Abstract
Loss of interleukin-7 (IL-2) receptor expression has been described in T lymphocytes from persons with human immunodeficiency virus (HIV) infection, potentially contributing to perturbations in T cell homeostasis. We investigated IL-7 receptor signaling by measuring signal transducer and activator of transcription 5 (STAT5) phosphorylation in CD4+ T cell subsets from HIV-infected persons. We determined that CD45RA− memory cell subsets (both CD27+ and CD27−) displayed the most robust immediate responses to IL-7, whereas naive CD4+ T cells sustained the signal most efficiently. Memory CD4+ T cells with a terminal phenotype (CD45RA+CD27−) responded poorly to IL-7 stimulation. Defects in signaling were observed in cells from viremic HIV-infected persons and were especially pronounced in CD45RA−CD27− memory subset. Although CD127 expression was diminished for T cells from HIV-infected persons, it was not directly related to IL-7 receptor signaling function. Instead, age was inversely related to IL-7 signaling in cells from both HIV-infected viremic subjects and healthy control subjects. Thus, HIV infection results in impaired IL-7 responsiveness, especially in memory CD4+ T cells, and this defect is likely compounded by aging.
Interleukin-7 (IL-7) plays an important role in T cell development and survival. Mice engineered with IL-7 [1] or IL-7 receptor gene deletions [2, 3] are lymphopenic and humans with IL-7 receptor α deficiencies also experience immunodeficiency [4]. IL-7 enhances thymocyte survival [5, 6] and is required for survival of memory and naive T cells [7–9]. Thus, perturbations in IL-7 and/or the IL-7 receptor are likely to have important consequences for immune homeostasis.
IL-7 receptor expression is diminished in cells from HIV-infected persons [10, 11], raising the possibility that IL-7 receptor signaling may be impaired in HIV disease. Our previous studies explored the effects of IL-7 stimulation on naive CD4+ T cells from HIV-infected persons during T cell receptor–driven cellular proliferation [12]. Our results suggested that naive T cells from HIV-infected persons responded to IL-7 stimulation with an intensity similar to that of cells from healthy control subjects. However, we did not directly measure IL-7 responsiveness in the absence of T cell receptor stimulation, nor did we investigate the signaling function of the receptor.
To mediate signaling, IL-7 interacts with the IL-7 receptor complex, consisting of the α chain (CD127) and the common γ chain, which is shared by several other cytokine receptors, including those for IL-2 and IL-15. Binding of IL-7 to the receptor results in activation of JAK3 and JAK1, which then leads to phosphorylation of signal transducer and activator of transcription (STAT) molecules, including STAT5 [13]. The importance of STAT5 signaling in T cell homeostasis has been demonstrated in transgenic mice, in which overexpression of STAT5b results in increased spontaneous proliferation of naive CD4+ and CD8+ T cells [14, 15]. Furthermore, mice with STAT5 gene deletion have reduced numbers of peripheral T lymphocytes [16, 17]. Thus, IL-7–mediated STAT5 activation is an important component to normal T cell homeostasis.
To examine the signaling response of CD4+ T cell subsets to IL-7 stimulation, we have defined CD3+CD4+ T cells on the basis of CD45RA/RO and CD27 coexpression. Memory CD45RO+ T cells can be subcategorized into CD27+ and CD27− subsets. CD27− cells can be generated from prolonged stimulation of T lymphocytes [18]. Functionally, CD27+ memory T cells possess greater proliferative potential when activated by T cell receptor engagement than CD27− memory T cells [19, 20]. Memory CD4+CD45RA+ T cells are enriched for CD57+ terminally differentiated cells [21]. On the basis of this understanding of T cell maturation and functionality, we have examined phosphorylated STAT5 (STAT5-P) induction in CD4+CD3+ T cell subsets, including CD45RA+CD45RO− CD27+ (naive), CD45RA−CD45RO+CD27+ (CD27+ memory), CD45RA−CD45RO+CD27− (CD27− memory) and CD45RA+ CD45RO−CD27− (terminal memory) T cells. We found that these cell subsets have distinct responses to IL-7 stimulation as measured by STAT5-P induction, and we observed reduced responsiveness to IL-7 stimulation in cells from HIV-infected persons, especially memory T cells. These results have important implications for CD4+ T cell homeostasis during HIV disease.
Subjects, Materials, and Methods
Subjects
Written consent was obtained from subjects, and studies were approved by the institution review board at University Hospital (Cleveland, OH). Cells were obtained from 9 healthy adult volunteers, 11 HIV-infected persons who had detectable plasma HIV RNA (load, >400 copies/mL), and 6 treated HIV-infected persons who had undetectable plasma HIV RNA (load, ≤400 copies/mL). Persons with undetectable plasma HIV RNA had been receiving antiretroviral therapy for at least 2 years (mean duration, 7.2 years). Only 1 subject with detectable plasma HIV RNA (load, 17,500 copies/mL) was receiving therapy at the time of the experiments. Further characteristics of the subjects are described in table 1.
Table 1.
Demographic and clinical characteristics of healthy control subjects and HIV-infected patients with or without viremia.
| Characteristic | Control subjects (n = 9) |
Viremic patients (n= 11) |
Aviremic patients (n = 6) |
P |
|---|---|---|---|---|
| Plasma HIV RNA load, copies/mL | … | 28,000 (1790–578,000) | <400 | |
| CD4+ cell count, cells/mL | ||||
| Overall | … | 341 (161–632) | 588 (280–874) | NS |
| Nadir | … | 263 (161–534) | 153 (25–230) | .007 |
| Age, years | 35 (30–48) | 45.5 (36-54) | 42.5 (40-50) | NS |
| CD38 expression, %a | 26 (16–37) | 42 (26–54) | 24 (13–48) | .003b; .018c |
NOTE. Data are median (range), unless otherwise indicated. NS, not significant.
Data are percentage of CD3+CD4+CD45RO− cells.
Between viremic patients and control subjects.
Between viremic and aviremic patients.
Antibodies and reagents
For STAT5-P analyses, anti–CD3-peridinin chlorophyll (BD Biosciences), anti–CD4-Pacific Blue (BD Biosciences), anti–CD45RA-phycoerythrin (BD Pharmingen), anti–CD27-FITC (BD Biosciences), and anti–STAT5-P Alexa Fluor 647 (BD Phosflow) were used to stain cells. Analyses with whole blood were performed with anti-CD3, anti-CD4, anti-CD45RO, anti-CD27, and anti-CD127 antibodies (BD Pharmingen). Recombinant IL-7 was provided by Cytheris.
Assessment of STAT5 phosphorylation
Peripheral blood mononuclear cells (PBMCs) were isolated over a Ficoll cushion. PBMCs (2 × 106 cells/well in 24-well plates) were incubated in medium (RPMI supplemented with 10% FBS, L-glutamine, and antibiotics) with or without IL-7 (5 ng, 0.5 ng, and 0.05 ng/mL) for 15 min at 37°C. Cell cultures were then treated with 100 μL of 16% ultrapure methanol-free formaldehyde (Polysciences) for 10 min at 37°C, transferred to polystyrene tubes, washed with PBS, and resuspended in 500 μL of cold 90% methanol for 30 min. Cells were washed and stained with antibodies for 60 min on ice before analysis with a BD LSRII flow cytometer. Additional cells from the original population incubated for 15 min with IL-7 were washed twice, replated in fresh culture media, and incubated at 37°C for 2 h in the absence of further IL-7 stimulation before harvesting for STAT5-P staining.
Statistical analyses
Cross-sectional analyses between subject categories were performed with Kruskal-Wallis multigroup comparison and Mann-Whitney U tests. Related samples were examined with Friedman multigroup analyses and Wilcoxon signed rank tests. Relationships between continuous variables were assessed with Spearman correlation tests.
Results
Diminished IL-7 receptor signaling in CD4+ T cell subsets from viremic HIV-infected persons
To assess IL-7 receptor signaling in CD4+ T cell subsets, PBMCs were incubated for 15 min in medium alone or in medium supplemented with various concentrations of IL-7. Some cells were stained for STAT5-P expression, whereas other cells from the same cultures were washed and replated for an additional 2-h incubation period. The latter was done to assess the duration of STAT-5P detection after soluble IL-7 was removed. STAT5 phosphorylation was measured as an increase in the percentage of positive cells or as an increase in mean fluorescence intensity (MFI) above the background levels observed for unstimulated cells (figure 1). The donors for these studies are described in table 1 and include healthy adults, viremic HIV-infected persons, and aviremic HIV-infected persons receiving antiretroviral therapy.
Figure 1.
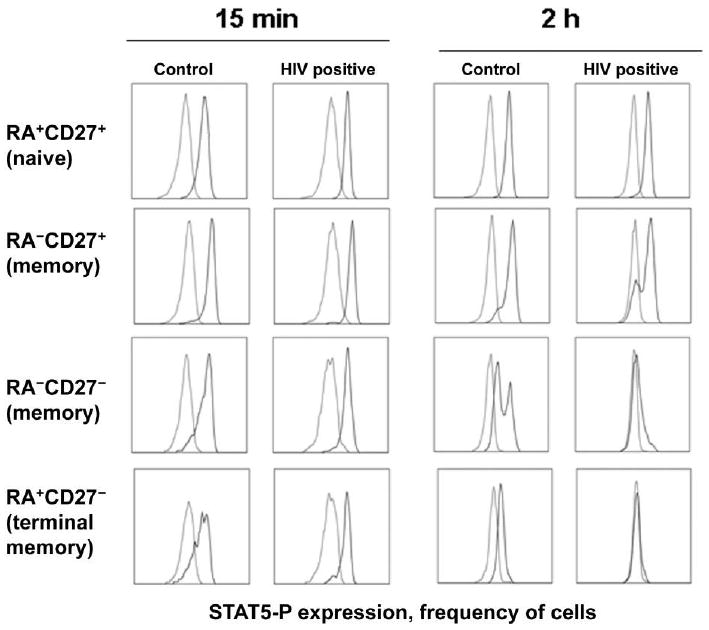
Induction of phosphorylated signal transducer and activator of transcription 5 (STAT5-P) by interleukin-7 (IL-7) in CD4+ T cell subsets. Peripheral blood mononuclear cells from a healthy control subject and an HIV-positive donor (plasma HIV RNA load, 7470 copies/mL; CD4+ cell count, 492 cells/μL) were incubated in medium alone (light gray) or in medium plus IL-7 (5 ng/mL; dark gray). Cells were examined for STAT5-P expression after 15 min by intracellular flow cytometry. Some cells that had been incubated with IL-7 for 15 min were washed twice and replated. These cells were assessed for STAT5-P expression after an additional 2 h of incubation. Cells were gated for lymphocyte forward and side scatter characteristics and further gated for coexpression of CD3 and CD4. CD3+CD4+ cells were then subdivided into subsets on the basis of coexpression of CD45RA and CD27, as indicated. Frequency distribution histograms showing STAT5-P staining are shown for cells in each of the subsets.
In cells from healthy control subjects and HIV-positive donors, the induction of STAT-5 phosphorylation by IL-7 as measured by the percentage of positive cells (figure 2) or by the MFI (figure 3) varied between CD4+ T cell subsets. In cells from healthy control subjects, the magnitude of STAT5-P induction after 15 min of incubation with IL-7 was greatest among CD27+ memory cells and CD27− memory cells, followed by naive and terminal memory subsets (P < .03 by the Wilcoxon signed rank test for comparisons of the percentages of positive cells and MFIs) (figures 2A and 3A). Thus, CD4+ T cell subsets have differential abilities to rapidly phosphorylate STAT5 after IL-7 stimulation, and terminal memory cells are particularly poor responders, compared with the other subsets.
Figure 2.
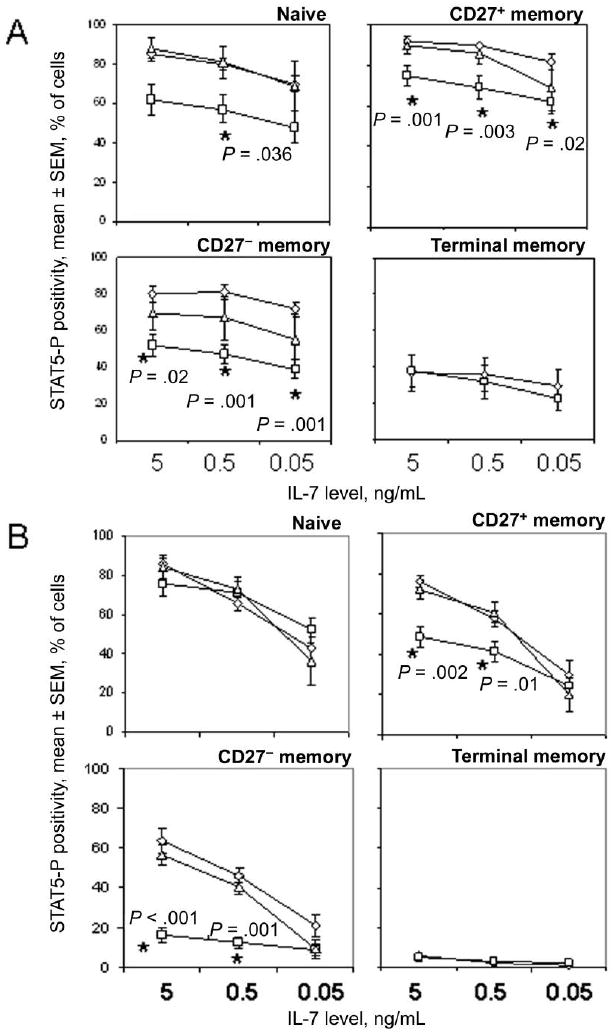
Diminished induction and maintenance of phosphorylated signal transducer and activator of transcription 5 (STAT5-P) signals in CD4+ T cell subsets from HIV-infected persons. Results are shown for cells derived from 9 healthy control subjects (diamonds), 11 viremic HIV-positive donors (HIV RNA load, >400 copies/mL; squares), and 6 aviremic HIV-positive donors (HIV RNA load, ≤400 copies/mL; triangles). Statistical analyses were performed with Kruskal-Wallis and Mann-Whitney U tests. The percentage of cells that expressed STAT5-P in medium alone was used to set the background. A, STAT5-P-positive cells after 15 min of incubation with interleukin-7 (IL-7). B, STAT5-P-positive cells after 15 min of incubation with IL-7 followed by 2 washes, replating, and an additional 2 h of incubation
Figure 3.
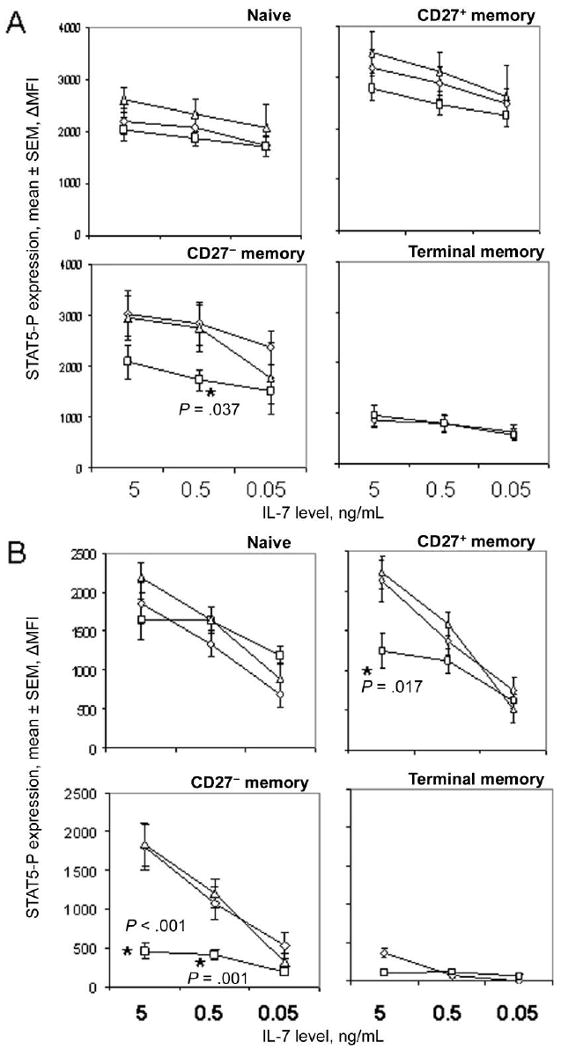
Diminished induction and maintenance of phosphorylated signal transducer and activator of transcription 5 (STAT5-P) signals in CD4+ T cell subsets from HIV-infected persons. The change in mean fluorescence intensity (ΔMFI) was determined by subtracting the MFI of STAT5-P staining in cells incubated in medium alone from that of cells incubated with interleukin-7 (IL-7). Results are shown for cells derived from 9 healthy control subjects (diamonds), 11 viremic HIV-positive donors (HIV RNA load, >400 copies/mL; squares), and 6 aviremic HIV-positive donors (HIV RNA load, ≤400 copies/mL; triangles). Statistical analyses were performed with Kruskal-Wallis and Mann-Whitney U tests. A, ΔMFI of STAT5-P expression in CD4+ T cell subsets after 15 min of incubation with IL-7. B, ΔMFI of STAT5-P expression in CD4+ T cell subsets after IL-7 stimulation for 15 min, followed by 2 washes, replating, and an additional 2 h of incubation.
Differences in IL-7 responsiveness were observed in cells from viremic HIV-positive donors and healthy control subjects. CD27+ memory, CD27− memory, and naive T cells but not terminal memory cells from HIV-infected persons displayed defects in the frequencies of cells induced to express STAT5-P after 15 min of IL-7 stimulation (figure 2A). Deficiencies in patient samples were less obvious when analyzing induction of STAT5-P by MFI, although statistically significant defects in CD27− memory cells from viremic donors were still observed with this approach (figure 3A). Cells from persons receiving antiretroviral therapy and having plasma HIV RNA loads of <400 copies/mL responded as well as cells from healthy control subjects to IL-7 stimulation, suggesting that defects in IL-7 receptor signaling that occur during HIV infection are at least partially reversible. Notably, there were insufficient numbers of terminal memory cells detected in samples from subjects receiving antiretroviral therapy to permit reliable analyses of STAT5-P signaling. Baseline expression of STAT5-P, measured in cells incubated for 15 min without stimulation and compared with expression in an isotype control stain, was not significantly different between patient and control samples (data not shown), with the exception of naive CD4+ T cells from viremic HIV-positive donors, which expressed a modest increase in STAT5-P staining intensity, compared with cells from healthy control subjects (change in MFI, 196 and 399 for patients and control subjects, respectively; P < .05). Overall, these results suggest that the frequencies of IL-7-responsive CD4+ T cells decrease during untreated HIV infection and that CD27− memory cells from viremic HIV-infected persons have the most severe deficiencies in IL-7 responsiveness.
The ability of cells to sustain a STAT5-P signal after removal of IL-7 was dependent on IL-7 concentration and T cell subset (figures 2B and 3B). Interestingly, 2 peaks of STAT5-P staining were noted in some cell populations at the 2 h time point (figure 1), suggesting heterogeneity in the ability of T cells in the subsets to maintain the signal. Analyses of cells from healthy control subjects indicated that, in contrast to the early induction of STAT5-P, which was most pronounced in the CD27+ and CD27− memory cells, sustained signaling of STAT5-P was most efficient in naive CD4+ T cells, followed by CD27+ memory cells, CD27− memory cells, and terminal memory cells. For example, in cells from healthy control donors, the average frequencies of STAT5-P-positive cells at 2 h were 100%, 82%, and 61% of the average frequencies observed at 15 min for naive T cells stimulated with 5 ng/mL, 0.5 ng/mL, and 0.05 ng/mL of IL-7, respectively, whereas values were 83%, 64%, and 36% for CD27+ memory cells; 80%, 57%, and 29% for CD27− memory cells; and 16%, 6%, and 3% for terminal memory cells. These values were significantly different at each IL-7 concentration when comparing naive T cells to any other subset (P < .05 by the Wilcoxon signed rank test for all comparisons) and when comparing either CD27+ or CD27− memory cells to terminal memory T cells (P < .03 for all comparisons).
STAT5-P signaling at 2 h was markedly reduced in CD27− memory cells from viremic HIV-infected persons, compared with cells from healthy control subjects and aviremic HIV-positive donors (figures 2B and 3B). STAT5-P expression in CD27+ cells from viremic HIV-infected persons was also clearly impaired at 2 h at higher concentrations of IL-7. Terminal memory cells displayed a relative failure to maintain STAT5-P signaling at 2 h even in cells from healthy control subjects. In contrast to the impairments in memory subsets, naive CD4+ T cells from viremic HIV-positive donors sustained STAT5-P signaling as efficiently as cells from healthy control subjects at all concentrations of IL-7 tested. These results suggest that T cell subsets regulate STAT5 signaling differently and that CD27− memory and CD27+ memory cells from viremic HIV-infected persons are deficient in their capacity to maintain STAT5-P signals.
Notably, HIV-infected persons (viremic and aviremic) maintained significantly higher frequencies of CD27− memory T cells in the CD4+ T cell population than did healthy control subjects (figure 4A). Thus, HIV infection resulted in a skewing of CD4+ T cell populations toward higher frequencies of CD27− memory cells, in which the IL-7 signaling defects appeared to be most pronounced.
Figure 4.
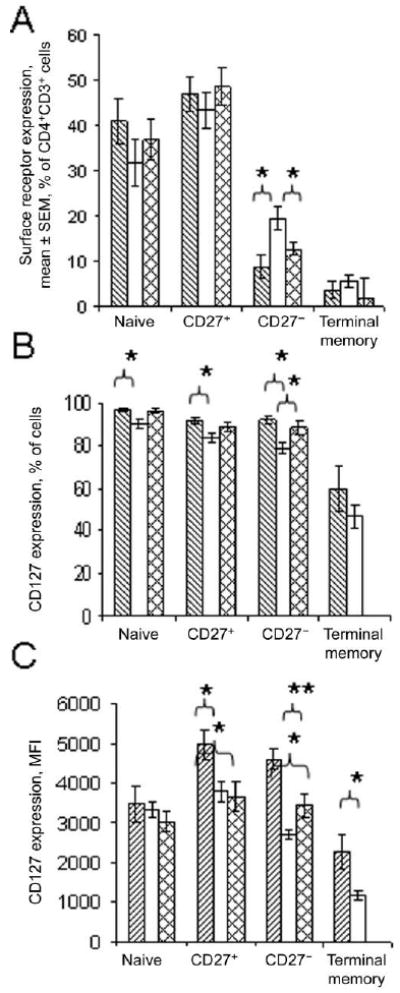
Enrichment of CD27− memory T cells and diminished expression of CD127 in CD4+ T cell subsets from HIV-infected persons. Whole blood was analyzed by staining cells for expression of CD3, CD4, CD45RO, CD27, and CD127. Data are shown for 9 healthy control subjects (lined bars), 11 viremic HIV-positive donors (HIV RNA oad, >400 copies/mL; empty bars), and 6 aviremic HIV-positive donors (HIV RNA load, ≤400 copies/mL; crosshatched bars). Statistical analyses comparing cells from HIV-positive donors and control subjects were performed with Kruskal-Wallis and Mann-Whitney U tests. A, Percentages of CD27+CD45RO− naive, CD27+CD45RO+ memory, CD27−CD45RO+ memory, and CD27−CD45RO− terminal memory cells in the CD4+CD3+ T cell subset. *P < .02. B, Percentage of cells expressing CD127. *P < .05. C, Mean fluorescence intensity (MFI) of CD127 expression in CD4+ T cell subsets. *P < .05 and **P < .005. In C and B, insufficient numbers of events were collected to permit analyses of CD127 expression on terminal memory cells from the aviremic HIV-positive patient population.
Deficiencies in IL-7 receptor expression in CD4+ T cell subsets from viremic HIV-positive donors
We next asked whether STAT5-P signaling was associated with expression of IL-7 receptor in freshly isolated cells from these subjects. Cells were gated as CD3+CD4+ and further distributed into subsets on the basis of surface expression of CD27 and CD45RO. Because CD45RO and CD45RA have reciprocal expression patterns in T cells [22], we designated the subsets as CD45RO−CD27+ (naive), CD45RO+CD27+ (CD27+ memory), CD45RO+CD27− (CD27− memory), and CD45RO−CD27− (terminal memory) cells. As reported by others [10], we found evidence of diminished IL-7 receptor expression in T cells from HIV-infected persons (figure 4B and 4C). The percentages of cells expressing CD127 were significantly reduced in CD27+ memory, CD27− memory, and naive CD4+ T cell subsets from viremic HIV-positive donors, compared with percentages in T cell subsets from control donors (figure 4B). The intensity of CD127 staining measured by MFI also tended to be reduced in memory CD4+ T cells from HIV-positive donors but not in the subset of naive T cells (figure 4C).
Normal regulation of IL-7 receptor expression in CD4+ T cell subsets from HIV-infected persons
Diminution of IL-7 receptor expression during HIV disease has been associated with increased serum concentrations of this cytokine [23, 24], suggesting that exposure to greater concentrations of IL-7 in vivo may result in the reduction of cell surface CD127 expression. We considered the alternative hypothesis that cells from HIV-infected persons may have intrinsic differences in the capacity to modulate receptor expression in the presence or absence of IL-7. To assess this possibility, we investigated CD127 regulation by cells from HIV-infected persons and healthy control subjects after incubation for 18 h in medium alone or with IL-7 (5 ng/mL). From previous reports involving mouse cells [25] and human cells [24], we expected that removal of cells from the in vivo cytokine milieu would result in increased surface expression of CD127, whereas incubation in the presence of IL-7 would result in down-modulation of CD127 surface expression. As anticipated, CD127 expression increased in cultured CD4+ T cells above the levels measured in freshly isolated cells. Interestingly, although all cultured CD4+ T cell subsets displayed evidence of increased CD127 surface expression above baseline levels, the relative increase in CD127 expression was most prominent in terminal memory cells (figure 5A). CD27− and CD27+ memory cells increased surface CD127 expression to a similar extent with in vitro culture, whereas naive CD4+ T cells increased CD127 significantly less than any other subset. These trends were observed in cells from healthy control subjects and HIV-infected persons. The relative increase in CD127 expression was not significantly different between CD4+ T cell subsets from HIV-infected persons and those from healthy control subjects, suggesting that cells from HIV-infected persons retain the capacity to increase surface CD127 expression in the absence of IL-7 stimulation.
Figure 5.
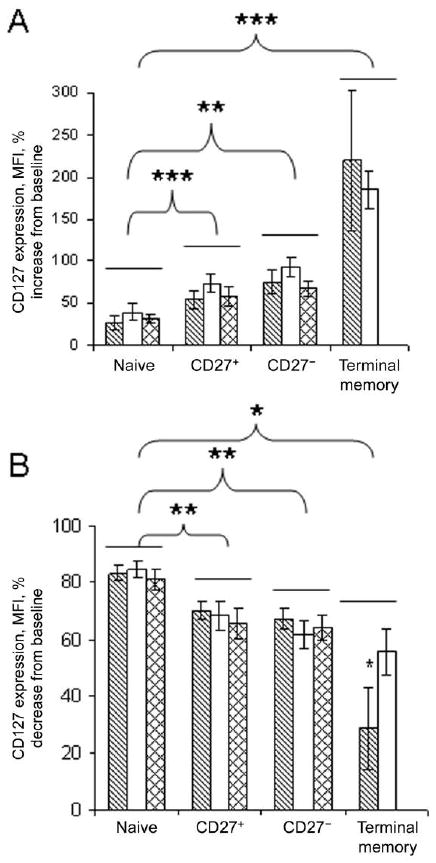
Normal regulation of CD127 expression in CD4+ T cells from HIV-infected persons. Peripheral blood mononuclear cells were stained immediately after isolation for detection of CD127 expression or were incubated overnight with or without 5 ng/mL of interleukin-7 (IL-7). No significant differences were found between cells from patients and cells from control subjects. However, values for terminal memory cells were significantly different from those for CD27+ and CD27− memory cells in the control samples. Statistical analyses were performed with Friedman and Wilcoxon signed rank tests for related samples. *P < .02, **P < .03, and ***P < .05. A, Percentage increase of CD127 mean fluorescence intensity (MFI) above the levels determined in freshly isolated cells among CD4+ T cell subsets that had been incubated for 18 h in medium alone. B, Percentage decrease in CD127 MFI below the levels observed in freshly isolated cells after 18 h incubation in the presence of 5 ng/mL of IL-7.
We also assessed the relative down-modulation of CD127 in CD4+ T cell subsets after continuous exposure to IL-7 in overnight culture. Naive T cells displayed the most robust loss of CD127, approximating an 80% loss of staining intensity, compared with freshly isolated cells, whereas CD27+ memory and CD27− memory cells lost 60%–70% of the original staining intensity for CD127, and terminal memory cells lost 28% in control subjects and 43% in HIV-positive patients (figure 5B). There were no differences in the relative down-modulation of CD127 expression between cells from HIV-infected persons and cells from healthy control subjects. These results suggest that IL-7 receptor perturbations during HIV disease are probably not the result of intrinsic defects in T cells from HIV-infected persons.
CD127 expression is related to the nadir CD4+ cell count among CD27+ memory and naive CD4+ T cells, whereas induction of STAT-5P is related to age in viremic HIV-infected persons
Of the clinical indices of plasma HIV RNA load, CD4+ T cell count, nadir expression of CD4, age, and percentage of memory cells expressing CD38, we found that the percentage of cells expressing CD127 was directly related to the nadir CD4+ cell count in CD27+ memory T cell subsets (r = 0.702; P = .016) and naive CD4+ T cell subsets (r = 0.722; P = .005) from viremic HIV-positive donors. Although CD127 expression was diminished in CD4+ T cells from HIV-infected persons, we did not observe a direct relationship between receptor expression and STAT5-P signaling in any of the T cell subsets when measured as continuous variables (data not shown). Of the other indices examined, the most consistent relationship observed was an inverse association between age and STAT5-P signaling (table 2). For example, among CD27+ memory cells, the percentages that were STAT5-P positive after 15 min of incubation with IL-7 (5 ng/mL) were inversely related to age (r = −0.658; P = .028), and the ability of cells to sustain STAT5-P signals as measured by the percentage of cells positive for STAT5-P was inversely related to age among both CD27+memory cells (r = − 0.716; P = .02) and naive CD4+T cells (r = −0.635; P = .049). A similar analysis involving cells from healthy control subjects provided evidence that age influenced the responsiveness of CD4+ T cells to IL-7 in these subjects as well (table 2). Thus, the ability to signal via the IL-7 receptor is altered by HIV infection and likely diminishes with age.
Table 2.
Relationships between age and phosphorylated signal transducer and activator of transcription 5 (STAT5-P) expression 15 min and 2 h after interleukin-7 (IL-7) stimulation in CD4+ T cell subsets.
| Study group, T cell subset | 15 min | 2h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cells in subset, % | ΔMFI | IL-7 responsiveness, % decreasea | Cells subset in, % | ΔMFI | IL-7 responsiveness, % decreasea | |||||
| r | P | r | P | r | P | r | P | |||
| Viremic, HIV positive | ||||||||||
| Naive | −0.396 | .228 | −0.301 | .368 | … | −0.635 | .049 | −0.37 | .26 | 9.0 |
| CD27+ memory | −0.658 | .028 | −0.201 | .554 | 4.2 | −0.716 | .02 | −0.626 | .04 | 7.4 |
| CD27− memory | −0.060 | .862 | −0.137 | .688 | … | −0.021 | .953 | −0.543 | .08 | … |
| Control | ||||||||||
| Naive | −0.83 | .006 | −0.45 | .22 | 3.8 | −0.251 | .5 | −0.15 | .7 | … |
| CD27+ memory | −0.715 | .021 | −0.37 | .33 | 1.4 | −0.22 | .57 | −0.37 | .33 | … |
| CD27− memory | −0.27 | .47 | −0.483 | .19 | … | −0.756 | .018 | −0.7 | .036 | 9.6 |
NOTE. Data are for 11 viremic HIV-positive patients and 9 healthy control subjects. ΔMFI, change in mean fluorescence intensity.
Data indicate the percentage of cells that lost responsiveness to interleukin-7 stimulation (STAT5-P induction) over a 5-year period. Values were calculated from the slope of the linear regression lines, using Microsoft Excel
Discussion
HIV disease is characterized by numeric and phenotypic perturbations in CD4+ T cell subsets. For example, naive CD4+ T cells and CD8+ T cells decrease during HIV disease progression [26]. The loss of naive T cells may be a consequence of direct viral infection, reduced thymic output [27, 28], fibrosis of lymph node tissues [29], and increased immune activation that results in cell death or conversion into the memory pool [30, 31]. Conceivably, reduced IL-7 responsiveness also could play a role in the decrease of naive CD4+ T cells during HIV infection. However, our studies suggest that naive T cells largely maintain responsiveness to IL-7 and also have relatively preserved expression of CD127 even in persons with uncontrolled viral replication.
Memory T cells are also altered during HIV disease. These cells have increased rates of cellular proliferation [32–35] and also express heightened levels of activation markers that predict disease progression [36, 37]. Elevated immune activation and cellular turnover are distinctly absent in disease-resistant, SIV-infected sooty mangabeys [38], suggesting that perturbations in memory cell homeostasis may play an important role in pathogenesis. Current models of HIV and SIV pathogenesis suggest that effector memory T cells are rapidly depleted from mucosal sites [39, 40]. The loss of these cells may place an increased burden on central memory T cells to replenish tissue-associated memory cells via homeostatic proliferation and differentiation [41]. Diminished IL-7 responsiveness in memory T cells, therefore, could potentially contribute to reduced survival and inefficient replacement of tissue memory cells in SIV or HIV infection.
Similar to other groups, we found evidence of IL-7 receptor loss in CD4+ T cells from HIV-positive donors; however, the mechanism that mediates this reduction is still uncertain. IL-7 receptor expression is typically reduced on exposure to IL-7 or other cytokines that use the common γ chain for signaling [25]. Interestingly, findings from recent studies involving lymph node tissues suggest that IL-2 and IL-15 expression maybe increased at these sites during HIV disease [42]. Thus, along with increased IL-7 concentrations, exposure to other cytokines might also contribute to down-modulation of CD127 during HIV disease. Exposure to these inflammatory or IL-7-rich microenvironments could differentially affect CD127 expression in T cell subsets, depending on migratory and other intrinsic properties of these cells.
Among CD4+ T cell subsets, we found differences in IL-7 receptor signaling function as well as in receptor regulation. The immediate response to IL-7 appeared to be most robust in CD45RA− memory cells, whereas the sustainability of the signal was best demonstrated in naive CD4+ T cells. These differences may stem in part from the relative levels of CD127 expression on the surface of these cells but also raise the possibility of differences in the expression of molecules that might regulate STAT5 signaling, such as suppressor of cytokine signaling proteins [43, 44]. Our observations are consistent with those from studies of primary T cells maintained in long-term cultures with IL-7 [45]. In these studies, naive T cells were better able to maintain STAT5-P signaling over a period of 20 days, compared with memory T cells; STAT5-P signaling became deficient on day 27 of culture in both T cell subsets; and, similar to our findings, deficiencies in STAT5-P signaling were not fully explained by IL-7 receptor levels. Overall, it is possible that exposure of T cells to high concentrations of IL-7 in vivo may result in hyporesponsiveness to further stimulation that is not fully the consequence of receptor down-modulation. Therefore, further studies exploring signaling regulation downstream of the IL-7 receptor maybe informative in these T cell subsets.
Our results also uncover a unique characteristic of terminal memory CD4+ T cells in that such cells, even those from healthy control subjects, have a relatively poor response to IL-7 stimulation. These cells also express relatively low levels of CD127, suggesting that terminal memory cells may have a reduced requirement for IL-7 signaling for their survival or, alternatively, that loss of IL-7 responsiveness could lead to their eventual death.
We found an inverse relationship between IL-7 receptor signaling function and age. This relationship appears to exist in essentially all CD4+ T cell subsets, with the exception of terminal memory cells (data not shown) and is observed in cells from both HIV-positive donors and healthy control subjects. We did not find an association between age and CD127 receptor expression in CD4+ T cell subsets. However, this may in part stem from the relatively small sample size used here. The mechanism behind the age-associated decrease in IL-7 responsiveness is unknown, but it may play an important role in T cell homeostasis and HIV pathogenesis in older persons with HIV infection.
Recent studies in SIV-infected monkeys [46, 47] and in HIV-infected humans [48] indicate that IL-7 may be a promising therapeutic intervention that leads to increased CD4+ T cell counts and naive T lymphocyte counts [48]. Use of IL-7 in conjunction with antiretroviral therapy is likely to provide the most substantial benefit to patients because this will reduce the potential risk for IL-7 to promote viral replication [49, 50]. Also, as suggested by our observations, effective antiretroviral therapy will likely improve IL-7 responsiveness in cells from HIV-infected persons. Nonetheless, variability in the magnitude of this improvement and the age of the patient when treatment is initiated may factor into how well IL-7 mediates T cell reconstitution during HIV disease.
Acknowledgments
We thank Dr. Robert Asaad, for providing patient samples, and Cytheris, for providing rIL-7.
Center for AIDS Research (grant Al 36219); National Institutes of Health (grant Al 076174).
Footnotes
Potential conflicts of interest: none reported.
References
- 1.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–9. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 3.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giliani S, Mori L, de Saint Basile G, et al. Interleukin-7 receptor α (IL-7Rα) deficiency: cellular and molecular bases: analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev. 2005;203:110–26. doi: 10.1111/j.0105-2896.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano LA, Stoddart CA, Hanley MB, Wieder E, McCune JM. Effects of IL-7 on early human thymocyte progenitor cells in vitro and in SCID-hu Thy/Liv mice. J Immunol. 2003;171:645–54. doi: 10.4049/jimmunol.171.2.645. [DOI] [PubMed] [Google Scholar]
- 6.Okamoto Y, Douek DC, McFarland RD, Koup RA. Effects of exogenous interleukin-7 on human thymus function. Blood. 2002;99:2851–8. doi: 10.1182/blood.v99.8.2851. [DOI] [PubMed] [Google Scholar]
- 7.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 8.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 9.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercier F, Boulassel MR, Yassine-Diab B, et al. Persistent human immunodeficiency virus-1 antigenaemia affects the expression of interleukin-7Rα on central and effector memory CD4+ and CD8+ T cell subsets. Clin Exp Immunol. 2008;152:72–80. doi: 10.1111/j.1365-2249.2008.03610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 12.Bazdar DA, Sieg SF. Interleukin-7 enhances proliferation responses to T-cell receptor stimulation in naive CD4+ T cells from human immunodeficiency virus-infected persons. J Virol. 2007;81:12670–4. doi: 10.1128/JVI.00476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foxwell BM, Beadling C, Guschin D, Kerr I, Cantrell D. Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur J Immunol. 1995;25:3041–6. doi: 10.1002/eji.1830251109. [DOI] [PubMed] [Google Scholar]
- 14.Burchill MA, Goetz CA, Prlic M, et al. Distinct effects of STAT5 activation on CD4+ and CD8+ T cell homeostasis: development of CD4+CD25+ regulatory T cells versus CD8+ memory T cells. J Immunol. 2003;171:5853–64. doi: 10.4049/jimmunol.171.11.5853. [DOI] [PubMed] [Google Scholar]
- 15.Taylor DK, Walsh PT, LaRosa DF, et al. Constitutive activation of STAT5 supersedes the requirement for cytokine and TCR engagement of CD4+ T cells in steady-state homeostasis. J Immunol. 2006;177:2216–23. doi: 10.4049/jimmunol.177.4.2216. [DOI] [PubMed] [Google Scholar]
- 16.Seki Y, Yang J, Okamoto M, et al. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–70. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 17.Yao Z, Cui Y, Watford WT, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci USA. 2006;103:1000–5. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hintzen RQ, de Jong R, Lens SM, Brouwer M, Baars P, van Lier RA. Regulation of CD27 expression on subsets of mature T-lymphocytes. J Immunol. 1993;151:2426–35. [PubMed] [Google Scholar]
- 19.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yue FY, Kovacs CM, Dimayuga RC, Parks P, Ostrowski MA. HIV-1-specific memory CD4+ T cells are phenotypically less mature than cytomegalovirus-specific memory CD4+ T cells. J Immunol. 2004;172:2476–86. doi: 10.4049/jimmunol.172.4.2476. [DOI] [PubMed] [Google Scholar]
- 21.Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005;175:8415–23. doi: 10.4049/jimmunol.175.12.8415. [DOI] [PubMed] [Google Scholar]
- 22.Prince HE, York J, Jensen ER. Phenotypic comparison of the three populations of human lymphocytes defined by CD45RO and CD45RA expression. Cell Immunol. 1992;145:254–62. doi: 10.1016/0008-8749(92)90329-n. [DOI] [PubMed] [Google Scholar]
- 23.Rethi B, Fluur C, Atlas A, et al. Loss of IL-7Rα is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS. 2005;19:2077–86. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 24.Sasson SC, Zaunders JJ, Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and Increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis. 2006;193:505–14. doi: 10.1086/499309. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Yu Q, Erman B, et al. Suppression of IL7Rα transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 26.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–6. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21:757–68. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 29.Schacker TW, Brenchley JM, Beilman GJ, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol. 2006;13:556–60. doi: 10.1128/CVI.13.5.556-560.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delobel P, Nugeyre MT, Cazabat M, et al. Naive T-cell depletion related to infection by ×4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. J Virol. 2006;80:10229–36. doi: 10.1128/JVI.00965-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Mascio M, Sereti I, Matthews LT, et al. Naive T-cell dynamics in human immunodeficiency virus type 1 infection: effects of highly active antiretroviral therapy provide insights into the mechanisms of naive T-cell depletion. J Virol. 2006;80:2665–74. doi: 10.1128/JVI.80.6.2665-2674.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–41. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lempicki RA, Kovacs JA, Baseler MW, et al. Impact of HIV-1 infection and highly active antiretroviral therapy on the kinetics of CD4+ and CD8+ T cell turnover in HIV-infected patients. Proc Natl Acad Sci USA. 2000;97:13778–83. doi: 10.1073/pnas.250472097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohri H, Perelson AS, Tung K, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. J Exp Med. 2001;194:1277–87. doi: 10.1084/jem.194.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sieg SF, Rodriguez B, Asaad R, Jiang W, Bazdar DA, Lederman MM. Peripheral S-phase T cells in HIV disease have a central memory phenotype and rarely have evidence of recent T cell receptor engagement. J Infect Dis. 2005;192:62–70. doi: 10.1086/430620. [DOI] [PubMed] [Google Scholar]
- 36.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–7. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Silvestri G, Sodora DL, Koup RA, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–52. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 39.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–31. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 41.Okoye A, Meier-Schellersheim M, Brenchley JM, et al. Progressive CD4+ central memory T cell decline results in CD4+ effector memory insufficiency and overt disease in chronic SIV infection. J Exp Med. 2007;204:2171–85. doi: 10.1084/jem.20070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cornish AL, Chong MM, Davey GM, et al. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–61. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 44.Fujimoto M, Naka T, Nakagawa R, et al. Defective thymocyte development and perturbed homeostasis of T cells in STAT-induced STAT inhibitor-1/suppressors of cytokine signaling-1 transgenic mice. J Immunol. 2000;165:1799–806. doi: 10.4049/jimmunol.165.4.1799. [DOI] [PubMed] [Google Scholar]
- 45.Swainson L, Verhoeyen E, Cosset FL, Taylor N. IL-7R α gene expression is inversely correlated with cell cycle progression in IL-7-stimulated T lymphocytes. J Immunol. 2006;176:6702–8. doi: 10.4049/jimmunol.176.11.6702. [DOI] [PubMed] [Google Scholar]
- 46.Beq S, Nugeyre MT, Ho Tsong Fang R, et al. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol. 2006;176:914–22. doi: 10.4049/jimmunol.176.2.914. [DOI] [PubMed] [Google Scholar]
- 47.Nugeyre MT, Monceaux V, Beq S, et al. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J Immunol. 2003;171:4447–53. doi: 10.4049/jimmunol.171.8.4447. [DOI] [PubMed] [Google Scholar]
- 48.Levy Y, Weiss L, Viard JP, et al. Repeated r-hIL-7 doses improve T-cell recovery in HIV-1-infected patients enrolled in a phase I/II multicentric study. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 25–28 February 2007. abstract 127. [Google Scholar]
- 49.Managlia EZ, Landay A, Al-Harthi L. Interleukin-7 induces HIV replication in primary naive T cells through a nuclear factor of activated T cell (NFAT)-dependent pathway. Virology. 2006;350:443–52. doi: 10.1016/j.virol.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Smithgall MD, Wong JG, Critchett KE, Haffar OK. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J Immunol. 1996;156:2324–30. [PubMed] [Google Scholar]


