review our current understanding regarding the stimulated secretion of von Willebrand factor and P-selectin in pulmonary artery and capillary endothelium. This information is considered in the context of vasculitis and pneumonia, two pathophysiological processes to which the stimulated secretion of von Willebrand factor and P-selectin contribute.
Keywords: Thrombin, Permeability, Coagulation, Vasculitis, Pneumonia, Acute Lung Injury
Introduction
The pulmonary vascular tree is the largest vascular bed in the human body, as it comprises an area equal to 120 m2 1. Endothelium coats this vascular system, forming a continuous, semi-permeable barrier between blood and tissue. At the same time, pulmonary endothelium serves as a nonthrombogenic surface; controls vascular tone and tissue perfusion; and plays key roles in leukocyte trafficking and inflammation.
Today, the heterogeneous nature of lung endothelial cells along the arterial-capillary-venous axis is widely recognized both in vivo and in vitro. From developmental origins to morphological and functional attributes, the lung arterial endothelial cells (macrovascular circulation) and capillary endothelial cells (microvascular circulation) are two distinct biological entities (Figure 1). These attributes range from gene expression patterns 2; lectin binding capacity 3.; resting membrane potentials 4; cAMP dynamics 5; intracellular calcium signaling 6; and oxidant signaling and sensitivity 7, to intercellular junctions 8; cytoskeletal dynamics 9; caveolar density 10; semi-permeable barrier function 11; flow alignment 12; cell proliferation 13; and the response to inflammatory mediators 14. Recent evidence suggests that the heterogeneity of lung endothelium is even broader than previously recognized. Therefore, this brief review discusses new findings from our laboratory and other investigative groups that have advanced our understanding of the incredible diversity of lung endothelial cells, specifically considering the expression, location, and function of von Willebrand factor, P-selection, and Weibel-Palade bodies (WPb).
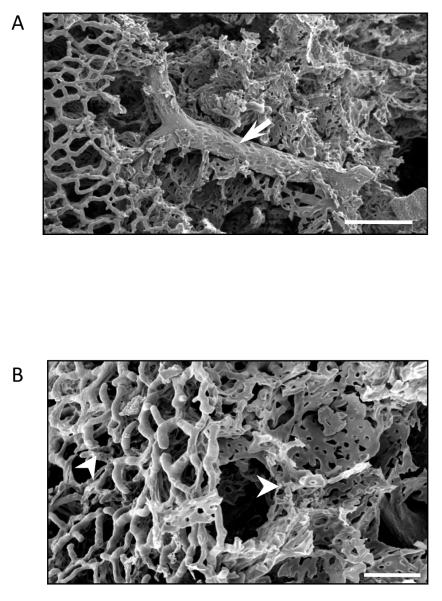
Figure 1.
Scanning electron micrograph of a methylmethacrylate casted lung reveals both the pulmonary macro- and microcirculation. A. Arrow points to extra-alveolar vessel. Scale 100 μm. B. Pulmonary microcirculation. White arrowheads point to arterioles and capillaries. Scale 50 μm. Electron micrographs courtesy of Dr. Diego Alvarez.
Defining Lung Endothelial Phenotypes: Macrovascular vs. Microvascular Pulmonary Endothelium
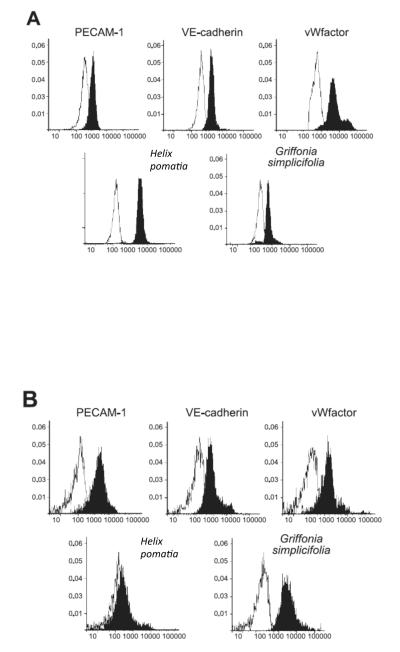
Blood flow through the pulmonary circulation is directly influenced by airway pressure. High airway pressure distinctly impacts the conduit and microvascular circulations, as it opens so-called extra-alveolar vessels and closes so-called alveolar vessels. This physiological demarcation occurs in small precapillary vessels that range between 25-100 μm in diameter. Interestingly, a similar functional demarcation can be resolved using lectin binding criteria, both in vivo and in vitro. When a dual lectin-binding approach is used in the intact pulmonary circulation, Helix pomatia and Griffonia simplicifolia co-localize in a region of the vascular tree with a diameter of approximately 25 μm. Upstream from this co-localization site endothelium binds to H. pomatia, while downstream, endothelium binds to G. simplicifolia 15. In culture, these lectin-binding properties do not change, and they are conserved regardless of cell passage number, as H. pomatia preferentially binds to lung macrovascular endothelial cells, while G. simplicifolia preferentially binds to lung microvascular endothelial cells (Figure 3) 3, 16. In the case of the pulmonary veins, the lectin-binding pattern of their endothelial coat has not been extensively tested, however, preliminary data suggests that Sambucus nigra discerningly binds pulmonary vein endothelial cells (Creighton JR, unpublished).
Figure 3.
Flow cytometry analysis of lung endothelium. Pulmonary artery endothelial cells bind to PECAM-1, VE-cadherin, von Wilebrand factor and Helix pomatia. Pulmonary capillary endothelial cells bind to PECAM-1, VE-cadherin, von Wilebrand factor and Griffonia simplicifolia. Adapted from 16, with permission.
Heterogeneity in the Stimulated Secretion of Von Willebrand Factor
This alveolar and extra-alveolar anatomic partition correlates with the presence or absence of the pro-thrombotic and pro-inflammatory WPb in pulmonary endothelia. WPb are secretory granules that store von Willebrand factor, factor XIIIa, P-selectin, and interleukin-8 17. Following endothelial activation or injury, WPb fuse with the cellular membrane releasing their contents in a regulated manner. While the pulmonary artery endothelium contains WPb, pulmonary capillaries do not 18. Despite the absence of WPb, pulmonary capillary endothelial cells express von Willebrand factor, factor VIII and P-selectin, suggesting that the lung capillaries have WPb-independent mechanisms of storing and secreting pro-thrombotic and pro-inflammatory factors. The precise intracellular locale for these important rheostatic regulators remains uncertain, as do the mechanisms for their stimulated secretion.
The physiological relevance of these fundamental endothelial cell anatomic features is still poorly understood. For example, both stimulated von Willebrand factor and factor VIII secretion contribute to hemostasis, yet it is unclear how or why these factors collect in the WPb of extra-alveolar endothelium, and fail to do so in capillary endothelium. One explanation is based on the idea that organelles are anatomically excluded from the cell periphery within capillaries. Indeed, pulmonary capillary endothelial cells cover a large surface area if viewed en face, with extremely thin cytoplasmic extensions that do not possess organelles; organelles are localized in the peri-nuclear region in capillaries. The expansive, thin cytoplasmic region is less than 100 nm in diameter, and resides on a basement membrane that is fused with an adjacenT-type I epithelial cell. This anatomic feature forms the basis of the alveolar-capillary membrane that optimizes gas exchange. While such an anatomic organization describes that organelles are restricted from the cell’s periphery, it provides no mechanistic insight into why pulmonary capillary endothelial cells fail to form WPb, and is therefore an unsatisfactory explanation. Indeed, it is generally believed that von Willebrand factor expression is sufficient to induce WPb formation, and recent work from Michaux and collegues support this contention, as expression of the full length von Willebrand factor induces the formation of WBb-like organeles in HEK293 cells 19.
WPb store multimers of von Willebrand factor. Such multimers form through an interaction within the von Willebrand factor D’ and D3 (D’-D3) domains. The D’-D3 domains have a number of crucial roles. This N-terminal region has been implicated in von Willebrand factor storage 20, multimerization (N-terminal interchain disulphide bond formation) 21, binding and stabilization of factor VIII 22, 22, binding the P-selectin lumenal domain, and triggering P-selectin recruitment to the WPb 23. In particular, the N-terminal interchain disulphide bond formation is critical for generation of the subcellular organelles that morphologically resemble WPbs 24. This domain may undergo splicing, resulting in a truncated von Willebrand factor form that renders it unable to multimerize, and hence, unable to generate the WPb. It remains unclear as to whether capillary endothelial cells remove the D’-D3 domain by splicing, providing a putative mechanistic explanation for their lack of WPb. Resolving this critical question will provide novel insight into the capillary endothelial cell phenotype, and enable more rigorous physiological studies to be conducted towards a comprehensive understanding of coagulation within the pulmonary microcirculation.
Once secreted, von Willebrand factor multimers interact with the exposed sub-intimal matrix, extend upon exposure to shear, and adhere to activated platelets to facilitate clot formation. Blood flow and shear stress throughout the lung circulation differs substantially. The pulmonary artery accommodates 100% of the cardiac output in a low-pressure environment (pulmonary artery pressure ≈ 25/8 mm Hg), with low shear stress in humans (Human: Pulmonary Artery Diameter ≈ 2.7 cm, Blood Flow = 5 L/min, Shear Stress = 1.72 dynes/cm2; Rat: Pulmonary Artery Diameter ≈ 1.5 mm, Blood Flow = 25 mL/min, Shear Stress = 50 dynes/cm2). In contrast, not all capillaries are perfused at rest, and shear stress in the perfused capillaries is estimated to be relatively high based on similar estimates in the systemic circulation (Cat Mesenteric Capillary: Capillary Diameter = 7 μm, Shear Stress ≈ 30 dynes/cm2); increasing cardiac output or increasing venous pressure recruits new capillary circuits. Veins accommodate 100% of the blood returning to the left atrium, again with low shear stress (Human: Pulmonary Vein Diameter ≈ 1 cm, Blood Flow = 1.25 L/min, Shear Stress = 8.49 dynes/cm2), yet these vessels differ from their systemic counterparts in that they lack valves, and are not influenced by a skeletal muscle pump. Rather, certain pulmonary veins are encased with cardiac muscle apparently derived from the left atrium 25. The function of this cardiac tissue remains poorly understood; although it is possible the vein-associated cardiac myocytes coordinate with the cardiac cycle to facilitate pulmonary venous return.
While clots form in all three major pulmonary vascular segments, e.g. arteries, capillaries, and veins, little consideration is given as to whether the unique attributes of endothelial cell phenotypes within arteries, capillaries and veins, or the vessel biophysics among these segments, contributes to coagulation. Indeed, it is not clear whether the presence of a WPb in extra-alveolar endothelium, and the absence of WPb in alveolar endothelium, confers a benefit to these segments of the pulmonary circulation. Nonetheless, indirect evidence is mounting that coagulation and thrombus formation may not occur by identical mechanisms in all vascular segments.
Hemostatic changes are a prominent finding in vasculitis 26. A study of vasculitis reveals that endothelial cell phenotype, and the microenvironment in which the endothelium resides, may contribute to the disease process. Behçet’s syndrome and Wegener’s granulomatosis cause both pulmonary macrovascular and microvascular compromise. However, Churg-Straus syndrome and microscopic polyangiitis appear to be restricted in their impact; where Churg-Strauss syndrome targets medium-sized arteries and veins of the macrocirculation, and microscopic polyaniitis only compromises arterioles, capillaries and venules of the microcirculation 27. Identical results are found in other vasculitides that uncommonly impact the pulmonary circulation: whereas necrotizing sarcoid granulomatosis, Takayasu’s arteritis, and giant cell arteritis cause macrovascular compromise; crytoglobulinemic vasculitis causes microvascular demise. Similarly, new information is arising regarding the link between deep venous thrombosis and pulmonary embolism, suggesting the pulmonary endothelium may support primary clot formation more frequently than previously appreciated. Nearly 30-40% of patients presenting with deep venous thrombosis had demonstrated evidence of asymptomatic pulmonary embolism, indicating the association between deep venous thrombosis and pulmonary embolism is more common than previously suspected 28, 29. In addition, a recent retrospective review of trauma patients undergoing computed tomographic pulmonary angiography and computed tomographic venography revealed that 19% of patients were diagnosed with pulmonary embolism, whereas only 7% had deep venous thrombosis, suggesting the thrombus originated from within the pulmonary circulation 30. Among the number of pulmonary embolism events that were resolved, eight were found in the main or lobar pulmonary arteries and 28 were observed in segmental or subsegmental branches. If the origin of these thrombi is within the pulmonary circulation, then given what we now know about endothelial heterogeneity in macro- and microvascular segments, we may consider that the mechanisms responsible for thrombus generation are distinct. A more systematic study of these disease processes, with careful consideration given to the vascular site that is impacted, will yield important new information about the disease process, and mechanisms of endothelial cell phenotype specification.
Heterogeneity in P-Selectin Surface Expression
P-selectin is packaged within the WPb, yet, like von Willebrand factor, its expression can be resolved in lung capillaries. Recently, Wu and colleagues addressed this important issue, and found that whereas little P-selectin is typically present on the capillary surface, thrombin rapidly induces P-selectin surface expression 31, 32. Using P-selectin antibodies conjugated to quantum dots, these investigators utilized transmission electron microscopy to demonstrate subcellular foci within capillary endothelium that are enriched with P-selectin immunoreactivity. While these subcellular foci are within the cytoplasm, they are not within conventional WPb, consistent with evidence documenting the absence of WPb in capillary endothelium 32. Whether or not P-selectin is contained within a distinct vesicular structure remains to be determined. However, the P-selectin pool does not co-localize with von Willebrand factor 32. The explanation for such “mis-targeting” of P-selectin and von Willebrand factor is presently unclear, and has not be experimentally addressed; it is possible that expression of a von Willebrand factor splice variant lacking the P-selectin lumenal domain could account for such mis-targeting.
The presence of P-selectin of lung capillaries has important physiological implications. Unlike the systemic circulation, the principal site of neutrophil trafficking from the blood into the tissue (and alveoli) is through capillaries 33. Infection results in the stimulated surface expression of P-selectin, which is necessary for neutrophils to adhere to capillary endothelium and initiate transmigration 34, 35. In this physiological context, the significance of P-selectin found within WPb of the extra-alveolar vessels is poorly understood, just as is the anatomic locale of capillary P-selectin.
The stimulated P-selectin surface translocation occurs following an increase in either cytosolic calcium or cAMP. Thrombin is a physiologically relevant first messenger that increases endothelial cell cytosolic calcium. Thrombin activates phospholipase C, which cleaves phosphatidyl inositol 4,5-bisphosphate into diacyclglycerol and inositol 1,4,5-trisphosphate (InsP3). Whereas diacylglycerol activates receptor operated calcium entry channels, InsP3 promotes calcium release from the endoplasmic reticulum and calcium entry through store operated calcium entry channels. These global calcium regulatory mechanisms initiate P-selectin surface expression in pulmonary artery endothelial cells 31, 32, although the precise calcium influx channel that is responsible for this effect in extra-alveolar endothelium is unknown.
Thrombin similarly induces P-selectin surface expression in pulmonary capillary endothelial cells, however in this case, neither receptor operated nor store operated calcium entry channels appear to provide the calcium source. Capillary endothelial cells express a T-type (Cav3.1) voltage gated calcium channel that extra-alveolar endothelial cells in the pulmonary circulation do not possess. In addition to activating both receptor-operated and store-operated calcium entry channels, thrombin induces membrane depolarization that, in capillary endothelial cell, is sufficient to activate the T-type calcium channel. Hence, thrombin stimulates calcium influx through the T-type calcium channel in capillary endothelial cells, and this calcium influx increases whole cell free cytosolic calcium. Using histology, fluorescence microscopy, and electron microscopy approaches, the Wu group resolved that thrombin promotes P-selectin surface expression in lung capillaries, and further, that this response depends upon the T-type calcium channel. Not only was the thrombin-induced P-selectin surface expression abolished by pharmacological blockade of the T-type calcium channel, this response was abolished in mice genetically deficient of the T channel 32, 36.
As neutrophil transmigration from the circulation into the distal airspace requires P-selectin, it is unclear whether this physiological process is similarly reliant upon function of the T-type calcium channel. Just recently studies were initiated to test this idea. Airway Pseudomonas aeruginosa inoculation causes pneumonia which progresses to sepsis and acute lung injury (Alvarez, Stevens; unpublished). Analysis of bronchoalveolar lavage fluid and histological assessments confirm neutrophil recruitment into alveoli in this model. However, in mice deficient of the T-type calcium channel, airway neutrophil recruitment is abolished, indicating a critical adaptive role for the T-type calcium channel that is essential for the neutrophil response to infection (Wu, unpublished).
On the Discrete Nature of Endothelial Cell Calcium Signals
Studies on the location and function of the T-type calcium channel reveal that its expression in endothelium is restricted to capillaries, as least in the pulmonary circulation 37. Calcium signals in endothelium cause endothelial cell barrier disruption, and thrombin is a widely acknowledged calcium agonist that increases permeability 38. However, thrombin, or protease activated receptor peptides, likely increase permeability across extra-alveolar endothelium, and not across alveolar endothelium 39. Studies undertaken to examine whether activation of the T-type calcium channel increases capillary endothelial cell permeability revealed no such effect 36. It therefore appears that the capillary endothelial cell T-type calcium channel provides a calcium source that is functionally coupled to P-selectin surface expression and neutrophil trafficking, but has no role in disrupting cell-cell or cell-matrix adhesion. In an in vitro study, release of von Willebrand factor – generally considered to co-localize with P-selectin in WPbs – was differentially controlled in pulmonary macro- and microvascular endothelial cells, where the α1G T-type channels mediated regulated von Willebrand factor release exclusively in pulmonary microvascular endothelial cells 40.
Whereas the T-type calcium channel is not implicated in endothelial cell barrier disruption, another channel abundantly expressed in capillary endothelium, the transient receptor potential 4 protein within the vanilloid subfamily (TRPV4), has been incriminated in control of the endothelial cell barrier. In this case, TRPV4 channel activation increases capillary permeability resulting in alveolar flooding 41, 42. An interesting aspect of these results is that, to date, TRPV4 activation has not been shown to induce inter-endothelial cell gaps, which represent the best-described mechanism of exudation. Rather, TRPV4 activation causes loss of cell-matrix association leading to sluffing, a prominent finding in acute lung injury. Mechanisms responsible for this loss of cell-matrix tethering are poorly understood. Collectively, we have learned an important lesson from these studies, as it has become clear that the activation of two different calcium channels, the T-type and the TRPV4 calcium channels, similarly increase free cytosolic calcium, but have highly specialized physiological functions 36.
The actions of these two channels can be further contrasted with those of the transient receptor potential channels within the canonical subfamily (TRPC) 43. In this case, a heteromeric channel comprised of at least TRPC1 and TRPC4 subunits is activated by thrombin and protease activated receptor peptides, resulting in increased endothelial cell permeability. However, unlike the TRPV4 channel, activation of the TRPC1/4-containing channel induces inter-endothelial cell gaps as a mechanism for exudation. Indeed, the systematic study of these three ion channels has taught us about heterogeneity of pulmonary endothelium, the fundamental cell biology of endothelium, and basic mechanisms relating to the response to inflammation, in particular neutrophil trafficking and permeability.
Conclusions
The past 10 years of research has begun to unravel a striking heterogeneity in the structure and function among endothelial cells in pulmonary arteries, capillaries and veins. Just recently, endothelial cell biologists have begun to recognize the discrepancies that exist in our knowledge regarding the expression of von Willebrand factor and P-selectin, and the relationship between these important pro-thrombotic, pro-inflammatory factors and their stimulated secretion in pulmonary arteries and capillaries. Future studies will be needed to mechanistically determine why von Willebrand factor and P-selectin are not organized into WPb in capillaries, and why they exist in discrete cytosolic loci within the capillary endothelium. Once established, this new knowledge in our basic understanding of endothelial cell structure and function will pave the way toward a better resolution of the mechanisms that contribute to thrombosis and neutrophil trafficking in inflammatory disorders such as vasculitis, pneumonia and acute lung injury.
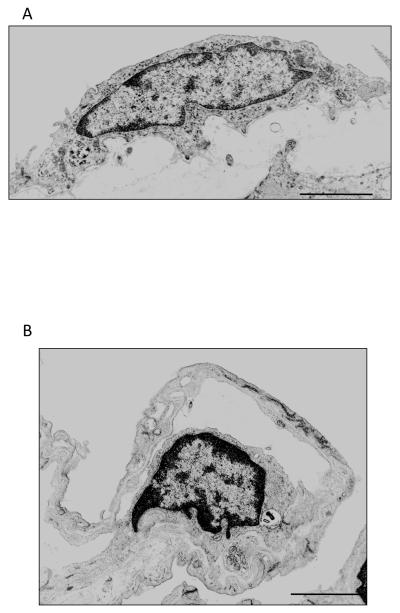
Figure 2.
Transmission electron micrographs of rat lung endothelium. A. Pulmonary artery endothelial cells. B. Pulmonary capillary endothelial cells. Scale 2 μm. Micrographs courtesy of Dr. Judy A. King.
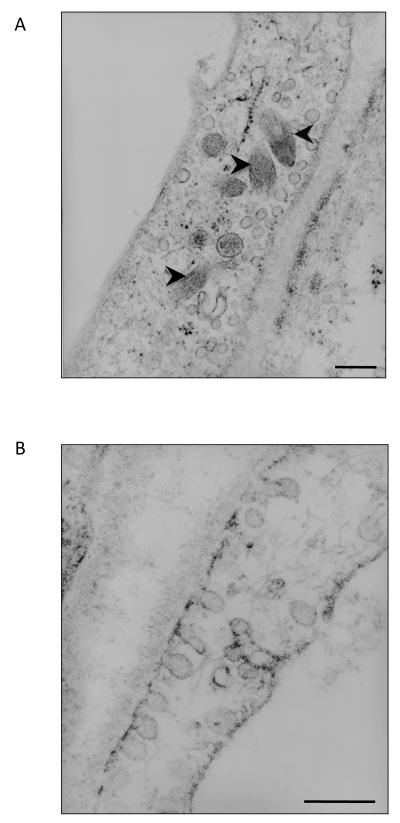
Figure 4.
Transmission electron micrographs of human lung endothelium. A. Pulmonary artery endothelial cells contain Weibel-Palade bodies (black arrowheads). B. Pulmonary capillary endothelial do not contain Weibel-Palade bodies; however, they contain large number of caveolea. Scale 200 nm. Micrographs courtesy of Dr. Judy A. King.
Acknowledgements
The authors wish to thank Dr. Abu-Bakr Al-Mehdi for his contributions to the completion of this manuscript, and Dr. James Stubbs for his review of the manuscript and his helpful insight. This work is supported by HL66299, HL60024, and HL76125.
References
- 1.Stan RV. Anatomy of the pulmonary endothelium. In: Voelkel NF, Rounds S, editors. The Pulmonary Endothelium. Wiley-Blackwell; West Sussex, UK: 2009. p. 25. [Google Scholar]
- 2.Chi JT, Chang HY, Haraldsen G, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100(19):10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King J. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvascular Research. 2004;67(2):139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Stevens T, Fouty B, Hepler L, et al. Cytosolic Ca2+ and adenylyl cyclase responses in phenotypically distinct pulmonary endothelial cells. Am J Physiol. 1997;272(1 Pt 1):L51–9. doi: 10.1152/ajplung.1997.272.1.L51. [DOI] [PubMed] [Google Scholar]
- 5.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. implications for control of barrier function. Am J Physiol. 1999;277(1 Pt 1):L119–26. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- 6.Cioffi DL, Wu S, Stevens T. On the endothelial cell I(SOC) Cell Calcium. 2003;33(5-6):323–36. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 7.Grishko V, Solomon M, Wilson GL, LeDoux SP, Gillespie MN. Oxygen radical-induced mitochondrial DNA damage and repair in pulmonary vascular endothelial cell phenotypes. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1300–8. doi: 10.1152/ajplung.2001.280.6.L1300. [DOI] [PubMed] [Google Scholar]
- 8.Ofori-Acquah SF, King J, Voelkel N, Schaphorst KL, Stevens T. Heterogeneity of barrier function in the lung reflects diversity in endothelial cell junctions. Microvasc Res. 2008;75(3):391–402. doi: 10.1016/j.mvr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res. 2009;77(1):53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86(1):279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 11.Parker JC, Stevens T, andall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L30–7. doi: 10.1152/ajplung.00317.2005. [DOI] [PubMed] [Google Scholar]
- 12.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvascular Research. 2004;68(1):1–12. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez DF, Huang L, King JA, ElZarrad MK, Yoder MC, Stevens T. Lung microvascular endothelium is enriched with progenitor cells that exhibit vasculogenic capacity. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L419–30. doi: 10.1152/ajplung.00314.2007. [DOI] [PubMed] [Google Scholar]
- 14.Cioffi D. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. The Journal of Cell Biology. 2002;157(7):1267–1278. doi: 10.1083/jcb.200204022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest. 2005;128(6):558S–564S. doi: 10.1378/chest.128.6_suppl.558S. [DOI] [PubMed] [Google Scholar]
- 16.Clark J, Alvarez DF, Alexeyev M, et al. Regulatory role for nucleosome assembly protein-1 in the proliferative and vasculogenic phenotype of pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L431–9. doi: 10.1152/ajplung.00316.2007. [DOI] [PubMed] [Google Scholar]
- 17.Lowenstein CJ, Morrell CN, Yamakuchi M. Weibel-palade bodies: Vesicular trafficking on the vascular highways. In: Aird WC, editor. Endothelial Biomedicine. Cambridge University Press; New York, NY: 2007. p. 657. [Google Scholar]
- 18.Fuchs A, Weibel ER. Morphometric study of the distribution of a specific cytoplasmatic organoid in the rat’s endothelial cells. Z Zellforsch Mikrosk Anat. 1966;73(1):1–9. [PubMed] [Google Scholar]
- 19.Michaux G, Hewlett LJ, Messenger SL, et al. Analysis of intracellular storage and regulated secretion of 3 von willebrand disease-causing variants of von willebrand factor. Blood. 2003;102(7):2452–2458. doi: 10.1182/blood-2003-02-0599. [DOI] [PubMed] [Google Scholar]
- 20.Haberichter SL, Jacobi P, Montgomery RR. Critical independent regions in the VWF propeptide and mature VWF that enable normal VWF storage. Blood. 2003;101(4):1384–1391. doi: 10.1182/blood-2002-07-2281. [DOI] [PubMed] [Google Scholar]
- 21.Voorberg J, Fontijn R, van Mourik JA, Pannekoek H. Domains involved in multimer assembly of von willebrand factor (vWF): Multimerization is independent of dimerization. EMBO J. 1990;9(3):797–803. doi: 10.1002/j.1460-2075.1990.tb08176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster PA, Fulcher CA, Marti T, Titani K, Zimmerman TS. A major factor VIII binding domain resides within the amino-terminal 272 amino acid residues of von willebrand factor. J Biol Chem. 1987;262(18):8443–8446. [PubMed] [Google Scholar]
- 23.Michaux G, Pullen TJ, Haberichter SL, Cutler DF. P-selectin binds to the D’-D3 domains of von willebrand factor in weibel-palade bodies. Blood. 2006;107(10):3922–3924. doi: 10.1182/blood-2005-09-3635. [DOI] [PubMed] [Google Scholar]
- 24.Wagner DD, Saffaripour S, Bonfanti R, et al. Induction of specific storage organelles by von willebrand factor propolypeptide. Cell. 1991;64(2):403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- 25.Michelakis ED, Weir EK, Wu X, et al. Potassium channels regulate tone in rat pulmonary veins. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1138–47. doi: 10.1152/ajplung.2001.280.6.L1138. [DOI] [PubMed] [Google Scholar]
- 26.Trifiletti A, Scamardi R, Bagnato GF, Gaudio A. Hemostatic changes in vasculitides. Thromb Res. 2009;124(3):252–255. doi: 10.1016/j.thromres.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Travis WD. Vasculitis. In: Dail DH, Tomashefski JF, Cagle PT, Farver CF, Fraire AE, editors. Dail and Hammar’s Pulmonary Pathology. Third ed Vol I. Nonneoplastic Lung Disease. Springer; New York, NY: 2004. p. 1088. [Google Scholar]
- 28.Moser KM, Fedullo PF, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA. 1994;271(3):223–225. [PubMed] [Google Scholar]
- 29.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56(4):727–31. doi: 10.1097/01.ta.0000119687.23542.ec. discussion 731-3. [DOI] [PubMed] [Google Scholar]
- 30.Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: Are they related? Arch Surg. 2009;144(10):928–932. doi: 10.1001/archsurg.2009.97. [DOI] [PubMed] [Google Scholar]
- 31.Zhou C, Liu B, Sellak H, Shin HS, Wu S. α-1G T-type Ca2+ channel controls plasma membrane P-selectin expression in pulmonary microvascular endothelial cells. FASEB J. 2007;21(6):977–17. [Google Scholar]
- 32.Zhou C, Chen H, King JA, et al. A distinct subcellular pool and an α-1G T-type Ca2+ channel regulated surface expression of P-selectin in pulmonary capillary endothelium. FASEB J. 2009;23:964.13. [Google Scholar]
- 33.Gebb SA, Graham JA, Hanger CC, et al. Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol. 1995;79(2):493–497. doi: 10.1152/jappl.1995.79.2.493. [DOI] [PubMed] [Google Scholar]
- 34.Forlow SB, Foley PL, Ley K. Severely reduced neutrophil adhesion and impaired host defense against fecal and commensal bacteria in CD18-/-P-selectin-/- double null mice. FASEB J. 2002;16(12):1488–1496. doi: 10.1096/fj.02-0230com. [DOI] [PubMed] [Google Scholar]
- 35.Wickel DJ, Mercer-Jones M, Peyton JC, Shrotri MS, Cheadle WG. Neutrophil migration into the peritoneum is P-selectin dependent, but sequestration in lungs is selectin independent during peritonitis. Shock. 1998;10(4):265–269. doi: 10.1097/00024382-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Wu S, Jian MY, Xu YC, et al. Ca2+ entry via alpha1G and TRPV4 channels differentially regulates surface expression of P-selectin and barrier integrity in pulmonary capillary endothelium. Am J Physiol Lung Cell Mol Physiol. 2009;297(4):L650–7. doi: 10.1152/ajplung.00015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S, Haynes J, Taylor JT, et al. Cav3.1 (alpha1G) T-type Ca2+ channels mediate vaso-occlusion of sickled erythrocytes in lung microcirculation. Circulation Research. 2003;93(4):346–53. doi: 10.1161/01.RES.0000087148.75363.8F. [DOI] [PubMed] [Google Scholar]
- 38.Garcia JG, Siflinger-Birnboim A, Bizios R, Del Vecchio PJ, Fenton JW, 2nd, Malik AB. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128(1):96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- 39.Troyanovsky B, Alvarez DF, King JA, Schaphorst KL. Thrombin enhances the barrier function of rat microvascular endothelium in a PAR-1-dependent manner. Am J Physiol Lung Cell Mol Physiol. 2008;294(2):L266–75. doi: 10.1152/ajplung.00107.2007. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Chen H, Lu F, et al. Cav3.1 (alpha1G) controls von willebrand factor secretion in rat pulmonary microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L833–44. doi: 10.1152/ajplung.00377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: A novel mechanism of acute lung injury. Circ Res. 2006;99(9):988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowe K, Alvarez D, King J, Stevens T. Phenotypic heterogeneity in lung capillary and extra-alveolar endothelial cells. Increased extra-alveolar endothelial permeability is sufficient to decrease compliance. Journal of Surgical Research. 2007;143(1):70–77. doi: 10.1016/j.jss.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cioffi DL, Lowe K, Alvarez DF, Barry C, Stevens T. TRPing on the lung endothelium. calcium channels that regulate barrier function. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]